Introduction
Ovarian cancer (OC) is the most common malignant
tumor involving the ovaries and remains associated with a high
mortality rate. Every year, ~191,000 cases are diagnosed worldwide
(1). Despite advances in therapy,
advanced OC maintains a five-year survival rate of ~40% (2). Thus, the necessity for novel
therapeutic modalities exists (3,4).
With the continuous development of molecular biology, gene therapy
for OC has become a notable field of study (5). Suicide gene therapy is one of the
most attractive tools. The most commonly used suicide gene approach
uses herpes simplex virus-thymidine kinase (HSV-TK), which has been
applied in several clinical trials (6,7).
This transforms nucleoside analogs, such as ganciclovir (GCV), into
monophosphorylated molecules, which are then converted into
triphosphorylated forms by cellular enzymes. These
triphosphorylated molecules are incorporated into elongated DNA,
causing premature chain termination and cell death (8).
Despite gene therapy providing an alternative
strategy for the prevention and treatment of cancer, its
application in the clinic remains limited, primarily due to the
lack of a safe and efficient gene delivery system (9). While viral and other classical
vectors are currently favored, non-viral delivery systems may offer
certain advantages for the delivery of therapeutic genes of
interest. Non-viral delivery systems that are utilized in gene
therapy for OC include injecting naked DNA, liposomes, polyplexes,
lipopolyplexes and nanoparticles, as well as gene gun and
ultrasound (US)/microbubble (MB)-mediated gene delivery (10). The injection of MBs into the blood,
followed by the application of US, has been proved to be safe for
humans (11). The theory behind
the use of MBs as gene ‘vectors’ is that a focused US beam can be
used to destroy the DNA-loaded MBs during their transit through the
microvascular circulation, leading to localized transduction once
the MB shell has been destroyed, while sparing non-targeted areas.
US-targeted MB destruction (UTMD) has been used to deliver genes to
cells in vitro and in vivo to treat diabetes,
cardiovascular disease, lung cancer, thyroid cancer and even tendon
diseases in experimental animal models (12–14).
In the present study, a mouse OC model was used to investigate the
potential inhibitory effects of the UTMD-mediated HSV-TK/GCV system
on OC in mice.
Materials and methods
Preparation of plasmid and MBs
The pORF-HSV-TK plasmid (Auragene Bioscience,
Changsha, China) was used in a polymerase chain reaction
amplification with upstream (GAATTCATGGCCTCGTACCCCGGC) and
downstream (CTCGAGTCAGTTAGCCTCCCCCATC) HSV-TK primers to obtain
~1.2 kb of the target HSV-TK fragment. The HSV-TK target gene was
then connected to a pMD-18T simple vector (Takara Biotechnology,
Co., Ltd., Dalian, China) to obtain the recombinant plasmid
pMD-18T-TK. The recombinant plasmid was transformed into DH5a
Escherichia coli competent cells (Auragene Bioscience) and
spread on a lysogeny broth agar plate for 16 h of culture. The
sequence was confirmed by the Beijing Genomics Institute (Beijing,
China). The positive clones were then selected for plasmid
extraction. The SonoVue® MB contrast agent SF6 was
purchased from Bracco (Milan, Italy); 90% of the MB diameters were
measured to be <6 μm. The average diameter of the MBs was 2.5
μm. The gene-loaded lipid MBs were prepared as described in the
study by Wang et al (15).
The MBs were cultured with poly-L-lysine (1 mg/ml) (Sigma, St.
Louis, MO, USA) at 37°C for 30 min. The subnatant was soaked,
removed and washed twice with phosphate-buffered saline (PBS).
Naked plasmid (1 mg/ml) was added and incubated at 37°C for 30 min,
and washed twice using PBS. This manipulation was repeated three
times. The plasmid concentration was measured to be 0.1 μg/μl.
Animal models
The animal experiments were performed in the Center
for Animal Experiments/Animal Biosafety Level III Laboratory of
Wuhan University (Wuhan, China), and were conducted in accordance
with the Guidelines for the Care and Use of Laboratory Animals of
Wuhan University. Eighty female BALB/c-nu mice, aged six weeks and
with a body weight of 14–16 g, were purchased from the Hubei Center
for Disease Control and Prevention (Wuhan, China). Mice were raised
in specific-pathogen free rooms under controlled conditions with a
temperature of 23±3°C and relative humidity of 50±20%. Each
BALB/c-nu mouse was inoculated with 2×106 cells/site OC
cells (SKOV3 cells, 107 cells/ml, presented by the
Medical Experimental Center of Zhongnan Hospital, Wuhan, China)
subcutaneously into the right armpit under isofluorane anesthesia.
