Introduction
Chronic obstructive pulmonary disease (COPD) is
characterized by a partially reversible and progressive airflow
limitation associated with an abnormal inflammatory response in the
lung. Chronic inflammation has an important function in the
development and progression of COPD, as well as extra-pulmonary
manifestations (1). Heterogeneity
is observed in terms of the progression and response to treatment,
which may be associated with the different inflammatory phenotypes
of the disease. Therefore, relevant biomarkers should be
investigated to understand the heterogeneity, natural history and
response of COPD to treatment. Potential biomarkers of COPD have
been the focus of studies on COPD for decades. For instance,
surfactant protein D (SP-D) is considered as a pulmonary-specific
biomarker, which may be used to track cardiopulmonary health status
(2). SP-D is produced
predominantly by type II pneumocytes; its expression is correlated
with pulmonary function and is increased in stable COPD (3), with higher levels observed during
acute exacerbation (4,5). Changes in SP-D level are associated
with the improvement of COPD symptoms (6). SP-D levels may be associated with
disease development and progression in other pulmonary diseases,
including community-acquired pneumonia (7), viral infection (8), asthma (9), acute respiratory distress syndrome
(10,11), lung cancer (12), pulmonary aspergillosis (13) and interstitial lung disease
(14). However, contradicting
results have also been reported (15–17).
Therefore, the present prospective study was conducted to
investigate the association of serum and sputum SP-D levels with
different clinical profiles of COPD and treatment response.
Materials and methods
Subjects
Patients with COPD were recruited from an outpatient
clinic in the First Affiliated Hospital of Guangzhou Medical
University (Guangzhou, China) between February and October, 2009.
The criteria for inclusion in the study comprised the following
points: i) Patients with COPD confirmed by a clinical history and
spirometry according to the criteria established by the Global
Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines
(18); ii) an age of 40–85 years;
iii) clinical stability for at least four weeks; and iv) no
long-term maintenance therapy, with the exception of the inhalation
of a short-acting bronchodilator as required. Patients with a
history or diagnosis of severe heart disease, asthma, lung tumor
and bronchiectasis or sequelae of tuberculosis were excluded from
the study. The subjects in the control group were recruited from
the Health Examination Department of the First Affiliated Hospital
of Guangzhou Medical University. These subjects underwent a normal
pulmonary function test and were free from respiratory tract
infection for four weeks. The present study was approved by the
Ethics Committee of Human Investigation of the First Affiliated
Hospital of Guangzhou Medical University. All the participants
provided informed consent.
Study design
This study was a prospective follow-up
investigation. All the patients with COPD who were involved in this
study were evaluated at the baseline and then one and three months
after the treatment. The treatment regimen was a combination of 500
μg fluticasone propionate and 50 μg salmeterol (Seretide
Accuhaler®; GlaxoSmithKline, Inc., Brentford, UK)
administered twice daily to the patients. Cough medicine and
expectorants were permitted during the study.
Measurement and evaluation
The variables evaluated included pulmonary function,
the modified Medical Research Council dyspnea scale (mMRC) and St.
George’s Respiratory Questionnaire (SGRQ) scores and serum and
induced sputum SP-D levels. Pulmonary function was evaluated prior
and subsequent to bronchodilator treatment according to the
criteria of the American Thoracic Society/European Respiratory
Society (19). Sputum induction
was performed as described by Beeh et al (20). The supernatants were collected and
frozen at −80°C until analysis. Blood samples were collected and
allowed to coagulate for ≥30 min, and subsequently centrifuged at
1,500 × g for 15 min at room temperature. The serum was frozen at
−80°C until analysis. Serum and sputum SP-D levels were determined
using commercially available ELISA kits from BioVendor-Laboratorní
Medicína a.s. (Brno, Czech Republic) according to the
manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed using the SPSS
13.0 statistical software package (SPSS, Chicago, IL, USA). The
normal distribution of variables was evaluated by the
Kolmogorov-Smirnov test. Serum and sputum SP-D levels were
non-normally distributed and log-transformed to achieve normality.
Data are expressed as the mean ± standard deviation. Univariate
analysis was performed using the Student’s t-test or Pearson
correlation. Pearson correlation was employed to determine the
correlation of serum or sputum SP-D levels with pulmonary function
and the mMRC or SGRQ scores to determine the cross-sectional data
at baseline and three months after treatment. Multivariate analysis
with stepwise linear regression was conducted to analyze the
correlation of the changes in SP-D levels with the changes in
pulmonary function and the mMRC or SGRQ scores, and other
confounding factors, including age and smoking history. All the
statistical tests were two-sided. P<0.05 was considered
statistically significant.
