Introduction
Chronic central pain (CCP) often occurs as a result
of spinal cord injury (SCI). It is characterized by spontaneous
pain, hyperalgesia (increased pain due to painful stimuli) and
allodynia (pain due to normally non-painful stimuli). Due to the
long-duration, slow recovery rate and difficult management, CCP is
regarded as one of the most obstinate pain syndromes and a major
challenge for patients following SCI (1).
An important consideration in the study of CCP
following SCI using an animal model is the pathological and/or
behavioral responses associated with human SCI. Numerous
experimental models, including photochemical, hemisection and clip
compression models, have been developed to replicate the complex
pathophysiological mechanisms of SCI (2–4).
Although these models share a number of pathological
characteristics with the human condition, the specific neural
substrates responsible for the injury-induced abnormal sensations
have yet to be elucidated. Yezierski et al demonstrated that
elevated excitatory amino acid levels were associated with SCI
(5). In addition, injection of
N-methyl-D-aspartate (NMDA) into the ventral horn of the spinal
cord has been found to induce marked neuronal degeneration and
acute inflammation in the grey and white matter (6). Previous studies have also
demonstrated that reactive oxygen species (ROS) are involved in
persistent pain, including neuropathic and inflammatory pain.
Coderre et al (7,8) reported that ROS are correlated with
the generation of allodynia. Using a peripheral nerve injury
(spinal nerve ligation) model, free radical scavengers, including
phenyl-N-t-butylnitrone (PBN) and 5,5-dimethyl-1-pyrroline-N-oxide,
were found to decrease mechanical allodynia (9). In addition, systemically or
intrathecally injected antioxidants were shown to reduce the
formalin-induced nociceptive response in the hindpaws of mice
(10). Furthermore, following
peripheral nerve injury, such as sciatic nerve transection, ROS
generation in the spinal cord was found to increase, while
superoxide dismutase (SOD) activity decreased, and the
mitochondrial ROS inside the spinal dorsal horn neurons in the
spinal nerve ligation model was found to decrease (11,12).
Using a neuropathic pain model, the enhancement of
NMDA receptor phosphorylation by ROS has become increasingly
studied as an important mechanism of central sensitization
(13,14). From these observations, it has been
hypothesized that the development of the allodynia mechanism may
result in an increase in ROS in the spinal cord and NMDA receptor
phosphorylation may induce central sensitization.
Therefore, in the present study, animal experiments
were performed to investigate whether NMDA receptor phosphorylation
is involved in the central sensitization of CCP rats following SCI.
In addition, SOD was administered to investigate whether the enzyme
inhibits the phosphorylation of NMDA receptors and alleviates CCP
following SCI.
Materials and methods
Animals
A total of 30 SPF grade Male Sprague-Dawley rats
(weight, 230–250 g) were provided by the Institute of Animal
Research of the Chinese Academy of Science (Beijing, China). The
experimental protocols were approved by the Animal Use and Care
Committee of Shandong University Health Science Center (Jinan,
China).
CCP model
Adult Sprague-Dawley rats were spinally contused at
L1, as previously described by Yezierski et al (15) and Allen et al (16). Briefly, under 10% chlorohydrate
anesthesia (3 ml/kg body weight, i.p.), the surgical field was
shaved and a longitudinal incision was made that exposed several
segments. A laminectomy was then performed at the two vertebral
segments (T13-L2). The spinal cord was contused using a 20-g copper
bar falling from 20 cm. The muscle and skin were then sutured and
the wound was treated with penicillin.
Pain behavior experiments
Behavioral tests were blindly performed by an
examiner, who was provided no information concerning the
experimental treatment. In order to observe the behaviors, the rats
were placed in a transparent acryl box installed on a wire net.
After a 15-min adaptation period, mechanical allodynia was
observed.