In this model, tumors typically grew to 0.2 cm3 by the
15th to 20th day after injection. Sixty mice were then selected and
randomly divided into four groups: i) HSV-TK plus MBs plus US
(n=15); ii) HSV-TK plus US (n=15); iii) HSV-TK (n=15); and iv) PBS
(n=15). Ten mice in each group were selected for a 14-day survival
rate observation.
US-assisted HSV-TK gene transfection in
vivo
The mixture of plasmid and MBs was injected through
the tail vein under isofluorane anesthesia on day 0. Only the HSV-T
+ MBs + US group received injection of the mixture of MBs and
plasmid. The mice were injected once every three days, three times
in total. PBS (200 μl) was administered in the PBS group and HSV-TK
(200 μl, 0.1 μg/μl plasmid) was administered in the other three
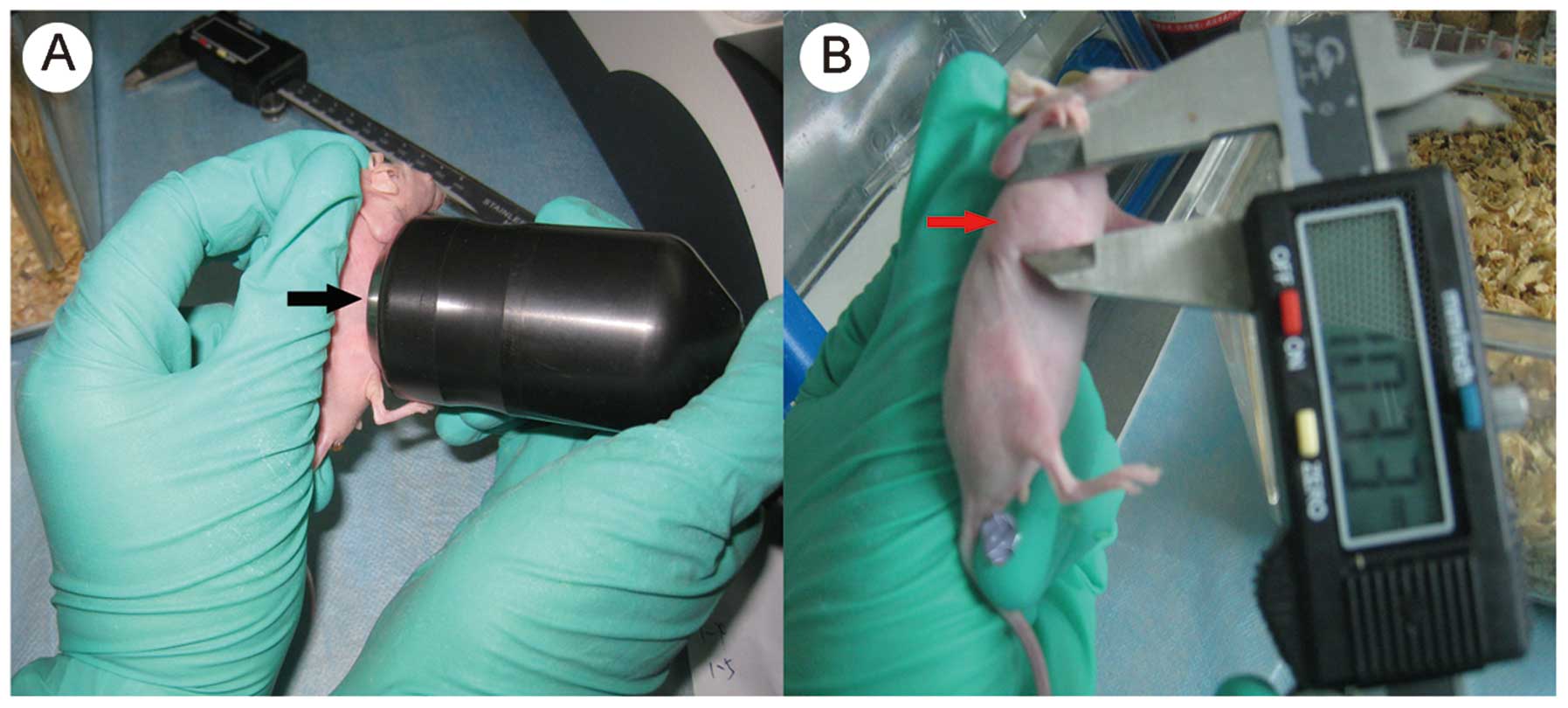
groups. A US transducer (Siemens, Berlin, Germany) was applied on
the HSV-TK plus MBs plus US and HSV-TK plus US groups for
irradiation following the gene injection. The US probe was tightly
placed on the tumor mass to ensure that the whole tumor was exposed
to the US. The US frequency was 1 MHz and sound intensity was 2
W/cm2. The pulse irradiation method was used for 5 min,
with an interval time of 10 sec, according to the method described
in the study by Zhou et al (16) (Fig.
