Introduction
Type 2 diabetes mellitus is a glycolipid metabolic
disorder that is characterized by high levels of blood glucose and
lipids in the context of insulin resistance and relative insulin
deficiency. It is a clinical syndrome caused by a combination of
environmental and genetic factors. When insulin resistance occurs,
the insulin signaling pathways of cells have a reduced ability to
respond to the action of the hormone insulin. To compensate for the
insulin resistance, the pancreas secretes greater amounts of
insulin to stimulate glucose uptake in the surrounding tissues. A
previous study has revealed that the abnormal accumulation of
lipids and the increase in inflammatory factors, including tumor
necrosis factor (TNF) α and interleukin (IL)6, play an important
role in the pathogenesis of insulin resistance (1).
As a member of the nuclear receptor superfamily, the
glucocorticoid receptor (GR) is a ligand-activated transcription
factor that regulates the associated gene expression of
glucocorticoids (2). In the
absence of glucocorticoids, the GR remains in the cytosol in the
form of complexes with a variety of proteins, including heat shock
protein 90 (Hsp90). Once glucocorticoids diffuse through the cell
membrane into the cytoplasm and bind to the glucocorticoid receptor
(GR), the heat shock proteins are released. The resulting activated
form of GR has two principal mechanisms of action: nongenomic and
genomic, as described below. It has been demonstrated that the GR
influences the key molecules of certain signaling pathways through
rapid nongenomic effects, including the phosphoinositide 3-kinase
(PI3K), c-Jun N-terminal kinase (JNK), 14-3-3 protein and T cell
receptor (TCR) signaling pathways, which are able to regulate the
expression of certain pro-inflammatory factors (3). The GR also acts as a transcription
factor to activate downstream gene expression in the nucleus via
two mechanisms (4). One direct
mechanism of action involves homodimerization of the GR, its
translocation through active transport into the nucleus and binding
to glucocorticoid response elements which are located in the
promoter regions of certain genes. It subsequently recruits
transcription factors or co-activators, changes the structure of
the chromosome and regulates the expression of genes associated
with sugar dysmetabolism (5). The
other mechanism of GR action occurs without dimerization or
combination with DNA. Activated GR complexes, with other
transcription factors attached, prevent these transcription factors
from binding to their target genes and thus repress the expression
of inflammatory genes (6).
The regulatory effect of the GR on inflammatory
genes has led to it becoming the main target for the development of
anti-inflammatory agents (7).
Currently, first-line anti-inflammatory agents, including
dexamethasone (Dex), exert anti-inflammatory effects by activating
GRs (8). As a complete agonist of
GR, Dex fully facilitates the entry of GRs into the nucleus. While
inhibition of the inflammation by Dex is efficient, the clinical
application of Dex is limited due to severe side-effects, including
central obesity (9).
The side-effects of Dex are a result of the
increased expression of gluconeogenic genes, which is caused by the
transcriptional activation of GR, while the anti-inflammatory
action results from the transrepression of GR (10). In the view of previous studies, the
development of a selective GR ligand with few side-effects has
become a new development direction and research strategy for the
treatment of chronic inflammation, which may be applied to the
remission of type 2 diabetes mellitus (11). Thus, the aim of the present study
was to develop a screening system for selective GR modulators and
carry out the screening and evaluation of a sample compound
library. The discovery of novel selective glucocorticoid receptor
ligands may lay a foundation for the further development of
therapeutic agents for chronic inflammation.
Materials and methods
Cell culture
Cultures of 293T (American Type Culture Collection,
Manassas, VA, USA) and human osteosarcoma (Thermo Fisher
Scientific, Inc., Rockford, IL, USA) cell lines were stably
transfected with GR-green fluorescent protein (GFP) in a carbon
dioxide incubator at 37°C in 5% CO2. The Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% fetal bovine serum
(FBS; Gibco®, Invitrogen Life Technologies, Grand
Island, NY, USA) and 10% penicillin-streptomycin
(Gibco®, Invitrogen Life Technologies) was changed every
day. Pancreatic enzyme (Gibco®, Invitrogen Life
Technologies) was applied every two days, and the cells were
cultured for 14 days in total. Following the subculture, cells in
the logarithmic growth phase were seeded in 96-well plates and
cultured in the incubator overnight.
Screening of compounds
The compounds in the compound sample library
[preserved in the laboratory at the Second People’s Hopital of
Jinan (Jinan, China)] were dissolved in serum-free DMEM to incubate
with the cells stably transfected with GR-GFP for 1 or 6 h.
