Introduction
Ischemia/reperfusion (I/R) injury is a common
occurrence during thrombotic disease. The effects of reperfusion
are typically beneficial but often lead to an inflammatory response
that causes further damage to viable tissue around the infarct, a
result thought to be associated with increased apoptosis,
especially in cardiomyocytes (1).
This form of damage has been demonstrated to lead to a loss in
cardiomyocytes and an increased infarct size (2).
There are a number of signaling pathways that
participate in cardiomyocyte apoptosis; however, the toll-like
receptor 4 (TLR4)/nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB) signal pathway is known to play one of
the key roles in I/R injury (3,4) and
has been observed to become markedly upregulated in failing and
ischemic myocardium (5).
Conversely, TLR4 deficiency increases the survival rate of
cardiomyocytes following myocardial ischemia via an
apoptosis-mediated effect (6). As
such, one of the primary goals of therapeutic intervention during
I/R injury is to increase myocardial protection by inhibiting
apoptosis.
Carvedilol is a non-selective β-blocker initially
used in the treatment of hypertension and angina (7). It has been shown to reduce the risk
of hospitalization and the mortality rate in patients with severe
chronic heart failure (CHF) (8).
In a number of clinical trials (9,10)
carvedilol has been demonstrated to exert a beneficial effect on
ventricular remodeling and in preserving the ejection fraction, as
compared with other blockers. These effects are considered to be
mediated through multiple mechanisms, including the inhibition of
cardiomyocyte apoptosis (11,12).
However, the association between the effect of carvedilol on
anti-apoptosis and the TLR4 signaling pathway is not yet known.
Therefore, the present study investigated the
possibility that the TLR4 signaling pathway is involved in the
anti-apoptotic effects of carvedilol. The effects of carvedilol
were investigated in the H9c2 cardiomyocyte cell line following
simulated I/R (SI/R) and the apoptosis rate was subsequently
observed in order to explore the possible internal association
between the effects of carvedilol and the TLR4 signaling
pathway.
Materials and methods
Cell culture
The rat H9c2 cardiomyocyte cell line was obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). The cells were maintained in Dulbecco’s modified Eagle’s
medium (DMEM, Hyclone, Logan, UT, USA) supplemented with 10% fetal
calf serum at 37.1°C under CO2 incubation. The medium
was replaced every 2–3 days and the cells were subcultured or
subjected to experimental procedures at 80–90% confluence.
SI/R injury model
Cardiomyocytes were randomly divided into seven
groups: i) control; ii) SI/R; iii) carvedilol (1 μM); iv)
carvedilol (5 μM); v) carvedilol (10 μM); vi) TLR4 inhibitor (20
μg/ml; an anti-TLR4 blocking antibody; CST Technologies, Boston,
MA, USA) and; vii) pyrrolidine dithiocarbamate (100 μmol/l PDTC;
NF-κB inhibitor) groups. In the control group, H9c2 cardiomyocytes
were cultured under normal conditions in 5% CO2
incubation. SI/R experiments were performed according to a method
previously described by Esumi et al in 1991 (13). Briefly, the cardiomyocytes were
exposed to ischemia by replacing the medium with an ‘ischemic
buffer’ designed to simulate the extracellular environment during
myocardial ischemia, with the approximate concentrations of
potassium, hydrogen and lactate ions that are observed to occur
in vivo [137 mmol NaCl, 12 mmol KCl, 0.49 mmol
MgCl2, 0.9 mmol CaCl2·2H2O, 4 mmol
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 20
mmol sodium lactate (pH 6.2)]. Cells were incubated in a
hypoxic/ischemic chamber also known as the Modular Incubator
Chamber (MIC-101; Billups-Rothenberg, Del Mar, CA, USA) at 37°C for
2 h in a humidified atmosphere of 5% CO2 and 95%
nitrogen. For the reoxygenation process the cells were superfused
in DMEM supplemented with 10% fetal calf serum at 37.1°C under 5%
CO2 incubation for 2 h.
