Introduction
Nerve growth factor (NGF) has essential roles in the
survival, development and differentiation of neurons in the nervous
system (1). It is known that NGF
also plays an important role in a variety of non-neuronal systems.
NGF and its receptors have been identified in normal prostatic
tissue, benign prostatic hyperplasia and prostatic cancer,
suggesting a potential role in the cell biology of these tissues
(2).
The prostate is the second most abundant source of
NGF following the central nervous system (3). In order for NGF to have an
antiproliferative effect, it is crucial that two NGF receptors,
namely p75 and tropomyosin-related kinase A are coexpressed. In
prostate tumorigenesis the expression of p75 is increasingly lost
and its disappearance is an indication of prostate adenocarcinoma.
Additionally, dysregulation of NGF signal transduction was
identified in a number of human tumors (4). NGF may also contribute to benign
proliferation, as it has been observed in chronic prostate and
chronic pelvic pain syndrome, since the abundance of NGF in
prostate secretions varied in proportion to pain severity.
It has been reported that NGF expression is absent
or present at low levels in the ventral rat prostate, whereas basal
levels of expression are present in the dorsal rat prostate.
Denervation of the rat prostate induces the expression of NGF in
the ventral prostate and increases the level of its expression in
the dorsal prostate (5). It has
previously been suggested that NGF localizes to the columnar
secretory epithelium lines of prostate tissue in rats (6).
Our previous study showed that two weeks of stress
induced by restraint water-immersion stress (WIRS) led to
hyperplastic morphological abnormalities in the ventral prostate of
adult male Wistar rats (7). In
addition, chemical sympathectomy with 6-hydroxydopamine (6-OHDA)
promoted the dilation of prostatic alveoli and atrophy of the
epithelium in all prostatic lobes.
In the present study, we hypothesized that NGF was
involved in the hyperplasia and atrophy responses observed in our
previous study. The purpose of the present study was to investigate
the changes in NGF levels in the prostate of the male rat in
response to chronic stress and sympathetic denervation.
Materials and methods
Animals and ethics statement
This study was carried out in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals of Shandong University (Jinan, China). The protocol was
approved by the Committee on the Ethics of Animal Experiments of
Shandong University (permit number, ECAESDUSM 20123008). All
surgery was performed under chloral hydrate anesthesia, and all
efforts were made to minimize suffering.
Male Wistar rats were purchased from the Animal
Center of Shandong University and were maintained on standard
laboratory food and water ad libitum with a 12-h light/dark
photoperiod. The rats were raised for one week and handled for 2
min daily to facilitate acclimation. The chemical sympathectomy
model with 6-OHDA (Sigma-Aldrich, St. Louis, MO, USA), as described
by Vaalasti et al (8), was
used on the ventral prostate of the rat, and the rats were
additionally subjected to WIRS, as described previously (9). Sexually mature rats (90 days of age)
were divided into four groups of eight rats each, as follows: i)
Untreated control group; ii) WIRS group (WIRS for 1 h/day for two
weeks); iii) 6-OHDA group (6-OHDA administered intravenously on
days 1 and 8 at a dose of 50 mg/kg); and iv) WIRS plus 6-OHDA group
(WIRS daily plus 6-OHDA on days 1 and 8 as above). The experiment
was terminated at 14 days; animals were anesthetized by chloral
hydrate intraperitoneally and then sacrificed by
exsanguination.
The prostatic lobes were exposed immediately by a
mid-abdominal incision. The ventral, lateral and dorsal prostatic
lobes of the rat were removed separately and immediately fixed in
10% formaldehyde for histology and immunohistochemical
observation.
Histopathology processing
The prostatic lobes were fixed in 10% formalin
solution for 10 h at room temperature. Thereafter, the samples were
routinely processed. Paraffin-embedded sections of 4 μm were cut
and stained by Harris’ hematoxylin and eosin.
Immunohistochemistry
The paraffin-embedded tissues were sectioned at 4 μm
thickness and mounted on poly-L-lysine-coated glass slides for
>24 h at 32°C. The sections were deparaffinized with xylene and
rehydrated in graded ethanol, then exposed to a microwave oven at
100°C for 15 min in sodium citrate buffer (0.01 M, pH 6.0) for
antigen retrieval. Antigen retrieval was followed by incubation in
3% H2O2 at room temperature and then in 5%
bovine serum albumin confining liquid. The tissue sections were
subsequently incubated overnight at 37°C with polyclonal antibodies
(1:100) against NGF (Boster Biological Technology Co., Ltd., Wuhan,
China). The negative control sections were incubated with
phosphate-buffered saline (PBS) in the absence of the primary
antibody for each reaction.
