Introduction
In addition to intestinal manifestations, ulcerative
colitis (UC) mainly affects the joints, skin, liver and eyes. UC
accompanied by pulmonary pathologies is rare in clinical settings;
however, the early detection of pulmonary complications is critical
for preventing the mortality of the patient (1). The rate of pulmonary complications
caused by inflammatory bowel diseases is ~0.21% (2). UC can be complicated by numerous
diseases, including interstitial lung disease (ILD) (3,4)
airway inflammation, airway stenosis (5), pulmonary vasculitis (6), pulmonary embolism, pulmonary bullae,
lung cysts (7) and pleural
adhesions (8). In the literature,
there were 24 cases of UC accompanied by ILD and 33 cases
accompanied by airway inflammation, in which there were 2 cases of
UC accompanied by airway inflammation, fibrosis of bronchi and
bronchiole, and bronchitis. Whilst UC accompanied by acute ILD is
rare, two cases of acute UC plus ILD were reported by Marten et
al (3) and Chikano et
al (4), in which high-dose
corticosteroid therapy was ineffective and the patients eventually
succumbed. In the present study, a case of UC accompanied by acute
ILD, airway disease, lung cysts and pleural adhesions was diagnosed
by the author. The disease remitted following administration of
cyclophosphamide combined with γ globulin in the case previously
mentioned. To further understand the clinical features of UC
accompanied by acute ILD, the present case of a male with UC
accompanied by acute ILD was reported and previous cases of UC
accompanied by ILD that were diagnosed on a pathological basis and
identified by a search of the English literature though PubMed were
analyzed retrospectively.
Case report
Clinical data
The patient was a male with an age of 58 years, a
height of 170 cm and a weight of 65 kg. The patient had a four-year
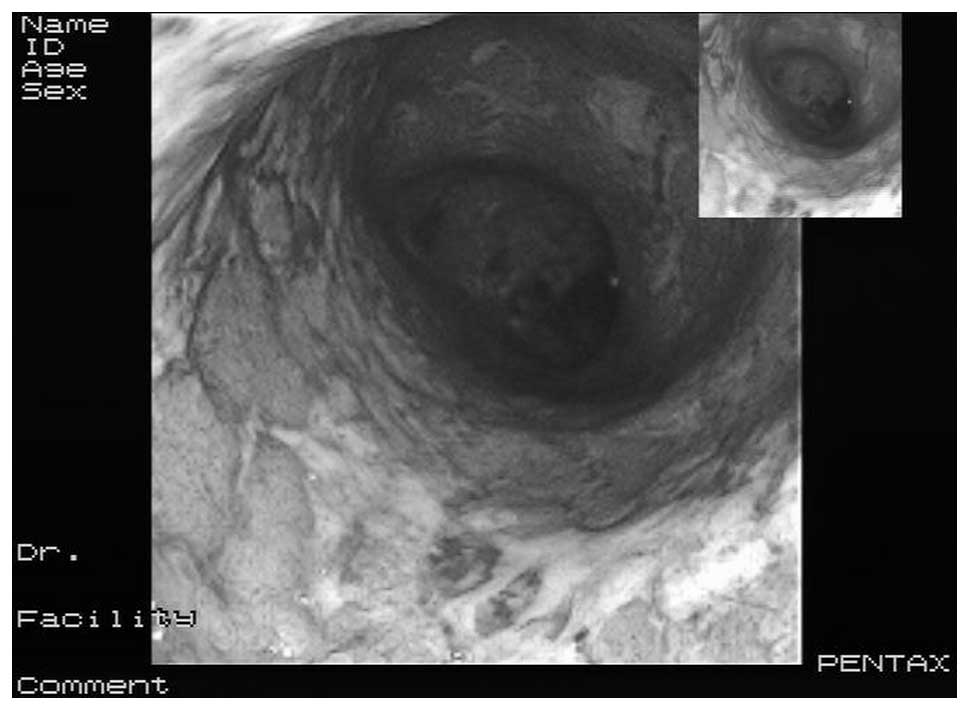
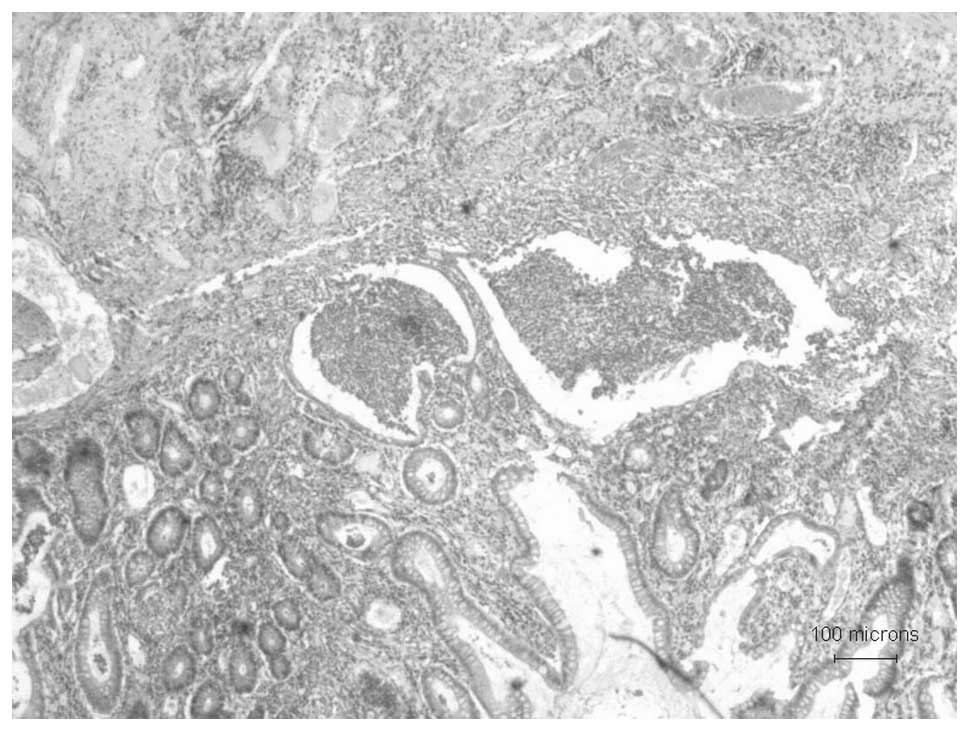
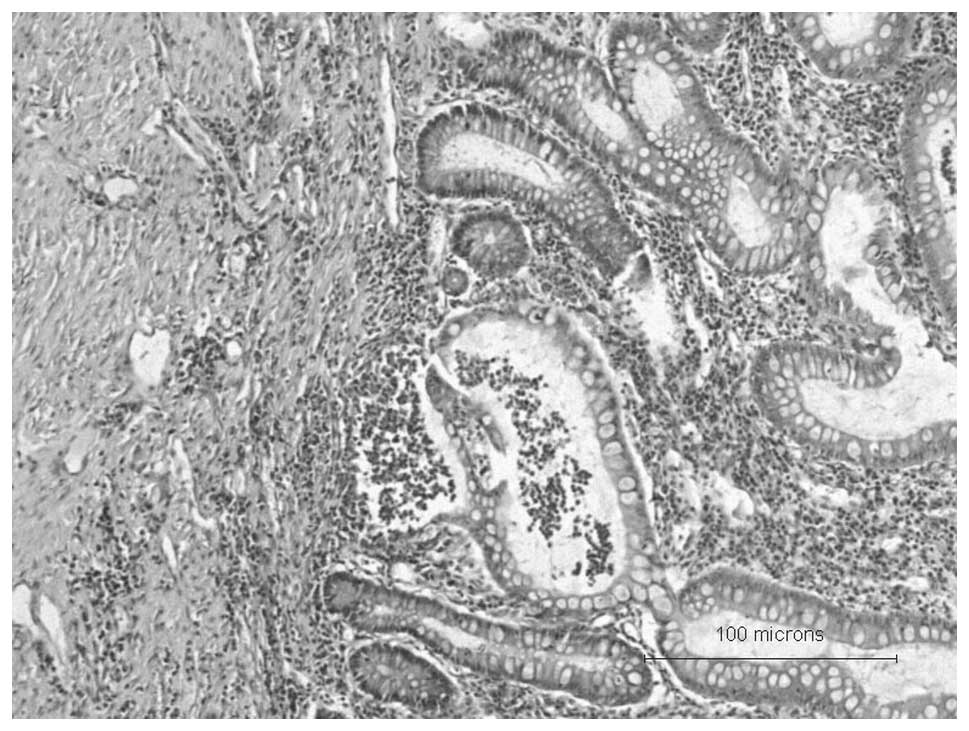
history of UC (colonoscopy images in Fig. 1 and colon biopsy histopathology
images in Figs. 2 and 3), and was admitted to hospital on
October 23, 2007, primarily due to dry cough and progressive
dyspnea that had been present for half a month. Four years prior to
the admission of the patient, the colonoscopy and pathological
diagnosis had indicated UC due to chronic diarrhea and bloody