Protein extraction and western blot
analysis
B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X
(Bax) protein concentrations were first determined using a
bicinchoninic acid (BCA; Bio-Rad, Hercules, CA, USA) protein assay
kit following the manufacturers’ instructions. Proteins were
separated on a 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) gel, transferred to a nitrocellulose
membrane, blocked for 30 min at 37°C with 5% skimmed dry milk and
incubated with a primary antibody overnight on a rocking platform.
The primary antibodies used were the following: Mouse monoclonal
against TLR4 (Abcam), rabbit against NFκB p50, rabbit against Bcl-2
and rabbit against Bax (all from Santa Cruz Biotechnology, Santa
Cruz, CA, USA). Membranes were subsequently washed three times with
Tris-buffered saline and Tween 20 (TBST) and incubated with the
secondary antibody in TBST solution for 30 min at 37°C after which
they were washed as above. The secondary antibodies used were the
following: Peroxidase-labeled goat anti-rabbit IgG and
peroxidase-labeled rabbit anti-mouse IgG (both from Zhongshan
Company, Beijing, China). Immunoblots were developed using an
enhanced chemiluminescent reagent kit (Abcam, Cambridge, UK). The
bands were scanned and quantified by densitometric analysis using
an image analyzer (Tanon2500, Shanghai, China).
Flow-cytometric analysis
Dual staining with Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) (Bestbio, Shanghai,
China) was conducted to detect cell apoptosis. Flow cytometric
analysis was performed 24 h after reperfusion. The procedures were
carried out in accordance with the manufacturers’ instructions.
Cells were harvested by trypsinization, washed twice with
phosphate-buffered saline (PBS) and resuspended in binding buffer
prior to the addition of Annexin V-FITC/PI. The mixture was
incubated for 15 min in the dark at room temperature. Subsequently,
cellular fluorescence was measured by bivariate flow cytometry
using a FACScan system (BD Biosciences, Franklin Lakes, NJ, USA)
and analyzed with CellQuest™ software (BD Biosciences).
Annexin V-FITC/PI dual staining discriminated between intact cells
(Annexin V−/PI−), apoptotic/early apoptotic
cells (Annexin V+/PI−) and necrotic/late
apoptotic cells (Annexin V+/PI+).
Fluorescence quantitative polymerase
chain reaction (qPCR)
The amounts of TLR4 and NF-κB were measured using
fluorescence qPCR. At the end of each experiment, cells were
collected and the total RNA was isolated using Gibco®
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA). The total RNA (8 μl) was reverse transcribed and 1 μl of
the product was subjected to qPCR in the presence of specific
primers. The sequences of the primers were as follows: TLR4
forward: 5′-TCATGCTTTCTCACGGCCTC-3′ and reverse:
5′-AGGAAGTACCTCTATGCAGGGAT-3′; NF-κB forward:
5′-ACGATCTGTTTCCCCTCATC-3′ and reverse: 5′-TGCTTCTCTCCCCAGGAATA-3′;
β-actin: forward: 5′-CGCGAGTACAACCTTCTTGC-3′ and reverse:
5′-CGTCATCCATGGCGAACTGG-3′). The conditions for all qPCR reactions
were optimized using an Applied Biosystems® 7500 iCycler
iQ system (Invitrogen Life Technologies) for a 20-μl reaction using
the following 40-cycle program: 95°C for 10 min, 95°C for 15 sec
and 60°C for 1 min. All samples were amplified simultaneously in
triplicate in a one assay-run. In each reaction, β-actin was
included as an internal standard and the relative quantitative gene
expression was calculated using the 2−ΔΔCt method.