Following the removal of DMEM, cells were treated with a fixative
containing Hoechst 33342 (Beyotime Institute of Biotechnology,
Haimen, China), at a final concentration of 1 mg/ml, and methanal,
at a final concentration of 4%, for 30 min. Cells were washed three
times with phosphate-buffered saline (PBS) and analyzed using the
IN Cell Analyzer 1000 (GE Healthcare, Bethesda, MD, USA). Dimethyl
sulfoxide (DMSO) served as the negative control and Dex (Sigma, St.
Louis, MO, USA) served as the positive control.
Luciferase assay
A luciferase assay was performed to determine the
effect of compound Q40 on GR transcriptional activity and its
ability to recruit co-activators. The 293T cells in the logarithmic
growth phase were seeded onto 24-well plates and cultured in the
carbon dioxide incubator. When cell density reached 50–70%, the
medium was changed to serum-free DMEM. A Calcium Phosphate
Transfection kit (Beyotime Institute of Biotechnology) was used to
transfer relevant plasmids into the cells. The GR-ligand-binding
domain (LBD), UAS-TK-Luc reporter and pRL-SV40 control plasmid were
transferred into the 293T cells during the investigation of the
ability of GR to recruit co-activators. GR full-length plasmids,
the glucocorticoid response element-luciferase (GRE-Luc) and
pRL-SV40 control plasmid were transferred into the 293T cells
during the detection of the influence of compounds on GR
transcriptional activity. The culture medium was replaced with
complete medium with 10% FBS following a 6-h transfection. The
compounds in the compound sample library were dissolved in the
complete medium and the cells were incubated in the medium for 18
h. Following the removal of DMEM, cells were washed once with PBS.
A total of 100 μl cell lysis solution was added to each well to
lyse the cells at 37°C. Cells were harvested within the 20 min
subsequent to lysis. A luciferase assay was performed according to
the manufacturers’ instructions (Promega Corporation, Madison,
Wisconsin, USA).
Statistical analysis
SPSS software, version 16.0 (SPSS, Inc, Chicago, IL,
USA) was used to carry out the statistical analysis. Data were
compared using the Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
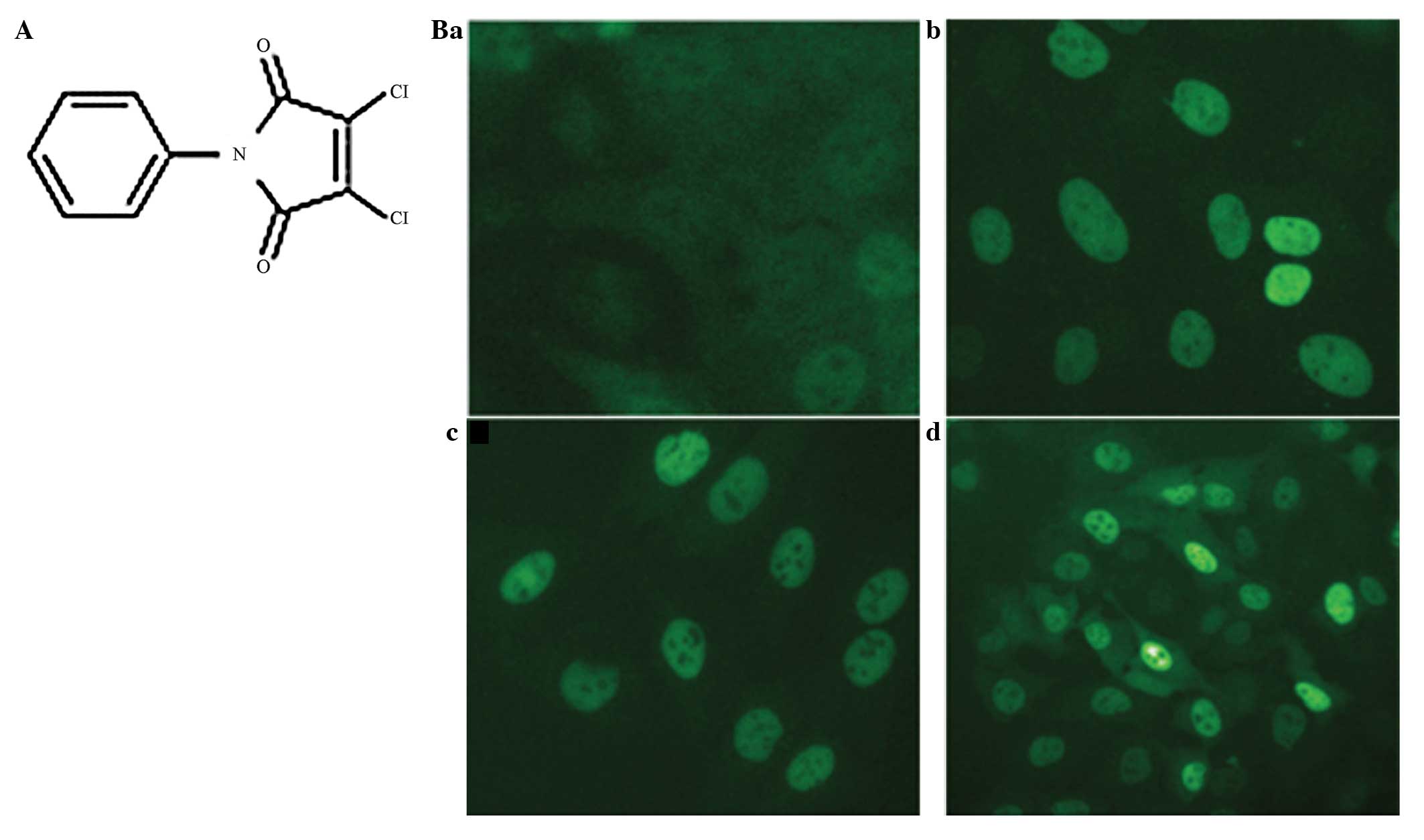
Compound Q40 significantly promotes GR
nuclear translocation
Following screening of the compounds in the compound
sample library, the compound Q40 was identified to significantly