1). Each mouse was intraperitoneally injected with 0.2 ml (100
mg/kg/day) GCV (Roche, Basel, Switzerland) from 24 h after
irradiation. The GCV injection was performed in the HSV-TK groups
every 24 h lasting for two weeks. The animals were sacrificed 24 h
after the last GCV injection and tissues were harvested for
pathological and biochemical examinations.
Evaluation of tumor inhibitory
effects
The maximum and minimum diameters of the tumor mass
were measured with a digital caliper every two days. The size of
the tumors was measured twice using the same method by two
different experimenters. Tumor volume and inhibition rate (IR) were
calculated on day 14 using the following formulae: Tumor volume
(mm3)=(a × b2)/2, where ‘a’ refers to the
maximum diameter (mm) and ‘b’ refers to the minimum diameter (mm);
and IR = (average tumor size in the control group - mean tumor
volume in the treatment group)/mean tumor volume in the control
group × 100%. The growth curve was drawn according to the size of
the tumor volume. The weight of the mice was also noted for the
evaluation of their condition.
Detection of apoptotic cells
Caspase-3 protease activity in the tumor tissue was
measured using a caspase-3 colorimetric assay kit according to the
manufacturer’s instructions (Beyotime Institute of Biotechnology,
Shanghai, China). Briefly, the homogenates of the liver tissues
were centrifuged, and protein (1 g) was incubated with
Asp-Glu-Val-Asp-p-nitroanilide and reaction buffer for 90 min at
37°C. Absorbance measured at 405 nm was representative of caspase-3
activity. A terminal deoxynucleotidyl transferase-mediated dUTP
nick end labeling (TUNEL) assay was carried out using a commercial
kit (Roche Diagnostics GmbH, Mannheim, Germany). Apoptotic cells
were identified by a brown stain over the nucleus. A total of ~200
cells were counted per field, five fields were examined per slide
and five slides were examined per group. The apoptotic index (AI)