Statistical analysis
All values are expressed as mean ± standard
deviation. The results were analyzed using analysis of variance
(ANOVA) for multiple comparisons followed by two-sided Dunnett’s or
Student-Newman-Keuls tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Apoptosis rate of cardiomyocytes
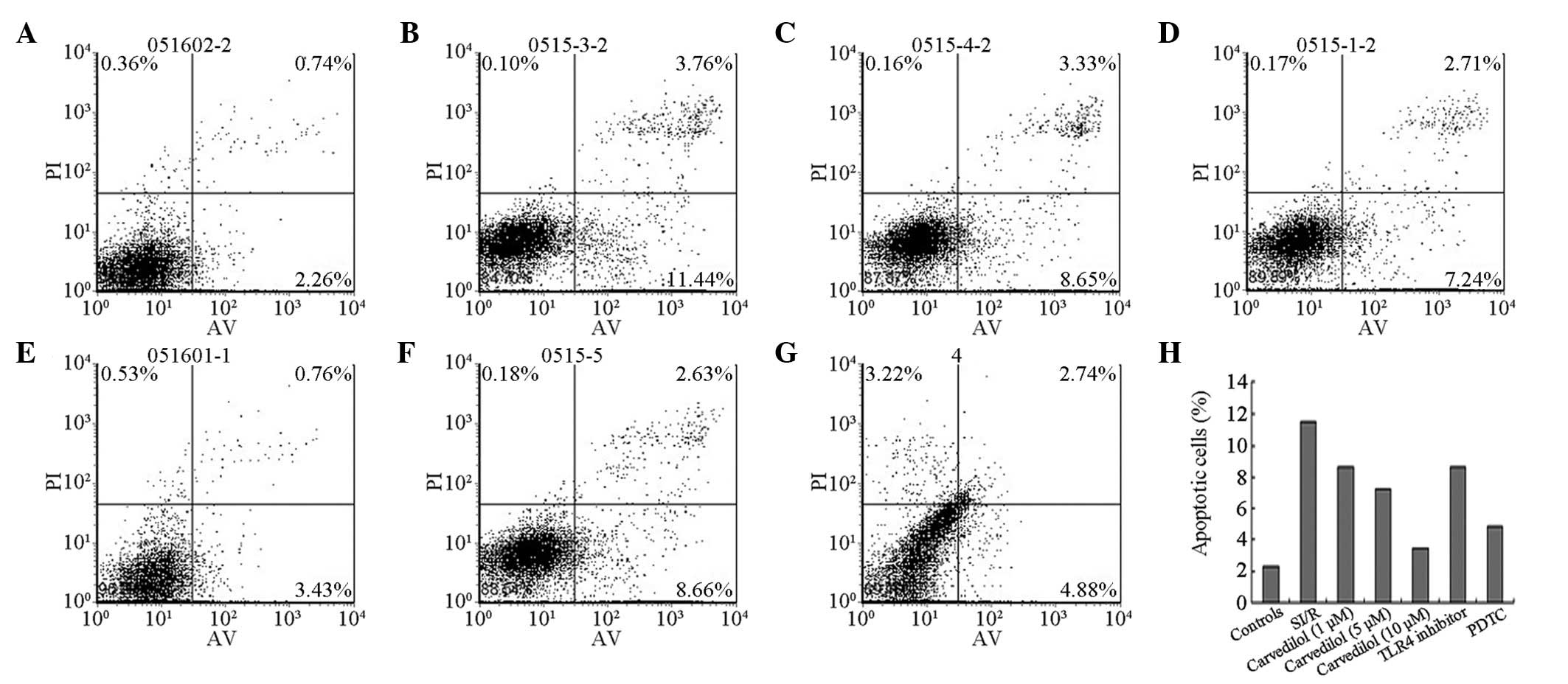
Flow-cytometric analysis demonstrated that
carvedilol exhibited anti-apoptotic effects (Fig. 1). Following 24 h of reperfusion,
the cardiomyocyte apoptosis rate was markedly increased in the SI/R
group when compared with that of the control group (P<0.01). The
two lower concentrations of carvedilol (1 and 5 μM) decreased the
apoptotic index to a similar extent, whereas the high dose (10 μM)
had a much larger apoptosis-inhibiting effect when compared with
the degree of apoptosis in the SI/R group (P<0.01). When the
activation of TLR4 was inhibited by the TLR4 antibody and the
activation of NF-κB was inhibited by PDTC, the apoptotic index of
the H9c2 cells was significantly decreased compared with that of
the SI/R group .