A Dynamic Plantar Aesthesiometer (Ugo Basile,
Comeril, Italy), operated using an automated Von Frey’s method, was
used for the measurement of mechanical allodynia. Once the animals
had adapted to the wire net, a straight metal Von Frey filament
(diameter, 0.5 mm) was placed at the plantar surface of the
ipsilateral hindpaw and the force (maximum force, 50 g) was
increased gradually until a withdrawal response was observed, in
order to measure the force required. This was repeated four times
with a minimum interval of 10 sec to measure the paw withdrawal
threshold. Mechanical allodynia was examined and the average
threshold was calculated for the contralateral hindpaw in the same
manner. The measurement data prior to SCI was set as the baseline
value and the ipsilateral and contralateral data were measured on
day 14 following SCI, when mechanical allodynia was the
highest.
Immunohistochemistry
Rats were anesthetized using 10% chlorohydrate (4.5
ml/kg body weight, i.p.; Yangzhou Aoxin Regent Factory of China,
Yangzhou, China). The rats were then perfused transcardially with
150–200 ml normal saline followed by 200 ml fixative, consisting of
4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS; pH
7.4). The L1 spinal segment was removed, postfixed in 4%
paraformaldehyde in PBS for 4–6 h, and then transferred to 20%
sucrose for 24–48 h. Transverse sections of 12-μm thickness were
cut using a cryostat. One out of every 5–6 sections through the L1
spinal segment was collected and mounted on gelatin-subbed slides
for immunohistochemical labeling for the phosphorylated NMDA
receptor subunit 1 (pNR1). A total of six sections from each rat
were collected, with 10 rats in each group.
The sections were stained immunohistochemically for
pNR1 using the Avidin Biotin Complex method (Hebei Yongchuan
Chemical Industry of China, Hebei, China). To reduce the
possibility of diaminobenzidine tetrahydrochloride (DAB) reacting
with endogenous peroxidases in the red blood cells in the tissues,
and additionally to increase the penetration of the antibody into
the tissue, the tissue sections were rinsed in 0.3%
H2O2 methanol solution for 30 min prior to
incubation with pNR1 antisera (Upstate Biotechnology, Lake Placid,
NY, USA). The tissue sections were then incubated sequentially with
rabbit anti-pNR1 (1:1,600; Upstate Biotechnology, Lake Placid, NY,
USA) overnight at 4°C, biotinylated goat anti-rabbit immunoglobulin
G for 1 h at room temperature and avidin-biotin-peroxidase reagent
using a Histostain™-SP kit (SP-9001-3; Zymed Laboratories, Inc.,
South San Francisco, CA, USA) for 30 min at room temperature. All
incubation steps were preceded by three rinses in PBS for 5 min.
The tissue samples was immunoreacted for pNR1 in 0.05% DAB and
0.03% H2O2 in 0.01 M PBS for 2–3 min to yield
a brown reaction product. The DAB step was preceded and followed by
three rinses with 0.01 M PBS for 5 min. The sections were
dehydrated in a series of dilutions of ethanol in water and xylene,
and the slides were mounted. To confirm the specificity of the
immunolabeling, control slides were exposed to diluted normal goat
serum (manufactured in New Zealand). Control slides that omitted
the primary antibody were consistently negative. The specificity of
the antisera was tested in a previous study (17).