For the immunoreaction, biotinylated goat
anti-rabbit secondary antibodies and streptavidin-biotin complex
(Boster Biological Technology Co., Ltd.) were used and the color
reaction was detected using
3,3′-diaminobenzidine-H2O2 substrate
according to the manufacturer’s instructions. The semi-quantitative
assessment of NGF expression was performed using the Image-Pro Plus
software 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). The
positive area was expressed by mean optical density.
Statistical analysis
The evaluation of differences among the groups was
performed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). The
statistical analysis was conducted using the two-way analysis of
variance test, and P≤0.05 was considered to indicate a
statistically significant difference.
Results
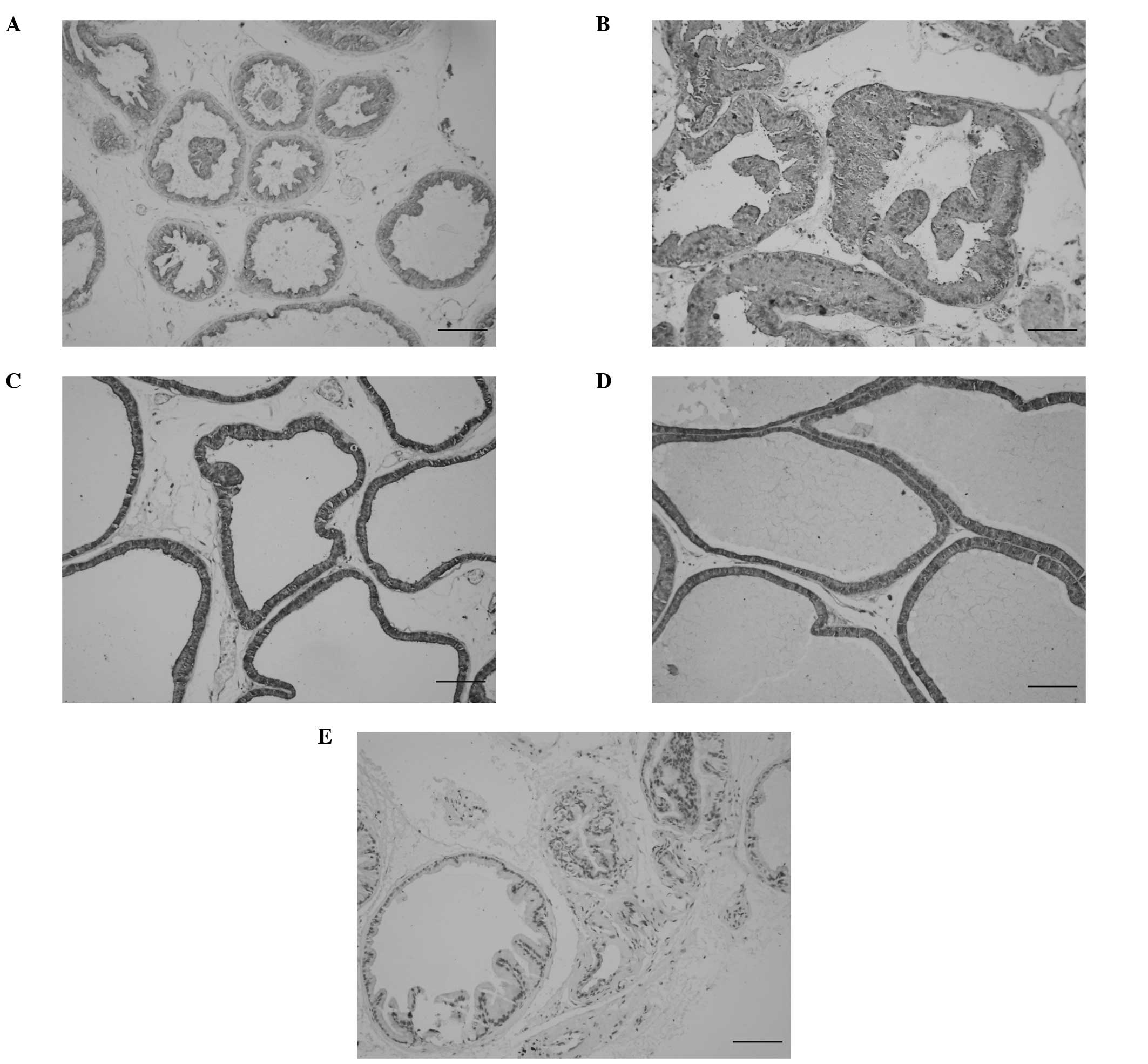
In the untreated control rats, the acini of each
prostatic lobe were rounded with secretion and lined by a single
layer of epithelium. An immunopositive reaction for NGF was
detected in the columnar secretory epithelium lines of the prostate
(Fig. 1A). Chronic stress induced
a proliferative change in the epithelium of the ventral lobes,
which was characterized by intraluminal villous enfolding, as well
as the appearance of epithelial nodules, piling up of epithelial
cells and a loss of cell polarity (Fig. 1B). Chemical sympathectomy led to
the dilation of the prostatic alveoli and atrophy of the epithelium
in all lobes (Fig. 1C). The level
of NGF increased significantly following stress or denervation in
the ventral lobes, with higher levels following the latter
(P<0.05). The dorsolateral lobes were almost unaffected in
response to stress and denervation (Table I). In the WIRS plus 6-OHDA group,
the ventral prostatic acini were dilated and no hyperplasia was
observed. In this group, the NGF level in the ventral lobes was
higher than that in the lobes in the untreated group (Fig. 1D). No immunostaining was detected
in the control sections in which PBS was substituted for the
primary antibody (Fig. 1E).