was calculated as follows: AI = (number of apoptotic cells/total
number of tumor cells) × 100%.
Western blot analysis
Proteins were extracted using a protein extraction
reagent (Sigma), following the manufacturer’s instructions. The TK
protein was detected by western blotting. Concentrated gel (40
ml/l), separation gel (100 ml/l), pre-stained protein marker (3.0
μl) and sample total proteins (50 μg/hole) were prepared. The
samples were added into 100 ml/l SDS for SDS-PAGE. The gel was
removed when the bromophenol blue ran to the bottom, and the
protein was synchronously transferred to the polyvinylidene
difluoride membrane at 20 V for 50 min. This was then sealed for 4
h with 50 ml/l skimmed milk and Tris-buffered saline with Tween 20
(TBST) at room temperature (RT) following the trans-membrane
procedure. The primary antibody was subsequently added (TK1
polyclonal antibody, 1:500; Abcam, Cambridge, UK) prior to
incubation for 2 h at RT and maintenance overnight at 4°C. TBST was
used to wash the membrane four times for 10 min/time. The
horseradish peroxidase-conjugated secondary antibody (rabbit
anti-rat antibody, 1:5,000; Boster Immunoleader, Wuhan, China) was
then added for incubation, followed by agitation at RT for 2 h,
washing of the membrane, imaging and exposure. The protein bands
were normalized to GAPDH, and all blots were quantified with
Quantity One software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Experimental data are presented as the mean ±
standard deviation. The data were processed by the statistical
analysis software SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
The analysis of variance was used to assess the IR. The
Kaplan-Meier method was applied for the survival analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Therapeutic effects in vivo
As the tumor size increased, the mice exhibited
evident appetite loss, activity reduction, body weight loss and