Results
Characteristics of the subjects
Sixty-five patients with COPD (61 male and 4 female)
and 26 control subjects (22 male and 4 female) were recruited in
this study. The baseline demographic and clinical characteristics
of the patients involved are summarized in Table I. Twenty-four patients dropped out
of the study during the first month and another three subjects
withdrew during the next two months. The withdrawal of these 27
subjects was due to the following factors: i) Acute exacerbation of
COPD (five subjects); ii) hospitalization caused by other disorders
(five subjects); iii) refusal to continue (nine subjects); and iv)
could not be contacted (eight subjects).
 | Table ICharacteristics of the subjects. |
Table I
Characteristics of the subjects.
| Variable | COPD group | Control group |
|---|
| Number
(male/female) | 65 (61/4) | 26 (22/4) |
| Age, years | 66.6±8.1 | 66.7±10 |
| Smoking index, pack
years | 41.7±19.7 | 22.5±2.8a |
| Course of disease,
years | 8.26±7.15 | 8.96±15.6a |
| FEV1,
liters | 1.08±0.54 | 3.02±0.81a |
| FEV1 %
pred, % | 42.93±18.14 | 108.2±17.82a |
| FVC, liters | 2.33±0.76 | 2.94±0.61a |
| FEV1/FVC,
% | 45.65±12.13 | 80.74±6.18a |
| mMRC, score | 2.38±1.33 | |
| SGRQ, score |
| Total | 49.54±19.53 | |
| Symptom | 55.87±17.77 | |
| Activity | 66.45±26.68 | |
| Impact | 37.54±21.30 | |
| Serum SP-D,
ng/ml | 45.46±37.78 | 31.68±12.04b |
| Induced sputum SP-D,
ng/ml | 173.23±186.93 | 89.59±70.29b |
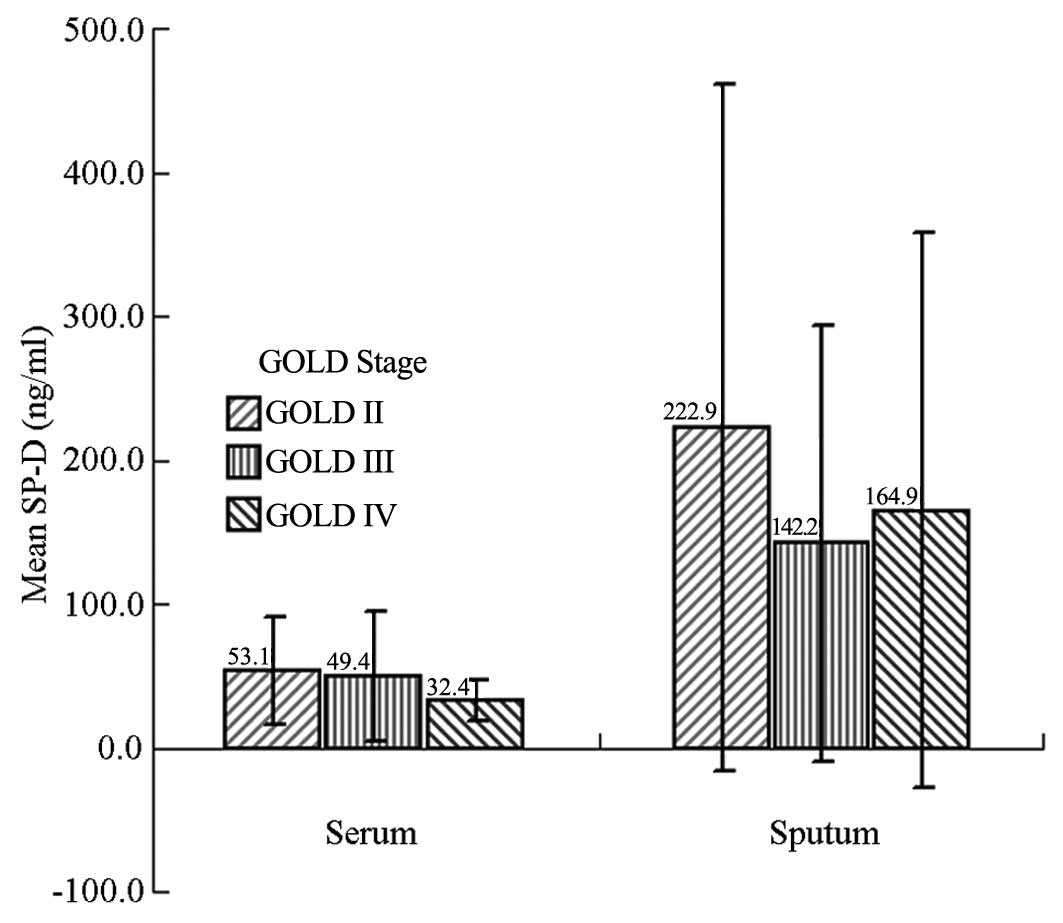
Serum and sputum SP-D levels
The serum SP-D levels in the different stages of
airflow obstruction according to the GOLD guidelines were
53.1±37.4, 49.4±44.9 and 32.4±14.2 ng/ml in stages II, III and IV
COPD, respectively. The sputum SP-D levels were 222.9±238.9,
142.2±151.7 and 164.9±192.7 ng/ml in the same stages, respectively.