promote GR nuclear translocation. A total of 20 μm Q40 was able to
promote the entry of GR-GFP into the nucleus in the shortest time
of 1 h (Fig. 1). DMSO served as
the negative control and Dex as the positive control. As the
regulating effect of GR is mainly exerted in the nucleus, a
mammalian two-hybrid system and transactivation assay were used to
investigate the effect of compound Q40 on the GR.
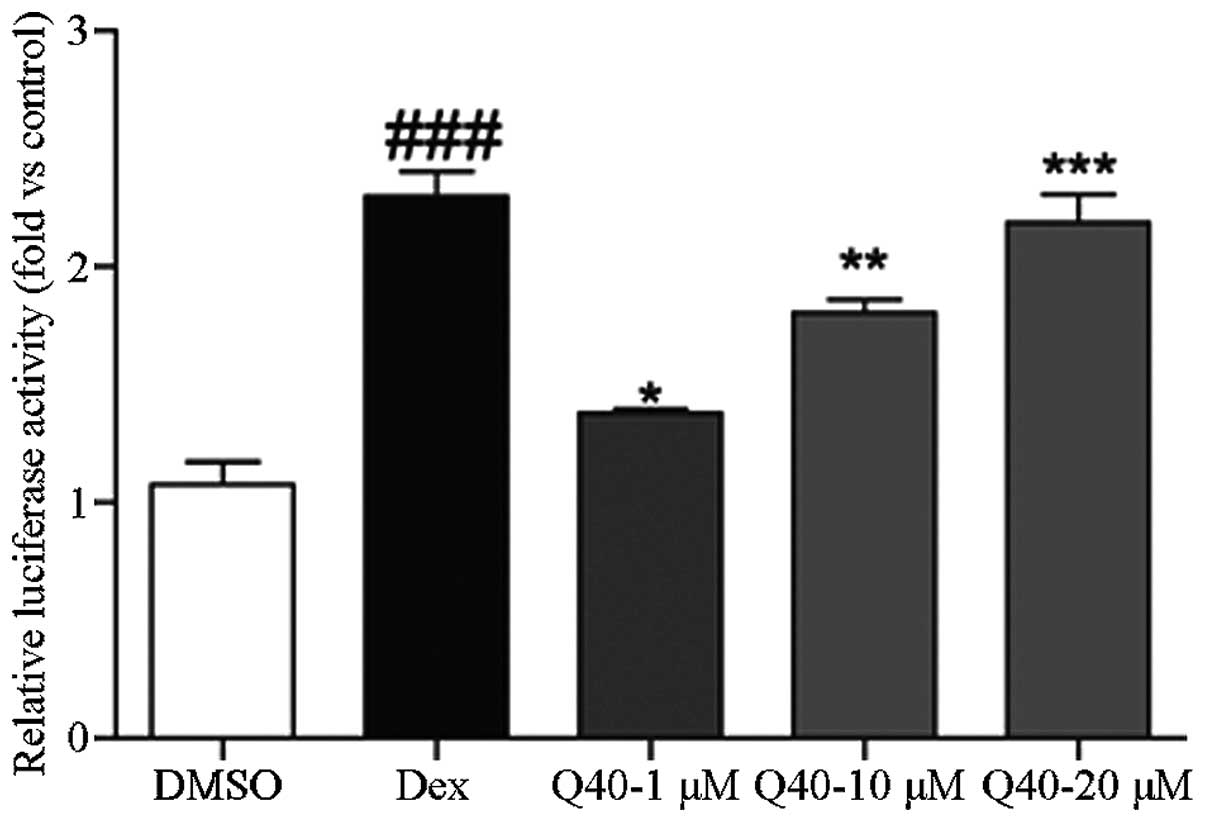
Compound Q40 is a selective regulator of
the GR
A mammalian two-hybrid system was used to
investigate the effect of compound Q40 on the ability of GRs to
recruit co-activators. At 6 h following transfection, the 293T
cells were treated with different concentrations of compound Q40
(1, 10 and 20 μmol/l), DMSO or Dex for 18 h. The luciferase assay
revealed that compound Q40 increased the ability of GRs to recruit
co-activators in a concentration-dependent manner (Fig. 2).
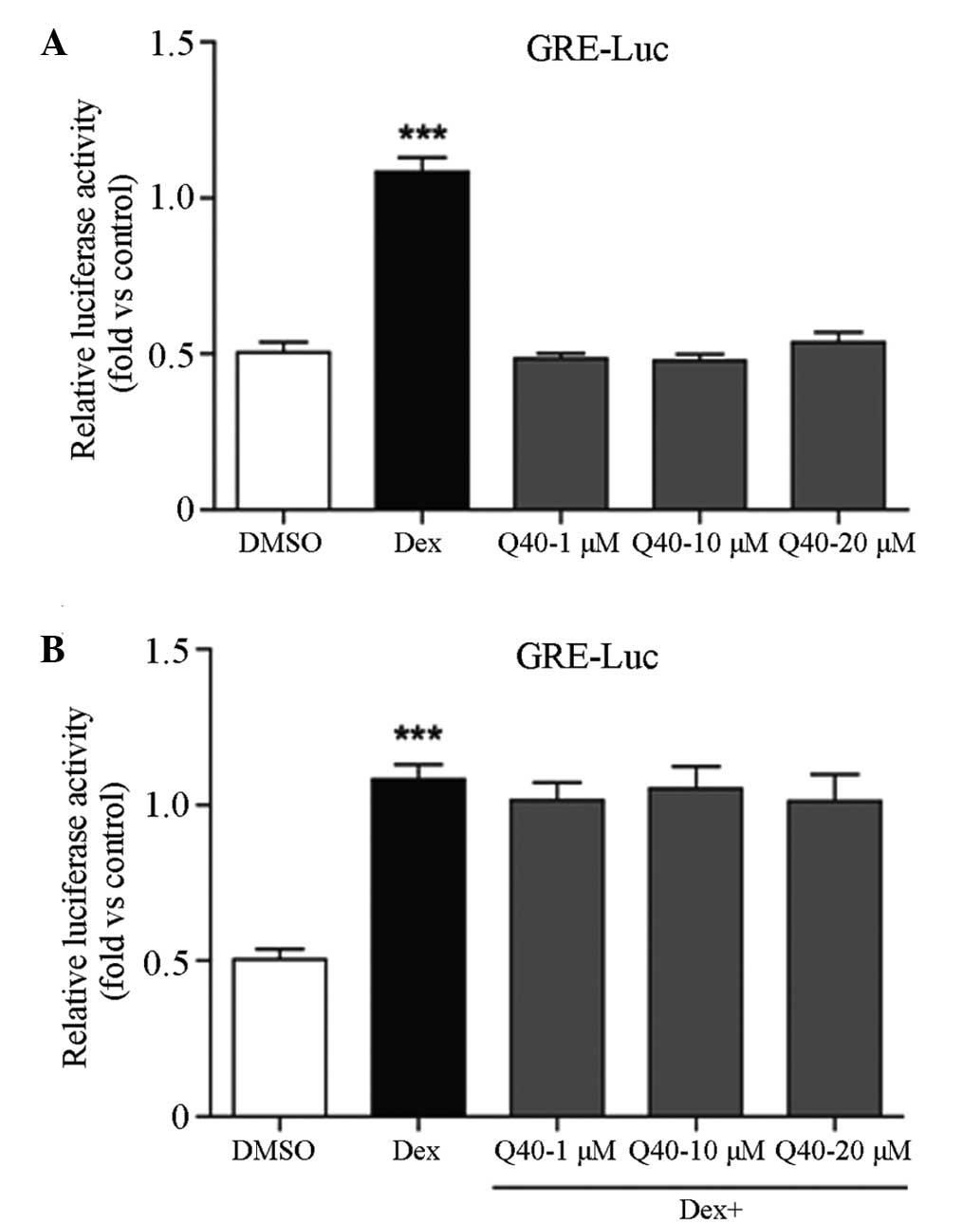
Compound Q40 has no effect on GR
transcriptional activity
At 6 h following transfection, 293T cells were
treated with various concentrations of compound Q40 (1, 10 and 20
μmol/l), DMSO or Dex for 18 h. The luciferase assay revealed that
compound Q40 had no effect on the transcriptional activity of GR.