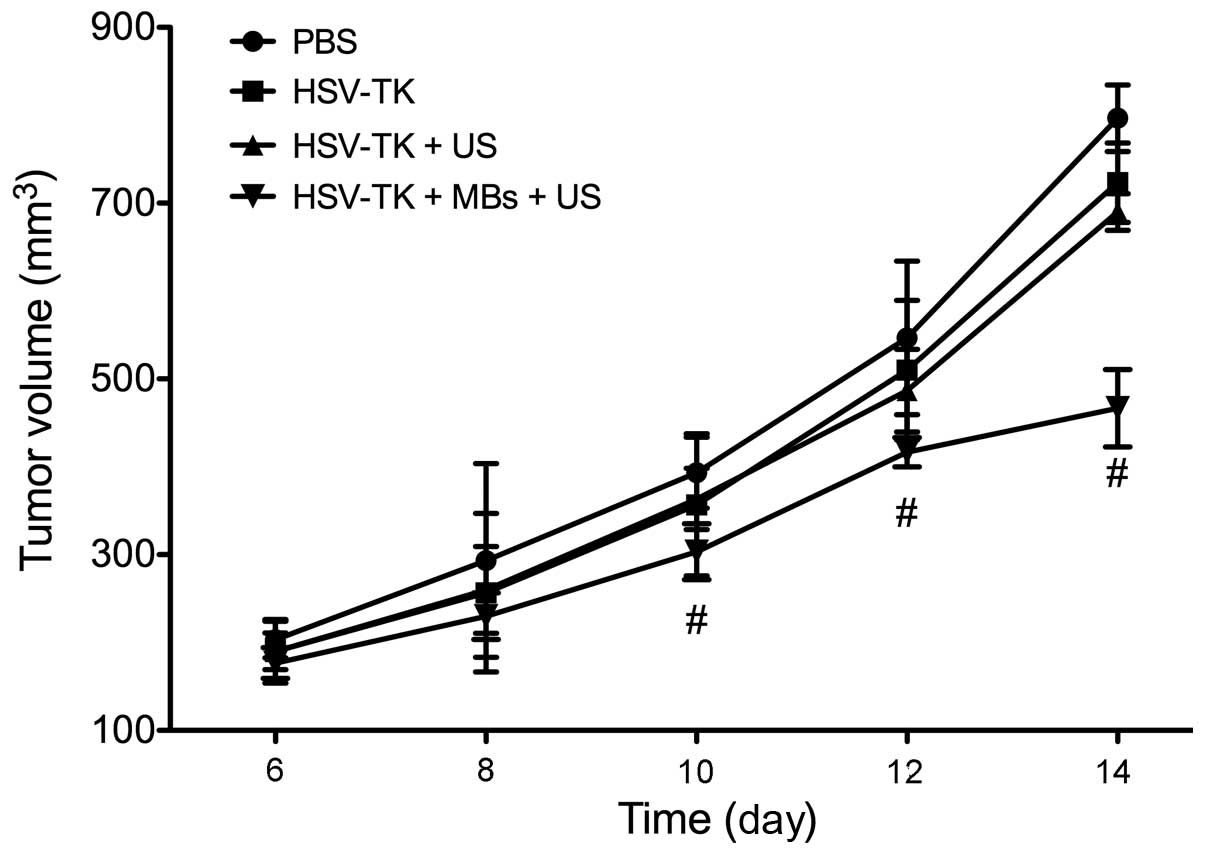
humpback posture. From the tumor growth curve, it was observed that
the tumor growth in the HSV-TK plus MBs plus US group slowed
significantly. Compared with the tumor sizes of the PBS group, the
tumor sizes of the HSV-TK plus MBs plus US group were significantly
lower during the last four days of observation (P<0.01)
(Fig. 2). The tumor IRs of the
HSV-TK plus MBs plus US, HSV-TK plus US, HSV-TK and PBS groups were
40.23±10.28, 22.20±3.73, 15.34±3.77 and 0%, respectively. In
addition, it was observed that the tumor mass in the HSV-TK plus
MBs plus US group was more easily separated from the normal tissue,
was surrounded by fluid at the time of separation and exhibited a
smooth surface. However, the tumors in the control group were
difficult to separate without damaging the surrounding skin and
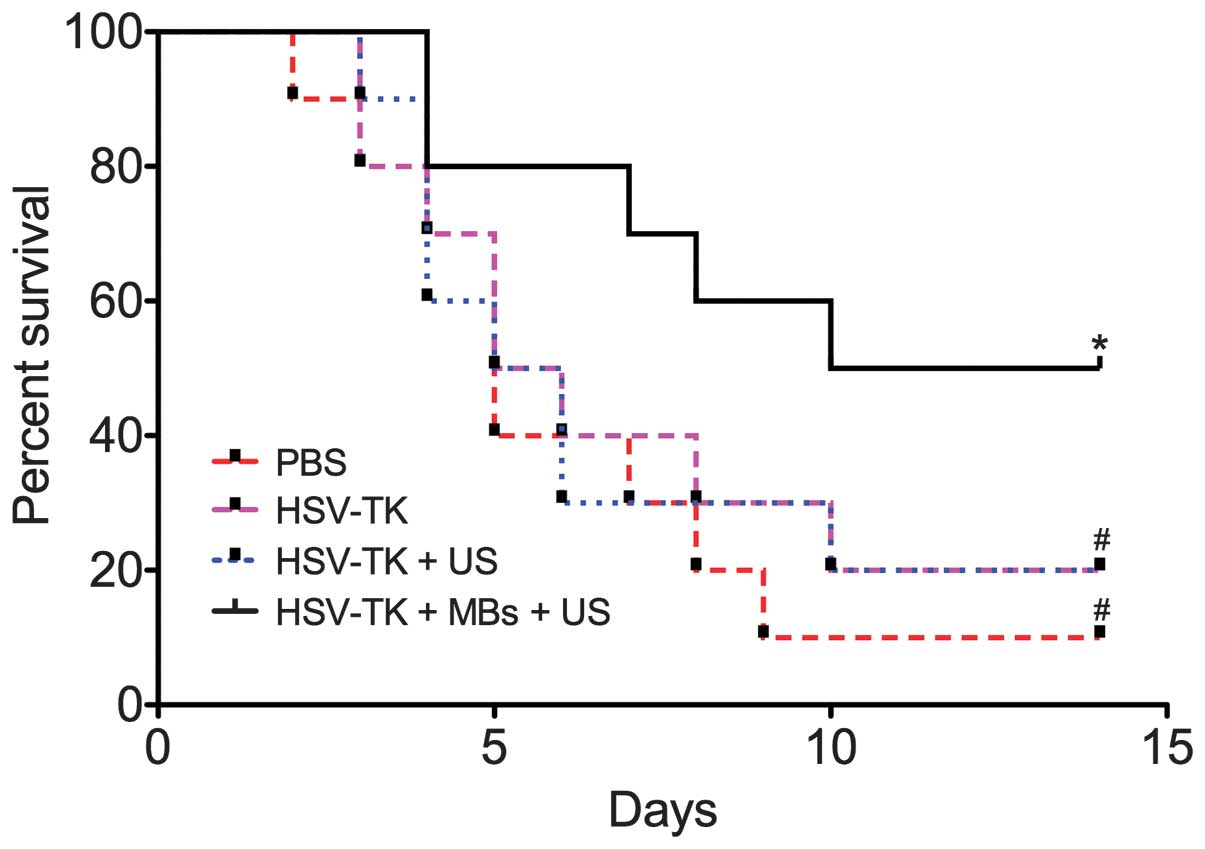
tissues. The survival rate observation results showed that the
HSV-TK plus MBs plus US group had a significantly higher survival
rate (P<0.01) compared with the control group (Fig. 3).
Apoptosis
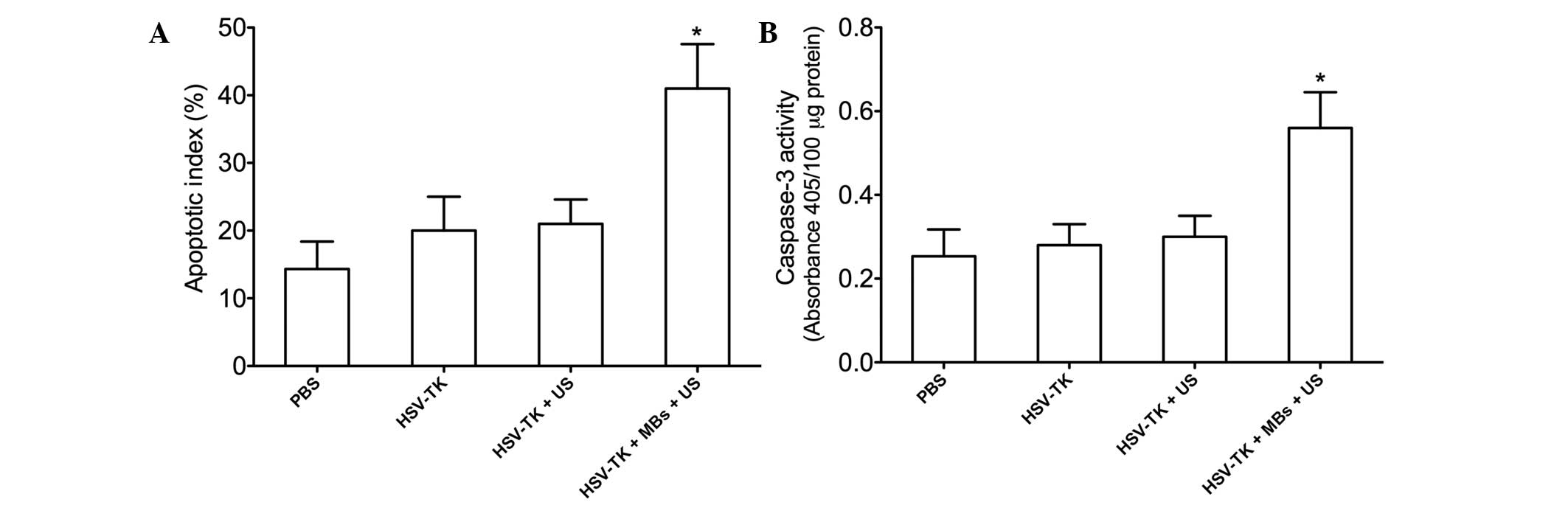
TUNEL staining was performed to detect tumor cell
apoptosis in each group. The TUNEL-positive cells (apoptotic cells)
were stained brown. The tumor cells in each group underwent
apoptosis to different degrees. The tumor cell apoptosis in the
HSV-TK plus MBs plus US group was the most evident. The AI was
highest (42.20±5.68%) in the HSV-TK plus MBs plus US group, which
was significantly higher than that in the HSV-TK plus US
(22.52±3.12%, P<0.01), HSV-TK (20.20±4.52%, P<0.01) and PBS
(14.65±3.88%, P<0.01) groups (Fig.
4A). In addition, the caspase-3 activity in the HSV-TK plus MBs
plus US group was significantly higher than that in the other
groups (P<0.05, respectively) (Fig.
4B).
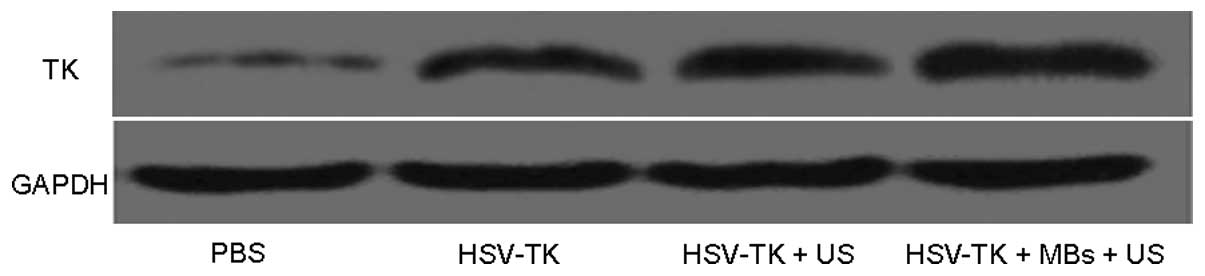
HSV-TK gene transfer in vivo
The TK protein expression was detected in tumor