Statistical analysis
Data were analyzed using SPSS 12.0 software (SPSS,
Inc., Chicago, IL, USA) and results are presented as the mean ±
standard error of the mean. Comparisons between the mean values of
groups were analyzed using t-tests, and one-way or two-way analysis
of variance where appropriate, followed by the Students
Newman-Keul’s test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of SOD on rats with CCP
Behavioral tests were performed at day 14 following
SCI. Rats with SCI that had a paw withdrawal threshold of <20 g
were considered to have CCP. The CCP rats were randomly assigned
into three groups, with 10 rats in each group. Intraperitoneal
injection of 4,000 U/kg SOD (SOD group; n=10) and 1 ml normal
saline (NS group; n=10) were administered. SOD was obtained from
Sigma-Aldrich (St. Louis, MO, USA) and was resolved in NS prior to
the injection. In addition, 10 rats with CCP received no treatment
as a control group. The dose of NS and SOD were based on previous
conventional studies associated with neuropathic pain models
(17,18). The paw withdrawal threshold was
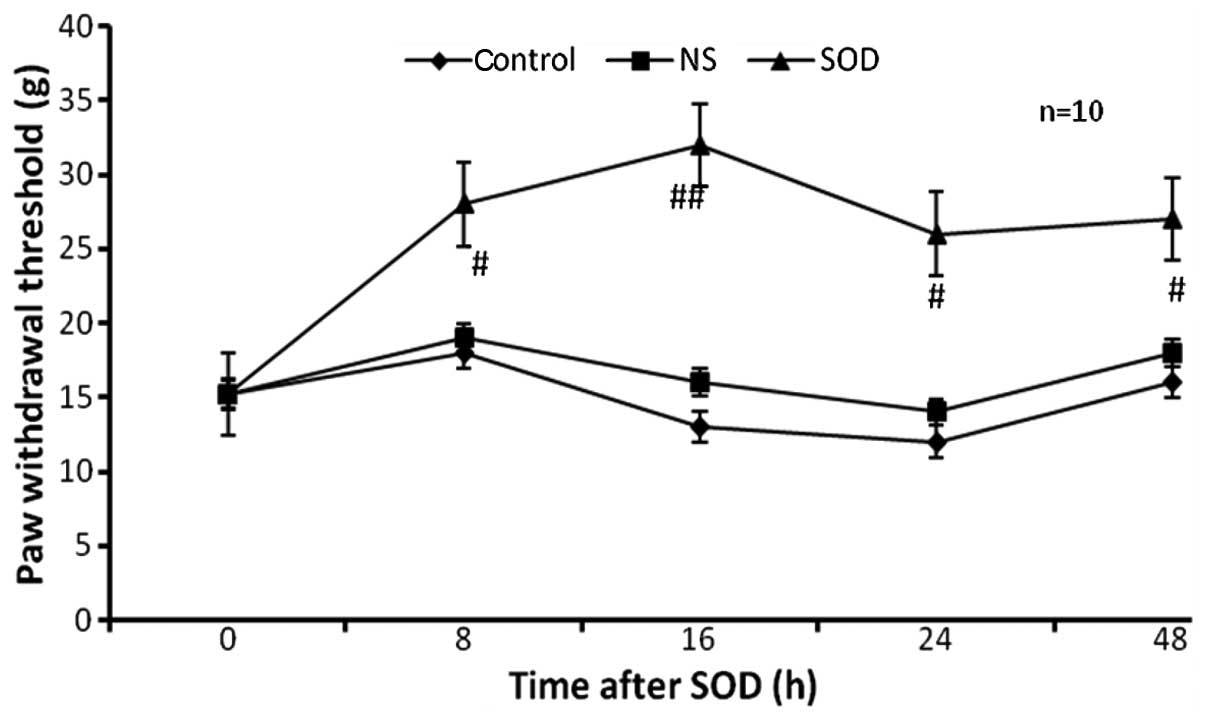
observed at 8, 16, 24 and 48 h following injection with SOD
(Fig. 1). In the control and NS
groups, the paw withdrawal threshold was stable over a period of 48
h. By contrast, in the SOD group, the paw withdrawal threshold
increased markedly between a basal level of 15.2±1.8 and 30.4±1.6 g
(P<0.01) at 16 h following the injection of SOD, and remained
high for 8, 24 and 48 h (P<0.05).