 | Table IExpression of nerve growth factor in
prostatic lobes measured in different groups. |
Table I
Expression of nerve growth factor in
prostatic lobes measured in different groups.
| Prostate region | Untreated (OD) | WIRS (OD) | 6-OHDA (OD) | WIRS+6-OHDA (OD) |
|---|
| Ventral prostate | 132.9±2.3 | 139.4±5.1a | 146.2±6.2a,b | 140.4±7.0a |
| Lateral prostate | 135.0±3.3 | 133.0±7.0 | 137.0±5.6 | 138.1±3.5 |
| Dorsal prostate | 133.1±9.7 | 126.2±5.6 | 131.0±5.6 | 130.2±6.6 |
Discussion
Several species, including humans, exhibit a
relative abundance of NGF within the prostate (10,11).
Prostate tissue derived from normal, benign prostatic hyperplasia
and adenocarcinoma specimens has indicated that NGF immunoreactive
protein is localized in the stromal compartment (12). NGF peptides play a physiological
role in the control of prostate epithelial cell growth and
differentiation in a paracrine interactive manner (11). A previous study showed that
immunoreactive NGF was localized in the prostatic epithelium lines
of adult male rats (6). This is
consistent with the data in the present study.
Conflicting findings have been reported regarding
the expression of NGF induced by denervation. The analysis of NGF
expression in the rat prostate by quantitative polymerase chain
reaction techniques showed that this neurotrophin was absent or
present at low levels in the ventral prostate and present at basal
levels in the dorsal prostate. Furthermore, denervation of the rat
prostate induced the expression of NGF in the ventral prostate and
increased the level of its expression in the dorsal prostate
(5). Mesenteric arterial
denervation significantly reduced the NGF levels in the adventitial
layer and ganglia. Loss of the arterial nerve plexus resulted in a
reduction in NGF expression levels; however, NGF content in the
artery was not altered by sympathetic decentralization (13).
Our previous study demonstrated that sympathetic
nervous system overactivity is involved in the pathogenesis of
hyperplasia in the rat ventral prostate induced by chronic stress
(7). Chemical sympathectomy caused
apparent atrophy of the prostatic epithelium. NGF levels increased
in the ventral prostate following chronic stress or denervation.
The dorsal and lateral lobes were almost unaffected. These findings
suggest that NGF may be a contributing mechanism to the hyperplasia
in ventral lobes. Denervation leads to a significant increase in
NGF expression levels in these lobes, which suggests that NGF has
the physiological function of attempting to re-establish
appropriate innervation. A previous study revealed that NGF could
prevent prostate tumor growth through an indirect effect, possibly
innervation of the tumor neovasculature (14).
Hyperplastic morphological abnormalities in the
ventral prostate that progress with age have been observed in the
spontaneously hypertensive rat (SHR), a genetic model of
hypertension (15). SHRs exhibit
excessive basal and environmentally evoked sympathetic activity and
demonstrate increased production of NGF by smooth muscles such as
the bladder (16). Kim et
al (17) demonstrated the
elevation of urinary NGF levels in patients with an overactive
bladder. An increase in organ hyperinnervation is associated with
NGF overproduction; this can affect gland growth either directly or
in a neurally mediated manner.