tissues by western blot analysis. It was observed that a single
band appeared in each group at 25 kd. The band in the HSV-TK plus
MBs plus US group was the most evident (band intensity, 0.65±0.20),
characterized by a higher band intensity (P<0.01) (Fig. 5). The bands in the HSV-TK plus US
and HSV-TK groups were similar (band intensity, 0.20±0.05 and
0.18±0.04, respectively). However, no evident TK protein expression
was identified in the major organs, including the heart, lung,
liver and kidneys (data not shown).
Discussion
OC is the leading cause of mortality from
gynecological malignancies. Despite the fact that a response to
initial therapy is exhibited by the majority of patients presenting
with advanced disease, only 10–15% maintain a complete response
following first-line therapy (17). Thus, using the enhanced
understanding of the genetics and associated molecular pathways in
OC, novel targeted drugs with a greater therapeutic specificity
than standard chemotherapy are being developed (18). Different non-viral and viral
vectors utilized for improvements in targeted therapy have been
presented (3). In this study, UTMD
was used to deliver plasmid DNA into target cells. UTMD can focus
on specific tissues or organs directly and is beneficial for the
targeted delivery of the gene of interest (19). Thus, a targeted and sustained
antitumor effect is obtained. Furthermore, similar to non-viral
vectors, MBs have a number of advantages over viral systems,
particularly from the perspective of safety.
In the present study, western blot analysis was
performed to confirm the TK expression, and it was revealed that
UTMD could directly deliver the TK suicide gene to OC cells in this
mouse model. The results of the western blot analysis showed that
there was no evident TK protein expression in the major organs,
including the heart, lung, liver and kidneys, which meant that the
transfection of the TK gene was confined within the tumor cells.