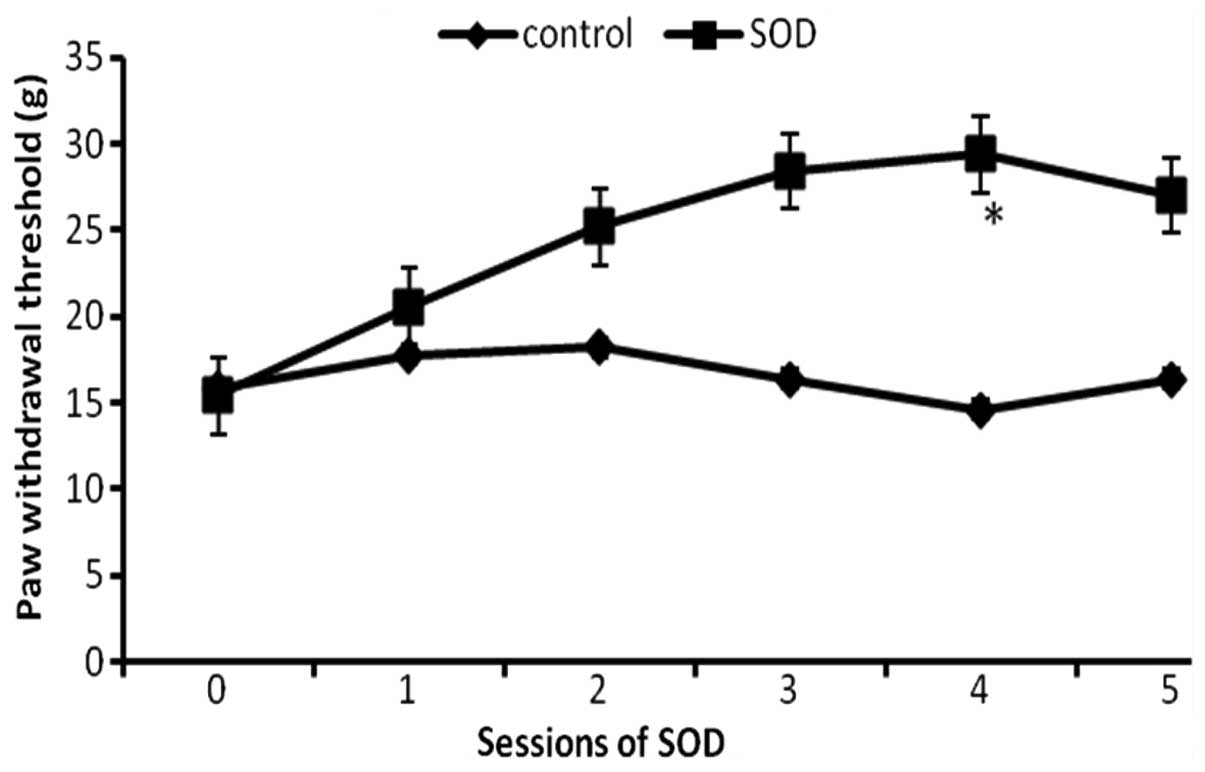
Effect of repeated injections of SOD on
rats with CCP
Since the aforementioned results demonstrated that
SOD inhibited CCP, it was then investigated whether SOD has a
cumulative analgesic effect with multiple treatments. The CCP rats
were divided into two groups (n=12) at day 16 following SCI. The
control group were restrained in the holder, while the SOD group
were administered SOD intraperitoneally every two days for five
sessions. The paw withdrawal threshold was assessed prior to each
administration of SOD (Fig. 2). At
the baseline, the paw withdrawal thresholds for the two groups were
almost identical; however, the withdrawal thresholds progressively
increased in the SOD group as compared with the control group
(P<0.05), indicating that the degree of CCP decreased with
repeated treatments of SOD.