No statistical differences were found in serum or sputum SP-D
levels among the different stages of airflow obstruction
(P>0.05) (Fig. 1).
Correlations between SP-D levels and
pulmonary function, mMRC and SGRQ scores in COPD
No significant correlation between serum and sputum
SP-D levels was observed (r=0.212, P=0.183). The serum or sputum
SP-D levels did not significantly correlate with pulmonary function
or the mMRC and SGRQ scores (P>0.05).
Effects of treatment with fluticasone
propionate/salmeterol on SP-D levels
A significant improvement was observed in the forced
expiratory volume in 1 sec (FEV1), forced vital capacity
and the mMRC and SGRQ scores after one month of treatment with
fluticasone propionate/salmeterol (P<0.05). Further improvement
was observed after three months of treatment. Although no
significant reduction in serum SP-D levels was observed after one
month, the serum SP-D levels decreased significantly from the
baseline after three months of treatment (t=2.217, P=0.031). No
significant reduction was found in sputum SP-D levels following
treatment, although the data showed a decreasing trend after three
months (P>0.05) (Table II).
The multivariate analysis showed no significant correlation between
the decrease in serum or sputum SP-D levels with the changes in
pulmonary function or the mMRC and SGRQ scores.
 | Table IIEffects of treatments with fluticasone
propionate/salmeterol on pulmonary function, the mMRC or SGRQ
scores and SP-D levels. |
Table II
Effects of treatments with fluticasone
propionate/salmeterol on pulmonary function, the mMRC or SGRQ
scores and SP-D levels.
| Baseline | 1 month later | 3 months later |
|---|
| FEV1,
liters | 1.08±0.54 | 1.15±0.48a | 1.21±0.50a |
| FVC, liters | 2.33±0.76 | 2.51±0.60a | 2.55±0.69a |
| mMRC, score | 2.38±1.33 | 1.59±1.19b | 1.27±0.80c |
| SGRQ, score |
| Total | 49.54±19.53 | 35.38±18.10b | 28.27±13.31c |
| Symptom | 55.87±17.77 | 42.32±17.89c | 37.13±13.40c |
| Activity | 66.45±26.68 | 51.41±25.28c | 45.13±20.27b |
| Impact | 37.54±21.30 | 24.73±17.58b | 16.93±11.60c |
| Serum SP-D,
ng/ml | 45.46±37.78 | 38.17±21.18 | 30.72±13.95a |
| Induced sputum
SP-D, ng/ml | 173.23±186.93 | 171.94±187.07 | 160.39±159.71 |
Baseline SP-D levels in the prediction of
treatment response
The baseline sputum SP-D levels were associated with
the changes in SGRQ activity scores after three months of treatment
based on the univariate and multivariate analysis (r=−0.652,
P=0.012); however this result was not associated with the changes
in pulmonary function or the mMRC and SGRQ total, symptom or impact
scores (P>0.05) (Table III).