The mechanisms underlying UTMD-mediated gene transfer remain
incompletely understood. The liposome MB is composed of
polyethylene glycol and liposome, and the mean diameter of the
liposome MB is smaller than that of red blood cells or conventional
MBs; thus the liposome MB could be developed as a gene transfection
tool and delivered into specific tissues (20). MB disruption at the US frequencies
used in the present study can produce high-velocity fragmentation
of the MB shell, which may contribute to gene transfection
(21,22). This suggests that microporation of
the vessel wall and/or the enhancement of cell membrane
permeability may be responsible for transfection by UTMD.
A clear inhibitory effect on OC was observed in the
present study. Compared with the other groups, tumor cell apoptosis
in the HSV-TK plus MBs plus US group was more apparent. In
addition, the tumor growth was significantly inhibited in the
HSV-TK plus MBs plus US group. These findings established proof of
concept that UTMD can impact tumor biology. Furthermore, it was
observed that mice in the HSV-TK + MBs + US treatment group showed
improved appetite, enhanced activity and lower body weight loss
during this study. HSV-TK gene delivery into tumor cells followed
by treatment with the antiviral drug GCV is the most common
experimental and clinical model for gene therapy. The TK
metabolizes the GCV into a monophosphorylated form, which is
subsequently converted to a toxic triphosphate molecule by other
cellular kinases. This toxic GCV metabolite is then incorporated
into the DNA of replicating cells, leading to premature strand
termination, replication failure and cell death. This type of
pro-drug activation is widely described as a ‘suicide’ gene therapy
(6,23). As one of the driving forces of the
suicide gene strategy, ‘bystander effects’ have also been
identified as important mechanisms of cytotoxicity and could ensure
destruction of more tumor cells than the number that is actually
infected. Those cells that have not been transfected can be
supplemented by bystander effects, resulting in an anti-tumor
effect (24,25). Additionally, MBs play an important
role in enhancing the effect of gene therapy. A previous study
(21) demonstrated that plasmids
on the MB surface could be shielded from digestion by blood DNAses,
in contrast to naked plasmids which are quickly degraded by blood
DNAses upon vascular administration.
In conclusion, UTMD was used as a gene delivery
method in the treatment of mouse OC by HSV-TK/GCV. The anti-tumor
efficacy of HSV-TK and the mouse survival rate were markedly
improved following use of the UTMD-mediated HSK-TK/GCV system. The
UTMD technology is expected to provide a novel strategy for
targeted OC therapy.
Acknowledgements
The authors would like to thank the Medical
Experimental Center and the Rehabilitation Department of Zhongnan
Hospital. This study was financially supported by the Natural
Science Foundation of Hubei Province (no. 2008CDB149).
References
|
1
|
Runnebaum IB and Stickeler E:
Epidemiological and molecular aspects of ovarian cancer risk. J
Cancer Res Clin Oncol. 127:73–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin HW, Tu YY, Lin SY, Su WJ, Lin WL, Lin
WZ, Wu SC and Lai YL: Risk of ovarian cancer in women with pelvic
inflammatory disease: a population-based study. Lancet Oncol.
12:900–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rocconi RP, Numnum TM, Stoff-Khalili M,
Makhija S, Alvarez RD and Curiel DT: Targeted gene therapy for
ovarian cancer. Curr Gene Ther. 5:643–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yap TA, Carden CP and Kaye SB: Beyond
chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer.
9:167–181. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kimball KJ, Numnum TM, Rocconi RP and
Alvarez RD: Gene therapy for ovarian cancer. Curr Oncol Rep.
8:441–447. 2006. View Article : Google Scholar
|
|
6
|
Rainov NG: A phase III clinical evaluation
of herpes simplex virus type 1 thymidine kinase and ganciclovir
gene therapy as an adjuvant to surgical resection and radiation in
adults with previously untreated glioblastoma multiforme. Hum Gene
Ther. 11:2389–2401. 2000. View Article : Google Scholar
|
|
7
|
Shand N, Weber F, Mariani L, Bernstein M,
Gianella-Borradori A, Long Z, Sorensen AG and Barbier N: A phase
1–2 clinical trial of gene therapy for recurrent glioblastoma
multiforme by tumor transduction with the herpes simplex thymidine
kinase gene followed by ganciclovir. GLI328 European-Canadian Study
Group. Hum Gene Ther. 10:2325–2335. 1999.