stools. The patient was administered 5-aminosalicylic acid (0.5 g,
four times/day) orally for three and a half years, and the disease
remained in a stable condition. Four months prior to admission, the
5-aminosalicylic acid was terminated due to UC aggravation, which
remitted following the administration of prednisone (30 mg/day).
Half a month prior to admission, the prednisone dosage was reduced
to 15 mg/day, and symptoms of dry cough and progressive dyspnea
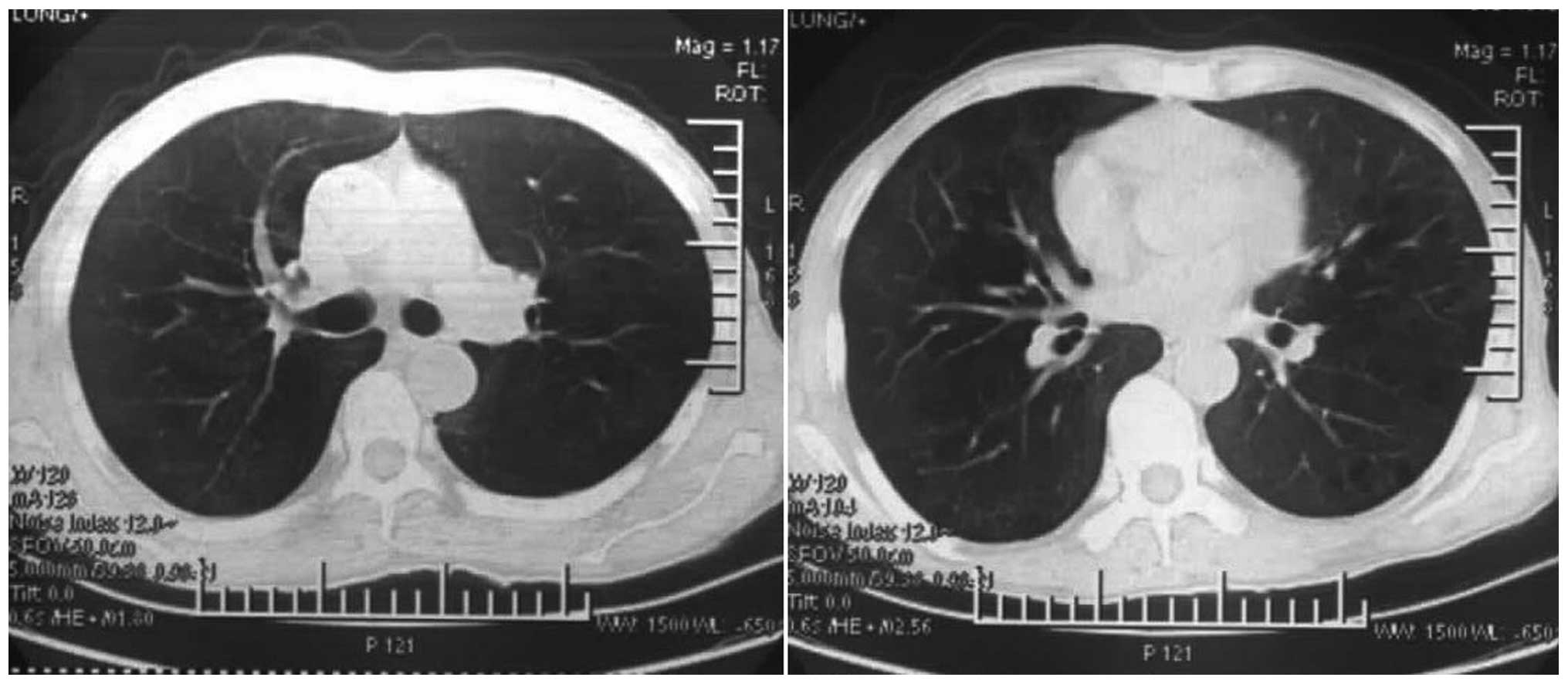
without fever appeared. The chest computed tomography (CT) was
normal on the seventh day after the respiratory difficulties
(Fig. 4); however, restrictive
ventilatory and diffuse pulmonary dysfunction were apparent, as
measured by a spirometer (Jaeger, Hoechberg, Germany). The chest CT
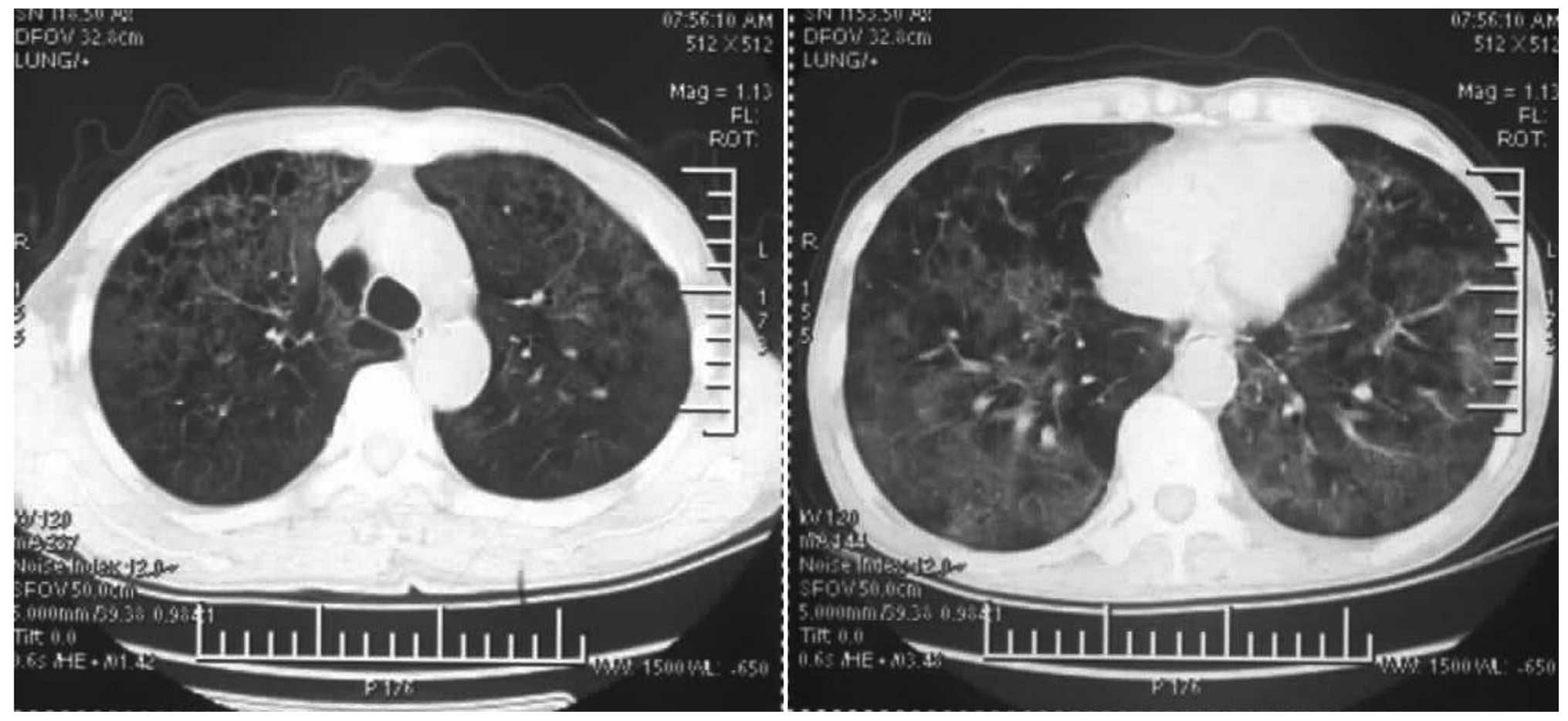
on the 11th day showed diffuse ground-glass shadows and nodules of
the hilar region in the bilateral lungs (Fig. 5). Levofloxacin, imipenem and
prednisone (30 mg/day) were prescribed by the local hospital for 14
days, but were ineffective. The patient was transferred to Qilu
Hospital of Shandong University (Jinan, China) on October 23, 2007
due to dyspnea. The patient had no previous history of