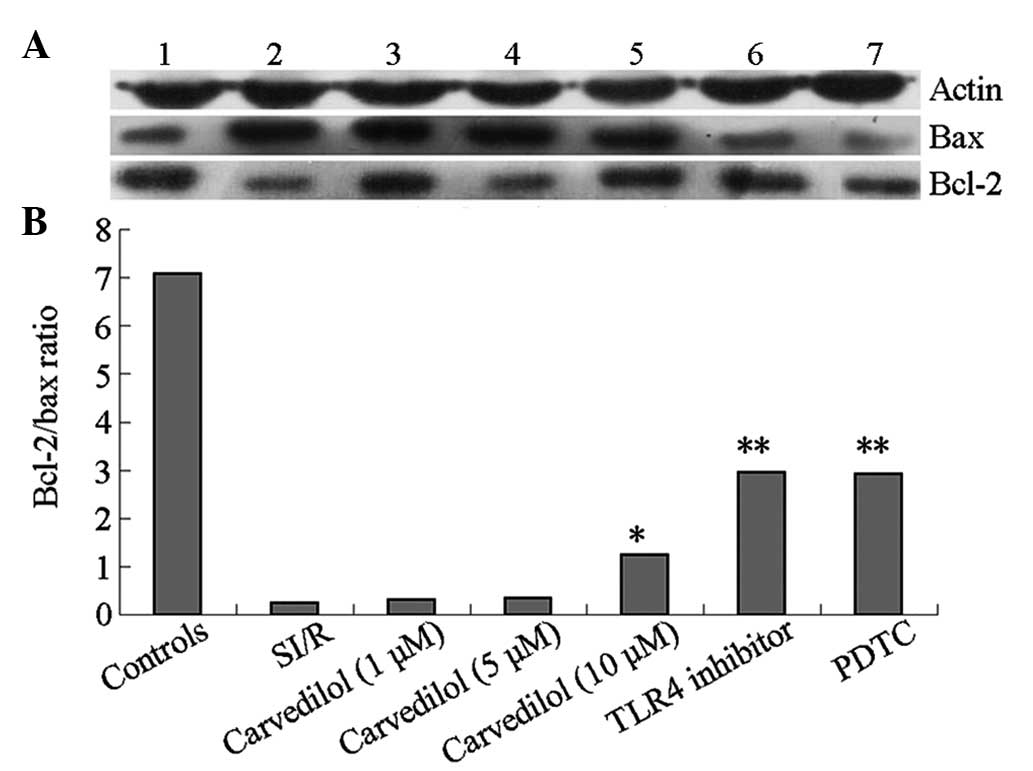
Expression levels of B-cell lymphoma 2
(Bcl-2) and Bcl-2-associated X protein (Bax)
The Bcl-2/Bax ratio was decreased in the current
SI/R injury model when compared with that of the control group
(Fig. 2). Carvedilol at low and
medium concentrations had no effect on the Bcl-2/Bax ratio during
reperfusion. However, a high concentration of carvedilol resulted
in a significant increase in the Bcl-2/Bax ratio compared with that
in the SI/R group; the increase was paralleled by those observed in
TLR4 inhibitor and NF-κB inhibitor groups, .