|
|
8
|
Aoi A, Watanabe Y, Mori S, Takahashi M,
Vassaux G and Kodama T: Herpes simplex virus thymidine
kinase-mediated suicide gene therapy using nano/microbubbles and
ultrasound. Ultrasound Med Biol. 34:425–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z, Liang K, Liu J, Xie M, Wang X, Lü
Q, Zhang J and Fang L: Enhancement of survivin gene downregulation
and cell apoptosis by a novel combination: liposome microbubbles
and ultrasound exposure. Med Oncol. 26:491–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaur T, Slavcev RA and Wettig SD:
Addressing the challenge: current and future directions in ovarian
cancer therapy. Curr Gene Ther. 9:434–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piscaglia F and Bolondi L: Italian Society
for Ultrasound in Medicine and Biology (SIUMB) Study Group on
Ultrasound Contrast Agents: The safety of Sonovue in abdominal
applications: retrospective analysis of 23188 investigations.
Ultrasound Med Biol. 32:1369–1375. 2006. View Article : Google Scholar
|
|
12
|
Chen S, Shimoda M, Wang MY, Ding J,
Noguchi H, Matsumoto S and Grayburn PA: Regeneration of pancreatic
islets in vivo by ultrasound-targeted gene therapy. Gene Ther.
17:1411–1420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujii H, Sun Z, Li SH, Wu J, Fazel S,
Weisel RD, Rakowski H, Lindner J and Li RK: Ultrasound-targeted
gene delivery induces angiogenesis after a myocardial infarction in
mice. JACC Cardiovasc Imaging. 2:869–879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delalande A, Bureau MF, Midoux P, Bouakaz
A and Pichon C: Ultrasound-assisted microbubbles gene transfer in
tendons for gene therapy. Ultrasonics. 50:269–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang ZX, Wang ZG, Ran HT, Ren JL, Zhang Y,
Li Q, Zhu YF and Ao M: The treatment of liver fibrosis induced by
hepatocyte growth factor-directed, ultrasound-targeted microbubble
destruction in rats. Clin Imaging. 33:454–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou S, Li S, Liu Z, Tang Y, Wang Z, Gong
J and Liu C: Ultrasound-targeted microbubble destruction mediated
herpes simplex virus-thymidine kinase gene treats hepatoma in mice.
J Exp Clin Cancer Res. 29:1702010. View Article : Google Scholar
|
|
17
|
Khaider NG, Lane D, Matte I, Rancourt C
and Piché A: Targeted ovarian cancer treatment: the TRAILs of
resistance. Am J Cancer Res. 2:75–92. 2012.PubMed/NCBI
|
|
18
|
Weberpals JI, Koti M and Squire JA:
Targeting genetic and epigenetic alterations in the treatment of
serous ovarian cancer. Cancer Genet. 204:525–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Xie M, Wang X, Lv Q and Ding S:
Effects of lipid shell microbubble on ultrasound mediated EGFP gene
delivery to transplanted tumors: initial experience. Chin-Ger J
Clin Oncol. 7:424–428. 2008. View Article : Google Scholar
|
|
20
|
Liu P, Gao YH, Tan KB, Liu Z and Zuo S:
Grey scale enhancement of rabbit liver and kidney by intravenous
injection of a new lipid-coated ultrasound contrast agent. World J
Gastroenterol. 10:2369–2372. 2004.PubMed/NCBI
|
|
21
|
Carson AR, McTiernan CF, Lavery L, Hodnick
A, Grata M, Leng X, Wang J, Chen X, Modzelewski RA and Villanueva
FS: Gene therapy of carcinoma using ultrasound-targeted microbubble
destruction. Ultrasound Med Biol. 37:393–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meijering BD, Juffermans LJ, van Wamel A,
Henning RH, Zuhorn IS, Emmer M, Versteilen AM, Paulus WJ, van Gilst
WH, Kooiman K, de Jong N, Musters RJ, Deelman LE and Kamp O:
Ultrasound and microbubble-targeted delivery of macromolecules is
regulated by induction of endocytosis and pore formation. Circ Res.
104:679–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Canine BF and Hatefi A: HSV-TK/GCV
cancer suicide gene therapy by a designed recombinant
multifunctional vector. Nanomedicine. 7:193–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicholas TW, Read SB, Burrows FJ and Kruse
CA: Suicide gene therapy with Herpes simplex virus thymidine kinase
and ganciclovir is enhanced with connexins to improve gap junctions
and bystander effects. Histol Histopathol. 18:495–507.
2003.PubMed/NCBI
|
|
25
|
Mesnil M and Yamasaki H: Bystander effect
in herpes simplex virus-thymidine kinase/ganciclovir cancer gene
therapy: role of gap-junctional intercellular communication. Cancer
Res. 60:3989–3999. 2000.
|