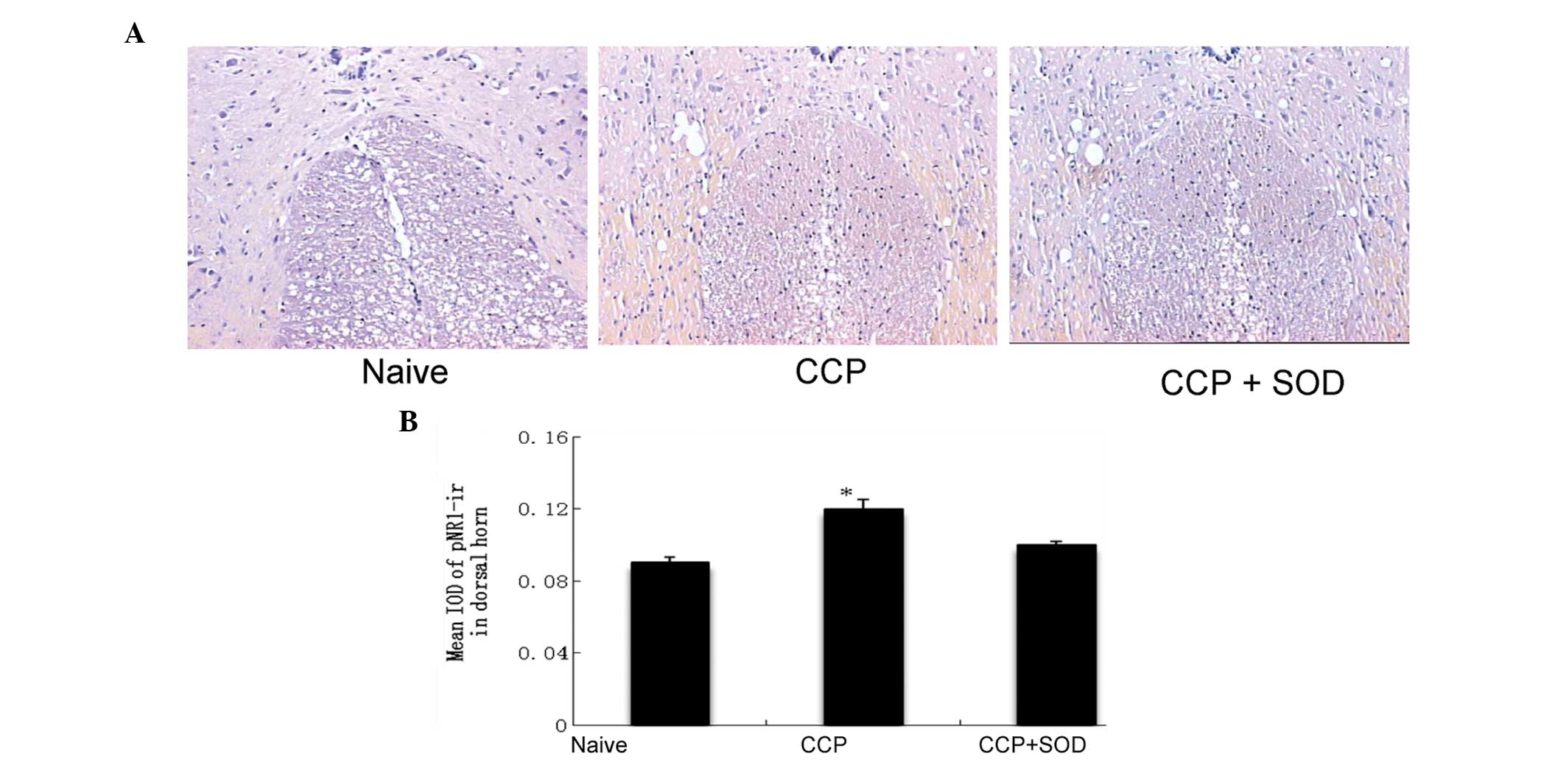
Measurement of pNR1
Following five sessions of SOD injections, the
expression levels of pNR1 were analyzed in the L1 spinal cord of
rats in the SOD, control and naive groups. The naive group had no
intervention. pNR1 immunoreactivity was enhanced significantly in
the superficial laminae of the spinal cord dorsal horn in the
control CCP rats when compared with the naive rats. However, no
statistically significant difference was observed between the SOD
and naive groups (Fig. 3A).