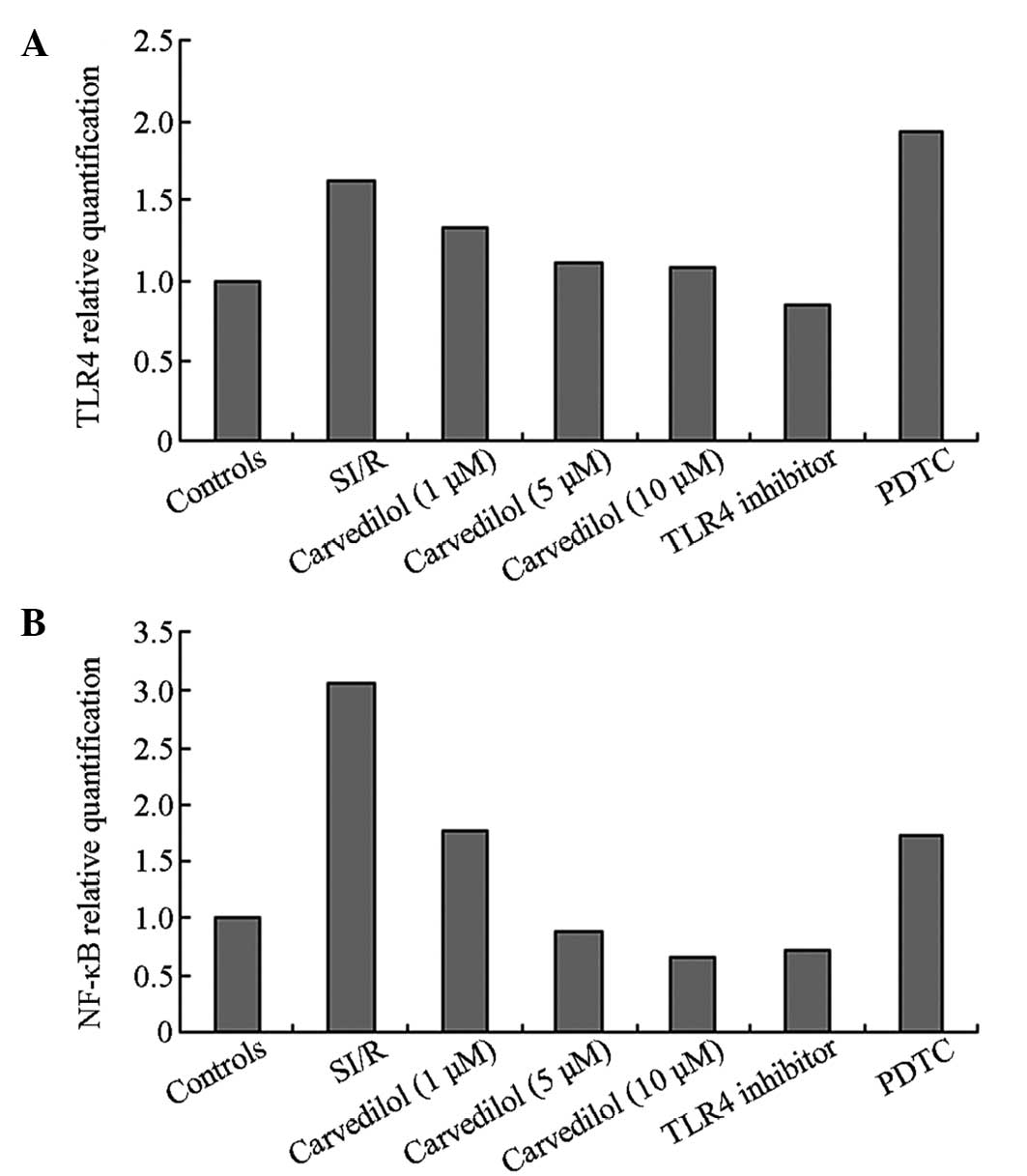
TLR4 and NF-κB gene expression
The gene expression levels of TLR4 and NF-κB were
upregulated in the SI/R model when compared with those in the
control group (Fig. 3). Following
the administration of carvedilol, the expression levels of TLR4 and
NF-κB significantly decreased compared with those in the SI/R
group. The highest concentration (10 μM) of carvedilol had the most
pronounced effect on gene expression out of all the carvedilol
treatments.
Discussion
Carvedilol is widely used in the treatment of
cardiac disease as a non-selective β-blocker. Previous studies have
demonstrated that carvedilol reduces the risk of ischemic events
following acute myocardial infarction (14). However, the particular pathways and
underlying mechanisms involved in this process have yet to be
elucidated. Apoptosis has been recognized as a component of a
number of common cardiac pathologies, including ischemia (15), and the TLR4/NF-κB pathway is
considered to be a major signal transduction pathway involved in
cardiomyocyte apoptosis (16,17).
In the present study, the cardioprotective effect of carvedilol was
confirmed, especially at high concentrations, and it was shown that
this effect is at least partially mediated by reductions in the
expression levels of TLR4 and NF-κB.
Carvedilol is considered to be superior to other
β-adrenoceptor blockers for the alleviation of heart failure at the
clinical level; this is likely to be through its action as a
regulator of apoptosis (18,19).