When the 293T cells were incubated with Q40 and Dex combined,
compound Q40 had no effect on the partial agonistic action of Dex
on GRE. This indicates that Q40 is not an antagonist of the GR
(Fig. 3).
Discussion
Type 2 diabetes mellitus has become a burden on
economic and social development due to its high morbidity rate. It
is a metabolic disorder in the context of insulin resistance and
relative insulin deficiency. Chronic inflammation plays a major
role in the occurrence of insulin resistance. The transcription and
expression of inflammatory factors, including TNF-α and IL-6, are
activated in patients with type 2 diabetes mellitus. Increasing the
sensitivity of insulin signaling through anti-inflammatory therapy
has become an important strategy for the development of therapeutic
agents for individuals with type 2 diabetes mellitus (12).
As a member of the nuclear receptor superfamily, the
GR is typical of the ligand-activated transcription factors. Upon
binding to its respective ligands, including glucocorticoids and
steroid hormones, the GR is activated. The activated GR is
transported into the nucleus to regulate the expression levels of
inflammatory and sugar dysmetabolism-related genes, including
nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB),
TNF-α, glucose 6-phosphatase (G6Pase) and phosphoenolpyruvate
carboxykinase (PEPCK) (13). Its
ability to regulate inflammatory gene expression has led to the GR
becoming the primary target for the development of
anti-inflammatory agents (14). As
a typical first-line anti-inflammatory agent, the clinical
application of Dex is limited due to severe side-effects, which are
induced by the transcriptional activation of GR (15,16).
The increased expression of gluconeogeneic genes, including G6Pase
and PEPCK, is the predisposing factor most commonly associated with
the side-effects of glucocorticoids (17,18).
Therefore, the screening of selective ligands of the GR is an
important strategy for the development of anti-inflammatory agents
with few side-effects.
In the present study, the IN Cell Analyzer 1000
platform was employed to select compounds that were able to promote
GR nuclear translocation. Following screening of all compounds in
the compound sample library, compound Q40 was identified to
accelerate the translocation of GR into the nucleus in a short
time. A mammalian two-hybrid system and transactivation assay were
performed to evaluate the regulatory effect of compound Q40 on GR
function. The results revealed that Q40 increased the ability of GR
to recruit co-activators in a concentration-dependent manner but
had no effect on the transcriptional activity of GR itself. In
light of these results, it is evident that compound Q40 is a
selective regulator of GR.
In the current study, compound Q40 demonstrated a
promotional effect on GR nuclear translocation and the ability of
GR to recruit co-activators. As the effect of GRs on the regulation
of inflammatory gene expression is carried out in the nucleus, the
ability of Q40 to translocate GRs to the nucleus indicates that it
may regulate the expression of inflammatory genes.
Since compound Q40 did not increase or have an
antagonistic effect on the transcriptional activity of GR, the
results indicated that compound Q40 did not enhance the regulatory
effect of the GR on the expression of genes associated with sugar
dysmetabolism. Thus, the results indicated that compound Q40 may
not cause sugar dysmetabolism-related side-effects. However,
identification of the biological function of compound Q40 requires
further study.