The baseline serum SP-D level was also not correlated with the
above parameters (P>0.05).
 | Table IIICorrelation between baseline sputum
surfactant protein D levels and changes in pulmonary function, and
the modified Medical Research Council dyspnea scale and SGRQ scores
in the multivariate analysis. |
Table III
Correlation between baseline sputum
surfactant protein D levels and changes in pulmonary function, and
the modified Medical Research Council dyspnea scale and SGRQ scores
in the multivariate analysis.
| Unstandardized
coefficients | Standardized
coefficients | | |
|---|
|
|
| | |
|---|
| Model | B | Standard error | β | t | P-value |
|---|
| (Constant) | 296.448 | 62.409 | | 4.750 | <0.001 |
| SGRQ activity score
change | −5.977 | 2.008 | −0.652 | −2.976 | 0.012 |
Discussion
COPD is a chronic inflammatory airway disorder with
systemic manifestations and significant heterogeneity. Evident
individual variations have been observed in the clinical
manifestations, frequency of acute exacerbation, disease
progression and response to treatment (21). The mechanism of heterogeneity in
COPD is complex. Research has focused on COPD biomarkers due to the
potential functions of these biomarkers in predicting the
progression or response to treatment, which is an important
parameter in evaluating the individualized management of patients
with COPD (21).
Biomarkers can provide a number of advantages,
including the objective measurement or evaluation of biological
processes and disease pathology, the prediction of disease
progression and the determination of pharmacological response to a
therapeutic intervention (22).
Studies have been conducted to investigate the biomarkers of COPD;
however, these studies have produced controversial results.
C-reactive protein (CRP) is one of the most widely studied
biomarkers (23) and possibly
correlates with COPD (24).
However, as CRP is not a pulmonary-specific protein, it appears
likely that CRP is an unspecific risk marker and not a causal risk
factor (17). In theory,
pulmonary-specific biomarkers, such as SP-D and Clara cell
secretory protein-16, could potentially be used as ideal biomarkers
of COPD (25). SP-D is composed of
three polypeptide chains of 43 kDa monomers and is produced
predominantly by type II pneumocytes; other cells, including
pulmonary Clara cells, endothelial cells and gastrointestinal tract
glandular cells, can produce small amounts of SP-D. SP-D exhibits
stable characteristics and good reproducibility (26,27).
Thus, SP-D is considered to be one of the most promising biomarkers
of COPD.
Multiple clinical studies (3,6) have
reported that serum SP-D levels were increased in patients with
COPD compared with those in smokers who did not exhibit airflow
limitation and non-smokers. Furthermore, SP-D levels decreased
after the subjects were treated with inhaled or oral
corticosteroids, and the changes in SP-D levels were correlated
with the improvement in symptoms. Shakoori et al (4) and Ju et al (5) reported that high expression of SP-D
is associated with acute exacerbations of COPD, and that SP-D
levels decrease gradually to the baseline 30 days after the onset
of exacerbation (5). These results
suggested that sputum SP-D may be a useful biomarker to assess
airway inflammation and serum SP-D may be a promising biomarker of
systemic inflammation in patients with COPD or in a subgroup of
these patients who are likely to benefit from corticosteroid
treatment. In the present study, serum SP-D levels were
significantly reduced subsequent to the subjects being treated with
fluticasone propionate/salmeterol for three months, although sputum
SP-D levels were not significantly reduced. However, the changes in
serum and sputum SP-D levels following the treatment did not
correlate with the improvement in pulmonary function or the mMRC or
SGRQ scores. A weak association was found between the sputum SP-D
baseline level and changes in SGRQ activity scores; therefore,
sputum SP-D alone could be used as a biomarker to predict the
response to treatment in stable COPD. The results of the present
study are consistent with those of Vestbo et al (16) and Engström et al (17). In a study known as the ‘Evaluation
of COPD Longitudinally to Identify Predictive Surrogate Endpoints’,
in which a total of 1,888 patients with COPD were recruited, the
serum SP-D levels were not associated with COPD disease severity
according to the GOLD guidelines, radiology emphysema score or
areas of low attenuation on computed tomography scans (15). Furthermore, SP-D level is not
associated with changes in FEV1 over time (16). By contrast, Sin et al
(3) reported that SP-D is
significantly correlated with pulmonary function in patients with
COPD. However, these inconsistent results remain incompletely
elucidated and may be multifactorial. Factors including the subject
characteristics, heterogeneity of the patients with COPD,
pathological characteristics, inflammatory profiles (28,29)
and presence of α1-antitrypsin deficiency may contribute to the
differences in the results of these studies (15,30).