Histological images were further analyzed densitometrically using
an HMIAS-2000 Microimaging Collection and Analysis System and the
mean integrated optical density values of pNR1 in the superficial
laminae (I–II) were measured (Fig.
3B). The increased expression of pNR1 immunoreactivity in the
CCP rats was significantly suppressed by treatment with SOD.
Discussion
In the present study, a long-lasting reduction in
CCP was observed following the injection of SOD. In addition, SOD
was shown to significantly decrease the expression of pNR1 in
sections of the dorsal horn. These observations indicate that
superoxide, mediated by the inflammatory reaction following SCI,
may cause hyperalgesia by inducing NMDA receptor phosphorylation in
spinal cord cells. Furthermore, SOD may decrease the
phosphorylation of the NMDA receptor, inhibiting the generation of
superoxide and reducing CCP.
ROS are involved in intracellular signaling
transduction pathways as the normal derivatives of oxygen
metabolism. ROS are balanced by the antioxidant capacity of the
body; however, when the balance is lost, ROS increase and cause
oxidative stress. Superoxide functions as a proinflammatory factor
by increasing endothelial permeability, generating chemotactic
factors, such as leukotriene B4, and inducing neutrophils.
Superoxide reacts with nitric oxide (NO) to form peroxynitrite,
which is involved in post-ischemia cellular injury by continuously
generating superoxide and inactivating intrinsic manganese SOD
(MnSOD) by nitration (20,21). The pain-associated role of ROS was
further investigated by Kim et al (22), who demonstrated that plasma
superoxide was inhibited by high levels of allopurinol prior to
ischemia in an SCI model (22).
These observations indicated that ROS are associated with an
increase in nociceptor sensitivity, as well as the pain
transmission pathway and mechanism, and may be involved in the
chronic pain of peripheral tissues. The development and maintenance
of chronic pain is known to be associated with central
sensitization. Previous studies have focused on superoxide and NO
as the ROS predominantly involved in central sensitization, as well
as the role of superoxide in association with mitochondrial MnSOD
(12,13). Park et al (12) demonstrated that mitochondrial ROS
increased in the spinal dorsal horn of a spinal nerve ligation
model. In addition, intraplantar injection of carrageenan in rats
caused hyperalgesia, and increased MnSOD nitration in the spinal
cord was found to decrease hyperalgesia. Following peripheral nerve
injury, the release of excitatory amino acids, including glutamate,
in the dorsal horn stimulates the NMDA receptor, resulting in the
production of superoxide and NO via the activation of enzymatic
cascades through the NMDA receptor. Peroxinitrate, formed as a
result of superoxide and NO, causes MnSOD nitration, resulting in
spinal cord hypersensitivity and an increase in central
sensitization mediated by NMDA. (13,23).
Physiologically, spinal cord central sensitization
is defined as the increased reactivity of the spinal dorsal horn to
peripheral nociceptor stimuli. The induction of spinal cord central
sensitization is associated with NMDA receptor subtypes, which have
previously been categorized. The NMDA receptor is activated via the
phosphorylation of NR1 (24). The
decrease in pNR1 immunoreactive neurons in the spinal dorsal horn
is consistent with the decrease in hyperalgesia following the
injection of PBN (9), which was
used as an antioxidant in neuropathic pain and inflammatory pain
models. Therefore, ROS are involved in central sensitization
through NMDA receptor activation.