It has been indicated that growth factor-mediated
pathways are modified during the development and progression of
prostate cancer (18). NGF has a
mitogenic effect on the prostate. The autocrine expression of NGF
has been revealed to be upregulated in the androgen refractory
TSU-prl cell line, which is a human prostate epithelial tumor cell
line (19).
In conclusion, the present study has demonstrated
alterations in the expression of NGF in the adult rat prostate in
response to stress and sympathetic denervation. Sympathetic nervous
system overactivity resulted in hyperplasia of the ventral prostate
and an increase in NGF expression, while denervation led to atrophy
of the epithelium and higher NGF levels in the ventral lobes. NGF
may thus be a contributing factor in the pathophysiology of the
prostate.
References
|
1
|
Snider WD: Functions of the neurotrophins
during nervous system development: what the knockouts are teaching
us. Cell. 77:627–638. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paul AB, Grant ES and Habib FK: The
expression and localisation of beta-nerve growth factor (beta-NGF)
in benign and malignant human prostate tissue: relationship to
neuroendocrine differentiation. Br J Cancer. 74:1990–1996. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papatsoris AG, Liolitsa D and Deliveliotis
C: Manipulation of the nerve growth factor network in prostate
cancer. Expert Opin Investig Drugs. 16:303–309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arrighi N, Bodei S, Zani D, et al: Nerve
growth factor signaling in prostate health and disease. Growth
Factors. 28:191–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McVary KT, McKenna KE and Lee C: Prostate
innervation. Prostate Suppl. 8:2–13. 1998. View Article : Google Scholar
|
|
6
|
Li C, Watanabe G, Weng Q, et al:
Expression of nerve growth factor (NGF), and its receptors TrkA and
p75 in the reproductive organs of the adult male rats. Zoolog Sci.
22:933–937. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang S, Fang X, Meng Y, et al:
Sympathetic nervous system overactivity in the Wistar rat with
proliferative lesions of ventral prostate induced by chronic
stress. Urol Int. 83:230–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaalasti A, Alho AM, Tainio H and Hervonen
A: The effect of sympathetic denervation with 6-hydroxydopamine on
the ventral prostate of the rat. Acta Histochem. 79:49–54. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karp JD, Smith J and Hawk K: Restraint
stress augments antibody production in cyclophosphamide-treated
mice. Physiol Behav. 70:271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphy RA, Watson AY and Rhodes JA:
Biological sources of nerve growth factor. Appl Neurophysiol.
47:33–42. 1984.PubMed/NCBI
|
|
11
|
Djakiew D, Delsite R, Pflug B, et al:
Regulation of growth by a nerve growth factor-like protein which
modulates paracrine interactions between a neoplastic epithelial
cell line and stromal cells of the human prostate. Cancer Res.
51:3304–3310. 1991.
|
|
12
|
Graham CW, Lynch JH and Djakiew D:
Distribution of nerve growth factor-like protein and nerve growth
factor receptor in human benign prostatic hyperplasia and prostatic
adenocarcinoma. J Urol. 147:1444–1447. 1992.PubMed/NCBI
|
|
13
|
Liu DT, Reid MT, Bridges DC and Rush RA:
Denervation, but not decentralization, reduces nerve growth factor
content of the mesenteric artery. J Neurochem. 66:2295–2299.
1996.PubMed/NCBI
|
|
14
|
Goda M, Atagi S, Amitani K, et al: Nerve
growth factor suppresses prostate tumor growth. J Pharmacol Sci.
112:463–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Golomb E, Rosenzweig N, Eilam R and
Abramovici A: Spontaneous hyperplasia of the ventral lobe of the
prostate in aging genetically hypertensive rats. J Androl.
21:58–64. 2000.PubMed/NCBI
|
|
16
|
Clemow DB, Steers WD, McCarty R and Tuttle
JB: Altered regulation of bladder nerve growth factor and neurally
mediated hyperactive voiding. Am J Physiol. 275:R1279–R1286.
1998.PubMed/NCBI
|
|
17
|
Kim JC, Park EY, Hong SH, et al: Changes
of urinary nerve growth factor and prostaglandins in male patients
with overactive bladder symptom. Int J Urol. 12:875–880. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Djakiew D: Dysregulated expression of
growth factors and their receptors in the development of prostate
cancer. Prostate. 42:150–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dalal R and Djakiew D: Molecular
characterization of neurotrophin expression and the corresponding
tropomyosin receptor kinases (trks) in epithelial and stromal cells
of the human prostate. Mol Cell Endocrinol. 134:15–22. 1997.
View Article : Google Scholar
|