Several limitations were encountered in the present
study. Firstly, the number of study subjects was smaller than that
in previous studies. Secondly, the observation period of three
months may be insufficiently long to detect the changes in lung
function and SP-D. Therefore, a large-scale and long-term study may
be necessary to investigate the potential function of SP-D as a
biomarker of COPD. SP-D could also be used as a potential biomarker
in combination with other biomarkers and could be administered to a
selected subgroup of patients with COPD. To date, the use of a
single biomarker of COPD is generally considered insufficient
(31). With the exception of the
lung function test, there are no well-validated biomarkers or
surrogate endpoints that can be used to establish efficacy of a
drug for COPD (21). The
combination of novel biomarkers with existing tools can optimize
the diagnosis (23), treatment
(31) and prognostic judgment
(32) of patients with COPD.
In conclusion, the results of the present study
showed that the serum SP-D levels decreased after three months of
treatment with a combination of salmeterol and fluticasone
propionate. The baseline sputum SP-D levels demonstrated a weak
correlation with treatment response. However, the baseline serum
and sputum SP-D levels were not associated with the severity of
airflow obstruction or the mMRC or SGRQ scores. No significant
correlation was found between serum and sputum SP-D levels. Changes
in serum and sputum SP-D levels were not associated with an
improvement in pulmonary function or the mMRC or SGRQ scores. SP-D
may be used in combination with other biomarkers or administered to
a selected subgroup of patients with COPD.
Acknowledgements
This study was supported by the ‘Changjiang Scholars
and Innovative Research Team in University Grant’ (no.
IRT0961).
References
|
1
|
Vestbo J, Hurd SS, Agustí AG, et al:
Global strategy for the diagnosis, management, and prevention of
chronic obstructive pulmonary disease: GOLD executive summary. Am J
Respir Crit Care Med. 187:347–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wulf-Johansson H, Thinggaard M, Tan Q, et
al: Circulating surfactant protein D is associated to mortality in
elderly women: a twin study. Immunobiology. 218:712–717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sin DD, Man SF, Marciniuk DD, et al; ABC
(Advair, Biomarkers in COPD) Investigators. The effects of
fluticasone with or without salmeterol on systemic biomarkers of
inflammation in chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 177:1207–1214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shakoori TA, Sin DD, Ghafoor F, Bashir S
and Bokhari SN: Serum surfactant protein D during acute
exacerbations of chronic obstructive pulmonary disease. Dis
Markers. 27:287–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ju CR, Liu W and Chen RC: Serum surfactant
protein D: biomarker of chronic obstructive pulmonary disease. Dis
Markers. 32:281–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sin DD, Leung R, Gan WQ and Man SP:
Circulating surfactant protein D as a potential lung-specific
biomarker of health outcomes in COPD: a pilot study. BMC Pulm Med.
7:132007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
García-Laorden MI, Rodríguez de Castro F,
Solé-Violán J, et al: Influence of genetic variability at the
surfactant proteins A and D in community-acquired pneumonia: a
prospective, observational, genetic study. Crit Care.
15:R572011.PubMed/NCBI
|
|
8
|
Boonarkart CH, Suptawiwat O, Uiprasertkul
M, et al: A reduced expression of surfactant protein D in the lungs
of fatal influenza H1N1 cases in 2009. Acta Virol. 56:253–255.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Atochina-Vasserman EN, Winkler C, Abramova
H, et al: Segmental allergen challenge alters multimeric structure
and function of surfactant protein D in humans. Am J Respir Crit
Care Med. 183:856–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
King BA and Kingma PS: Surfactant protein
D deficiency increases lung injury during endotoxemia. Am J Respir
Cell Mol Biol. 44:709–715. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ware LB, Koyama T, Billheimer DD, et al;
NHLBI ARDS Clinical Trials Network. Prognostic and pathogenetic
value of combining clinical and biochemical indices in patients
with acute lung injury. Chest. 137:288–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sin DD, Man SF, McWilliams A and Lam S:
Surfactant protein D and bronchial dysplasia in smokers at high
risk of lung cancer. Chest. 134:582–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Madan T, Reid KB, Clark H, et al:
Susceptibility of mice genetically deficient in SP-A or SP-D gene
to invasive pulmonary aspergillosis. Mol Immunol. 47:1923–1930.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ichiyasu H, Ichikado K, Yamashita A, et
al: Pneumocyte biomarkers KL-6 and surfactant protein D reflect the
distinct findings of high-resolution computed tomography in
nonspecific interstitial pneumonia. Respiration. 83:190–197. 2012.