In the present study, pNR1 expression was decreased
by which was inhibited the superoxide mediated by xanthine oxidase
(XO), superoxide and NO (precursors of peroxynitrite), indicating
that ROS are involved in the central sensitization mechanism
associated with the NMDA receptor in the SCI model. Previous
studies on the hippocampus and other cerebral regions have
demonstrated that long-term potentiation (LTP) in synaptic efficacy
may be produced postsynaptically by an alteration in patterns of
presynaptic stimulation. It has been demonstrated that intense,
recurrent and/or sustained noxious stimulation of C fibers leads to
an increase in synaptic efficacy and wide dynamic range neuron
excitability in the dorsal horn (25,26).
Processes leading to central sensitization are associated with the
processes underlying LTP and involve the engagement of NMDA
receptors. Using electrophysiological and pharmacological
approaches, NMDA receptors have been shown to have an important
role in neuropathic pain (27,28).
In the present study, a significant increase in pNR1
immunoreactivity was observed in the superficial laminae of dorsal
horns from rats with neuropathic pain in an SCI model. This
discrepancy may be accounted for by differences in the animal
models used, time points or the methods used for the detection of
pain behavior. Nociceptive behavior was assessed using the paw
withdrawal threshold. However, Zou et al (14) demonstrated that only pNR1 in the
spinal dorsal horn was enhanced following SCI, not NR1 itself. The
sensitization of spinal dorsal horn neurons is expressed very
rapidly and reversibly (14).
However, the sensitization of spinal horn neurons induced by SCI,
underlying chronic, pathological and painful states, lasts a long
time, and may be associated with alterations in gene expression of
NR1, and ultimately, morphological changes in NR1 expression.
In conclusion, superoxide produced by XO, and
superoxide and NO as precursors of peroxynitrite, were demonstrated
to be involved in the mediation of central sensitization associated
with the phosphorylation of the NMDA receptor in an SCI model.
These ROS contributed to the etiology and maintenance of mechanical
allodynia. Therefore, the observations indicate that SOD, an ROS
scavenger, may be used as a potential therapeutic agent to reduce
CCP in patients following SCI.
References
|
1
|
Hulsebosch CE, Hains BC, Crown ED and
Carlton SM: Mechanisms of chronic central neuropathic pain after
spinal cord injury. Brain Res Rev. 60:202–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu XJ, Hao JX, Aldskogius H, Seiger A and
Wiesenfeld-Hallin Z: Chronic pain-related syndrome in rats after
ischemic spinal cord lesion: a possible animal model for pain in
patients with spinal cord injury. Pain. 48:279–290. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gwak YS, Crown ED, Unabia GC and
Hulsebosch CE: Propentofylline attenuates allodynia, glial
activation and modulates GABAergic tone after spinal cord injury in
the rat. Pain. 138:410–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruce JC, Oatway MA and Weaver LC: Chronic
pain after clip-compression injury of the rat spinal cord. Exp
Neurol. 178:33–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yezierski RP, Liu S, Ruenes GL, Kajander
KJ and Brewer KL: Excitotoxic spinal cord injury: behavioral and
morphological characteristics of a central pain model. Pain.
75:141–155. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomes-Leal W, Corkill DJ, Freire MA,
Picanço-Diniz CW and Perry VH: Astrocytosis, microglia activation,
oligodendrocyte degeneration, and pyknosis following acute spinal
cord injury. Exp Neurol. 190:456–467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coderre TJ, Xanthos DN, Francis L and
Bennett GJ: Chronic post-ischemia pain (CPIP): a novel animal model
of complex regional pain syndrome-type 1 (CRPS-I; reflex
sympathetic dystrophy) produced by prolonged hindpaw ischemia and
reperfusion in the rat. Pain. 112:94–105. 2004. View Article : Google Scholar
|
|
8
|
Bodera P, Stankiewicz W, Zawada K,
Antkowiak B, Paluch M, Kieliszek J, Kalicki B, Bartosiński A and
Wawer I: Changes in antioxidant capacity of blood due to mutual
action of electromagnetic field (1800 MHz) and opioid drug
(tramadol) in animal model of persistent inflammatory state.