The present study demonstrated that carvedilol significantly
inhibited apoptosis following in vitro SI/R (2 h/24 h) in a
concentration-dependent manner. The authors suggest that these
dose-dependent anti-apoptotic effects of carvedilol should be taken
into consideration in order to improve the prognosis of patients
with I/R injury. However, further in vivo studies are
required in order to determine the ideal doses.
Bcl-2 and Bax genes are widely used to evaluate cell
survival/apoptosis following an apoptotic stimulus (20). Bax exhibits pro-apoptotic actions
whereas Bcl-2 has an anti-apoptotic effect. Therefore, the ratio of
Bax to Bcl-2 is an effective predictor of the apoptotic fate of a
cell (21). As predicted, in the
current study the Bcl-2/Bax ratio decreased during SI/R-induced
apoptosis (P<0.01, compared with the control group) and this
effect was blocked following the administration of carvedilol.
Furthermore, the present study revealed that a high concentration
of carvedilol (10 μM) had the largest effect on the ratio of
Bcl-2/Bax. These data suggest that high dosages of carvedilol are
able to significantly suppress the apoptotic effects of SI/R by
blocking regulatory proteins. Once again, the clinical implications
of using higher doses of carvedilol must be further studied in
order to clarify the effects of this cardioprotective drug.
The current study also investigated the mechanisms
that are potentially involved in the observed anti-apoptotic effect
of carvedilol. TLRs are a family of molecules that play a critical
role in the regulation of innate immunity, which is a significant
component of myocardial I/R (MI/R) injury. When inhibited, TLRs
protect against MI/R-induced damage in the heart (3,22).
Therefore, the authors hypothesized that the anti-apoptotic action
of carvedilol may be associated with a TLR4/NF-κB-mediated
response. The present study demonstrated that the expression levels
of TLR4 and NF-κB increased during SI/R. Furthermore, the
expression level of NF-κB decreased significantly following the
inhibition of TLR4 by the TLR4 antibody. These results were
verified by fluorescence qPCR. The results indicate that
carvedilol-mediated inhibition of apoptosis is regulated, to a
significant extent, through the TLR4/NF-κB pathway.
In conclusion, the present study demonstrated that
carvedilol inhibits SI/R-induced apoptosis in vitro in
cardiomyocytes via the TLR4/NF-κB-mediated pathway. These results
may be important in elucidating certain key factors involved in the
molecular mechanisms of the cardioprotective capabilities of
carvedilol.
Acknowledgements
This study was supported by the Natural Science
Foundation of Anhui Province (No. 070413103). The authors would
like to express their sincere gratitude to Dr Jianhua Zhang for her
valuable suggestions.
References
|
1
|
Jayachandran M, Brunn GJ, Karnicki K,
Miller RS, Owen WG and Miller VM: In vivo effects of
lipopolysaccharide and TLR4 on platelet production and activity:
implications for thrombotic risk. J Appl Physiol (1985).
102:429–433. 2007. View Article : Google Scholar
|
|
2
|
Zhong X, Li X, Qian L, et al: Glycine
attenuates myocardial ischemia-reperfusion injury by inhibiting
myocardial apoptosis in rats. J Biomed Res. 26:346–354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin J, Wang H, Li J, et al: κ-Opioid
receptor stimulation modulates TLR4/NF-κB signaling in the rat
heart subjected to ischemia-reperfusion. Cytokine. 61:842–848.
2013.
|
|
4
|
Kim SC, Stice JP, Chen L, et al:
Extracellular heat shock protein 60, cardiac myocytes, and
apoptosis. Circ Res. 105:1186–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao P, Wang J, He L, et al: Deficiency in
TLR4 signal transduction ameliorates cardiac injury and
cardiomyocyte contractile dysfunction during ischemia. J Cell Mol
Med. 13:1513–1525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riad A, Jäger S, Sobirey M, et al:
Toll-like receptor-4 modulates survival by induction of left
ventricular remodeling after myocardial infarction in mice. J
Immunol. 180:6954–6961. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leonetti G and Egan CG: Use of carvedilol
in hypertension: an update. Vasc Health Risk Manag. 8:307–322.