As a nuclear receptor, the GR is the main target for
first-line anti-inflammatory agents. Chronic inflammation plays a
major role in the occurrence of insulin resistance. Therefore,
screening selective ligands of the GR is an important strategy for
the further development of anti-inflammatory adjuvant therapy. The
results of the present study suggest that compound Q40 is a ligand
of GRs and exerts an agonistic action on GRs in the recruitment of
co-activators in a concentration-dependent manner, without sugar
dysmetabolism-related side-effects. Thus, compound Q40 may be used
as an anti-inflammatory adjuvant therapy with few side-effects in
patients with type 2 diabetes mellitus.
References
|
1
|
Mitrou P, Lambadiari V, Maratou E, et al:
Skeletal muscle insulin resistance in morbid obesity: the role of
interleukin-6 and leptin. Exp Clin Endocrinol Diabetes.
119:484–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oakley RH and Cidlowski JA: The biology of
the glucocorticoid receptor: new signaling mechanisms in health and
disease. J Allergy Clin Immunol. 132:1033–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deroo BJ and Archer TK: Glucocorticoid
receptor-mediated chromatin remodeling in vivo. Oncogene.
20:3039–3046. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nelson G, Wilde GJ, Spiller DG, et al:
NF-kappaB signalling is inhibited by glucocorticoid receptor and
STAT6 via distinct mechanisms. J Cell Sci. 116:2495–2503. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Segard-Maurel I, Rajkowski K, Jibard N,
Schweizer-Groyer G, Baulieu EE and Cadepond F: Glucocorticosteroid
receptor dimerization investigated by analysis of receptor binding
to glucocorticosteroid responsive elements using a monomer-dimer
equilibrium model. Biochemistry. 35:1634–1642. 1996. View Article : Google Scholar
|
|
6
|
Adcock IM and Caramori G: Cross-talk
between pro-inflammatory transcription factors and glucocorticoids.
Immunol Cell Biol. 79:376–384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Hejjaj WK, Numan IT, Al-Sa’ad RZ and
Hussain SA: Anti-inflammatory activity of telmisartan in rat models
of experimentally-induced chronic inflammation: Comparative study
with dexamethasone. Saudi Pharm J. 19:29–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terzic N, Vujcic M, Ristic-Fira A,
Krstic-Demonacos M, Milanovic D, Kanazir DT and Ruzdijic S: Effects
of age and dexamethasone treatment on glucocorticoid response
element and activating protein-1 binding activity in rat brain. J
Gerontol A Biol Sci Med Sci. 58:297–303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar RK, Herbert C, Thomas PS, et al:
Inhibition of inflammation and remodeling by roflumilast and
dexamethasone in murine chronic asthma. J Pharmacol Exp Ther.
307:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smoak KA and Cidlowski JA: Mechanisms of
glucocorticoid receptor signaling during inflammation. Mech Ageing
Dev. 125:697–706. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baschant U and Tuckermann J: The role of
the glucocorticoid receptor in inflammation and immunity. J Steroid
Biochem Mol Biol. 120:69–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bledsoe RK, Montana VG, Stanley TB, et al:
Crystal structure of the glucocorticoid receptor ligand binding
domain reveals a novel mode of receptor dimerization and
coactivator recognition. Cell. 110:93–105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicolaides NC, Galata Z, Kino T, Chrousos
GP and Charmandari E: The human glucocorticoid receptor: molecular
basis of biologic function. Steroids. 75:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schoneveld OJ, Gaemers IC and Lamers WH:
Mechanisms of glucocorticoid signalling. Biochim Biophys Acta.
1680:114–128. 2004. View Article : Google Scholar
|
|
15
|
Nissen RM and Yamamoto KR: The
glucocorticoid receptor inhibits NFkappaB by interfering with
serine-2 phosphorylation of the RNA polymerase II carboxy-terminal
domain. Genes Dev. 14:2314–2329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schäcke H, Berger M, Rehwinkel H and
Asadullah K: Selective glucocorticoid receptor agonists (SEGRAs):
novel ligands with an improved therapeutic index. Mol Cell
Endocrinol. 275:109–117. 2007.PubMed/NCBI
|
|
17
|
Rosen J and Miner JN: The search for safer
glucocorticoid receptor ligands. Endocr Rev. 26:452–464. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clark AR and Belvisi MG: Maps and legends:
the quest for dissociated ligands of the glucocorticoid receptor.
Pharmacol Ther. 134:54–67. 2012. View Article : Google Scholar : PubMed/NCBI
|