Pharmacol Rep. 65:421–428. 2013. View Article : Google Scholar
|
|
9
|
Kwak KH, Han CG, Lee SH, Jeon Y, Park SS,
et al: Reactive oxygen species in rats with chronic post ischemia
pain. Acta Anaesthesiol Scand. 53:648–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hacimuftuoglu A, Handy CR, Goettl VM, Lin
CG, Dane S and Stephens RL Jr: Antioxidants attenuate multiple
phases of formalin induced nociceptive response in mice. Behav
Brain Res. 173:211–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guedes RP, Bosco LD, Teixeira CM, et al:
Neuropathic pain modifies antioxidant activity in rat spinal cord.
Neurochem Res. 31:603–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park ES, Gao X, Chung JM and Chung K:
Levels of mitochondrial reactive oxygen species increase in rat
neuropathic spinal dorsal horn neurons. Neurosci Lett. 391:108–111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao X, Kim HK, Chung JM and Chung K:
Reactive oxygen species (ROS) are involved in enhancement of
NMDA-receptor phosphorylation in animal models of pain. Pain.
131:262–271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou X, Lin Q and Wills WD: Enhanced
phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal
horn and spinothalamic tract neurons after intradermal injection of
capsaicin in rats. J Neurosci. 20:6989–6997. 2000.
|
|
15
|
Yezierski RP: Pain following spinal cord
injury: the clinical problem and experimental studies. Pain.
68:185–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Allen JW and Yaksh TL: Tissue injury
models of persistent nociception in rats. Methods Mol Med.
99:25–34. 2004.PubMed/NCBI
|
|
17
|
Zou X and Willis WD: Role of protein
kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal
horn and spinothalamic tract neurons after intradermal injection of
capsaicin in rats. Neuroscience. 115:775–786. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levy D and Zochodne DW: Local nitric oxide
synthase activity in a model of neuropathic pain. Eur J Neurosci.
10:1846–1855. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caban A, Oczkowicz G and Cierpka L:
Influence of ischemic precondition and nitric oxide on
microcirculation and the degree of rat liver injury in the model of
ischemia and reperfusion. Transplant Proc. 38:196–198. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Halliwell B and Gutteridge JMC: Free
Radicals in Biology and Medicine. Oxford University Press; New
York: pp. 222007
|
|
21
|
Li C and Jackson RM: Reactive species
mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol
Cell Physiol. 282:C227–C241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim KW, Ha MJ, Jung KY, et al: Reactive
oxygen species and N-methyl-D-aspartate receptor-mediated central
sensitization in hindlimb ischemia/reperfusion injury-induced
neuropathic pain rats. Korean J Anesthesiol. 56:186–194. 2009.
View Article : Google Scholar
|
|
23
|
Muscoli C, Mollace V, Wheatley J, et al:
Superoxide-mediated nitration of spinal manganese superoxide
dismutase: a novel pathway in N-methyl-D-aspartate-mediated
hyperalgesia. Pain. 111:96–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mori H and Mishina M: Structure and
function of the NMDA receptor channel. Neuropharmacology.
34:1219–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Willis WD: Long-term potentiation in
spinothalamic neurons. Brain Res. 40:202–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sandkühler J: Learning and memory in pain
pathways. Pain. 88:113–118. 2000.
|
|
27
|
Boyce S, Wyatt A, Webb JK, et al:
Selective NMDA NR2B antagonists induce antinociception without
motor dysfunction: correlation with restricted localisation of NR2B
subunit in dorsal horn. Neuropharmacology. 38:611–623. 1999.
View Article : Google Scholar
|
|
28
|
Bedi SS, Yang Q, Crook RJ, et al: Chronic
spontaneous activity generated in the somatic of primary
nociceptors is associated with pain-related behavior following
spinal cord injury. J Neurosci. 30:14870–14882. 2010. View Article : Google Scholar : PubMed/NCBI
|