2012.PubMed/NCBI
|
|
8
|
Kanoupakis EM, Manios EG, Mavrakis HE, et
al: Electrophysiological effects of carvedilol administration in
patients with dilated cardiomyopathy. Cardiovasc Drugs Ther.
22:169–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le DE, Pascotto M, Leong-Poi H, Sari I,
Micari A and Kaul S: Anti-inflammatory and pro-angiogenic effects
of beta blockers in a canine model of chronic ischemic
cardiomyopathy: comparison between carvedilol and metoprolol. Basic
Res Cardiol. 108:3842013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mori Y, Nishikawa Y, Kobayashi F and
Hiramatsu K: Clinical status and outcome of Japanese heart failure
patients with reduced or preserved ejection fraction treated with
carvedilol. Int Heart J. 54:15–22. 2013.PubMed/NCBI
|
|
11
|
Chen-Scarabelli C, Saravolatz L Jr, Murad
Y, et al: A critical review of the use of carvedilol in ischemic
heart disease. Am J Cardiovasc Drugs. 12:391–401. 2012.PubMed/NCBI
|
|
12
|
Liu Q, Zhang J, Xu Y, Huang Y and Wu C:
Effect of carvedilol on cardiomyocyte apoptosis in a rat model of
myocardial infarction: a role for toll-like receptor 4. Indian J
Pharmacol. 45:458–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esumi K, Nishida M, Shaw D, Smith TW and
Marsh JD: NADH measurements in adult rat myocytes during simulated
ischemia. Am J Physiol. 260:H1743–1752. 1991.PubMed/NCBI
|
|
14
|
Dargie HJ: Effect of carvedilol on outcome
after myocardial infarction in patients with left-ventricular
dysfunction: the CAPRICORN randomised trial. Lancet. 357:1385–1390.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin YC, Kim CW, Kim YM, et al:
Cryptotanshinone, a lipophilic compound of Salvia
miltiorrriza root, inhibits TNF-alpha-induced expression of
adhesion molecules in HUVEC and attenuates rat myocardial
ischemia/reperfusion injury in vivo. Eur J Pharmacol.
614:91–97. 2009.PubMed/NCBI
|
|
16
|
Lin E, Freedman JE and Beaulieu LM: Innate
immunity and toll-like receptor antagonists: a potential role in
the treatment of cardiovascular diseases. Cardiovasc Ther.
27:117–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishikawa Y, Satoh M, Itoh T, Minami Y,
Takahashi Y and Akamura M: Local expression of Toll-like receptor 4
at the site of ruptured plaques in patients with acute myocardial
infarction. Clin Sci (Lond). 115:133–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poole-Wilson PA, Swedberg K, Cleland JG,
et al: Carvedilol Or Metoprolol European Trial Investigators:
Comparison of carvedilol and metoprolol on clinical outcomes in
patients with chronic heart failure in the Carvedilol Or Metoprolol
European Trial (COMET): randomised controlled trial. Lancet.
362:7–13. 2003. View Article : Google Scholar
|
|
19
|
Fiuzat M, Wojdyla D, Kitzman D, et al:
Relationship of beta-blocker dose with outcomes in ambulatory heart
failure patients with systolic dysfunction: results from the
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes
of Exercise Training) trial. J Am Coll Cardiol. 60:208–215. 2012.
View Article : Google Scholar
|
|
20
|
Soriano ME and Scorrano L: Traveling Bax
and forth from mitochondria to control apoptosis. Cell. 145:15–17.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Condorelli G, Morisco C, Stassi G, et al:
Increased cardiomyocyte apoptosis and changes in proapoptotic and
antiapoptotic genes bax and bcl-2 during left ventricular
adaptations to chronic pressure overload in the rat. Circulation.
99:3071–3078. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimamoto A, Chong AJ, Yada M, et al:
Inhibition of Toll-like receptor 4 with eritoran attenuates
myocardial ischemia-reperfusion injury. Circulation. 114(1 Suppl):
I270–I274. 2006. View Article : Google Scholar : PubMed/NCBI
|