View Article : Google Scholar
|
|
15
|
Lomas DA, Silverman EK, Edwards LD, et al;
Evaluation of COPD Longitudinally to Identify Predictive Surrogate
Endpoints study investigators. Serum surfactant protein D is
steroid sensitive and associated with exacerbations of COPD. Eur
Respir J. 34:95–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vestbo J, Edwards LD, Scanlon PD, et al;
ECLIPSE Investigators. Changes in forced expiratory volume in 1
second over time in COPD. N Engl J Med. 365:1184–1192.
2011.PubMed/NCBI
|
|
17
|
Engström G, Lindberg C, Gerhardsson de
Verdier M, et al: Blood biomarkers and measures of pulmonary
function - a study from the Swedish twin registry. Respir Med.
106:1250–1257. 2012.PubMed/NCBI
|
|
18
|
Global Initiative for Chronic Obstructive
Lung Disease (GOLD). Global Strategy for the Diagnosis, Management,
and Prevention of Chronic Obstructive Pulmonary Disease. http://www.goldcopd.org/uri.
Accessed December 31, 2011
|
|
19
|
Miller MR, Hankinson J, Brusasco V, et al;
ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J.
26:319–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beeh KM, Beier J, Kornmann O and Buhl R:
Sputum matrix metalloproteinase-9, tissue inhibitor of
metalloproteinase-1, and their molar ratio in patients with chronic
obstructive pulmonary disease, idiopathic pulmonary fibrosis and
healthy subjects. Respir Med. 97:634–639. 2003. View Article : Google Scholar
|
|
21
|
Sin DD and Vestbo J: Biomarkers in chronic
obstructive pulmonary disease. Proc Am Thorac Soc. 6:543–545. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Biomarkers Definitions Working Group.
Biomarkers and surrogate endpoints: preferred definitions and
conceptual framework. Clin Pharmacol Ther. 69:89–95. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koutsokera A, Stolz D, Loukides S and
Kostikas K: Systemic biomarkers in exacerbations of COPD: the
evolving clinical challenge. Chest. 141:396–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Durme YM, Verhamme KM, Aarnoudse AJ,
et al: C-reactive protein levels, haplotypes, and the risk of
incident chronic obstructive pulmonary disease. Am J Respir Crit
Care Med. 179:375–382. 2009.PubMed/NCBI
|
|
25
|
Hartl D and Griese M: Surfactant protein D
in human lung diseases. Eur J Clin Invest. 36:423–435. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dickens JA, Miller BE, Edwards LD, et al;
Evaluation of COPD Longitudinally to Identify Surrogate Endpoints
(ECLIPSE) study investigators. COPD association and repeatability
of blood biomarkers in the ECLIPSE cohort. Respir Res. 12:1462011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoegh SV, Sorensen GL, Tornoe I, et al:
Long-term stability and circadian variation in circulating levels
of surfactant protein D. Immunobiology. 215:314–320. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang IM, Stepaniants S, Boie Y, et al:
Gene expression profiling in patients with chronic obstructive
pulmonary disease and lung cancer. Am J Respir Crit Care Med.
177:402–411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Foreman MG, Kong X, DeMeo DL, et al:
Polymorphisms in surfactant protein-D are associated with chronic
obstructive pulmonary disease. Am J Respir Cell Mol Biol.
44:316–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
The Chronic Obstructive Pulmonary Disease
group of Chinese Thoracic Society. The diagnosis and treatment
guideline of chronic obstructive pulmonary disease. Zhonghua Jie He
He Hu Xi Za Zhi. 30:8–17. 2007.(In Chinese).
|
|
31
|
Braido F, Bagnasco D, Scichilone N, et al:
Biomarkers in obstructive respiratory diseases: an update.
Panminerva Med. 54:119–127. 2012.PubMed/NCBI
|
|
32
|
Celli BR, Locantore N, Yates J, et al;
ECLIPSE Investigators. Inflammatory biomarkers improve clinical
prediction of mortality in chronic obstructive pulmonary disease.
Am J Respir Crit Care Med. 185:1065–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|