cardiopulmonary or rheumatic diseases or other noteworthy medical
history, and no history of allergies, smoking, dust inhalation or
pet ownership.
Physical examination on admission
The patient had the following characteristics on
admission: Temperature, 36.8°C; heart rate, 98 beats per minute;
breathing frequency, 28 times/min; and blood pressure, 107/69 mmHg.
The patient was in a supine position and exhibited nervousness,
shortness of breath, cyanosis of the lips and fingers, rough sounds
in the lungs and feeble, moist breath. Laboratory tests were
performed subsequent to admission, and a routine blood test
revealed the following results: White blood cell count,
8.7×109; neutrophils, 77.4% and lymphocytes, 22.6%;
erythrocyte sedimentation rate, 36 mm/h; blood bacterial culture,
negative; purified protein derivative test, negative; mycoplasma,
chlamydia and legionella antibody, negative; cytomegalovirus and
Epstein-Barr virus antibody, negative; human immunodeficiency virus
antibody, negative; lactate dehydrogenase levels, 233 IU/l (a
normal range is 120–230 IU/l); C-reactive protein levels, 12.4 mg/l
(a normal range is 0–8 mg/l); rheumatism series antibodies and
anti-neutrophil cytoplasmic antibody, negative; and complement,
T-cell subsets and immunoglobulins, all within normal range. A
blood gas analysis without oxygen was conducted using a blood gas
analyzer (IRMA TruPoint® Blood Analysis system;
International Technidyne Corporation, Edison, NJ, USA), which
revealed the following results: pH, 7.464; partial pressure of
blood carbon dioxide (PaCO2), 32 mmHg; partial pressure
of blood oxygen (PaO2), 55 mmHg; and
HCO3− levels, 25 mmol/l. In addition, the
electrocardiogram and echocardiogram findings were normal. This
study was conducted in accordance with the Declaration of Helsinki
and with approval from the Ethics Committee of Qilu Hospital of
Shandong University. Written informed consent was obtained from all
participants.
Diagnosis and treatment
Subsequent to admission, 3 g cefoperazone-sulbactam,
1 g vancomycin, 300 mg ganciclovir and 160 mg methylprednisolone
were intravenously infused once every 12 h. However, the condition
of the patient deteriorated and respiratory distress appeared. The
blood gas analysis (6 l/min oxygen inhalation by mask) revealed the
following results: pH, 7.35; PaCO2, 45 mmHg;
PaO2, 57 mmHg and HCO3− levels, 27
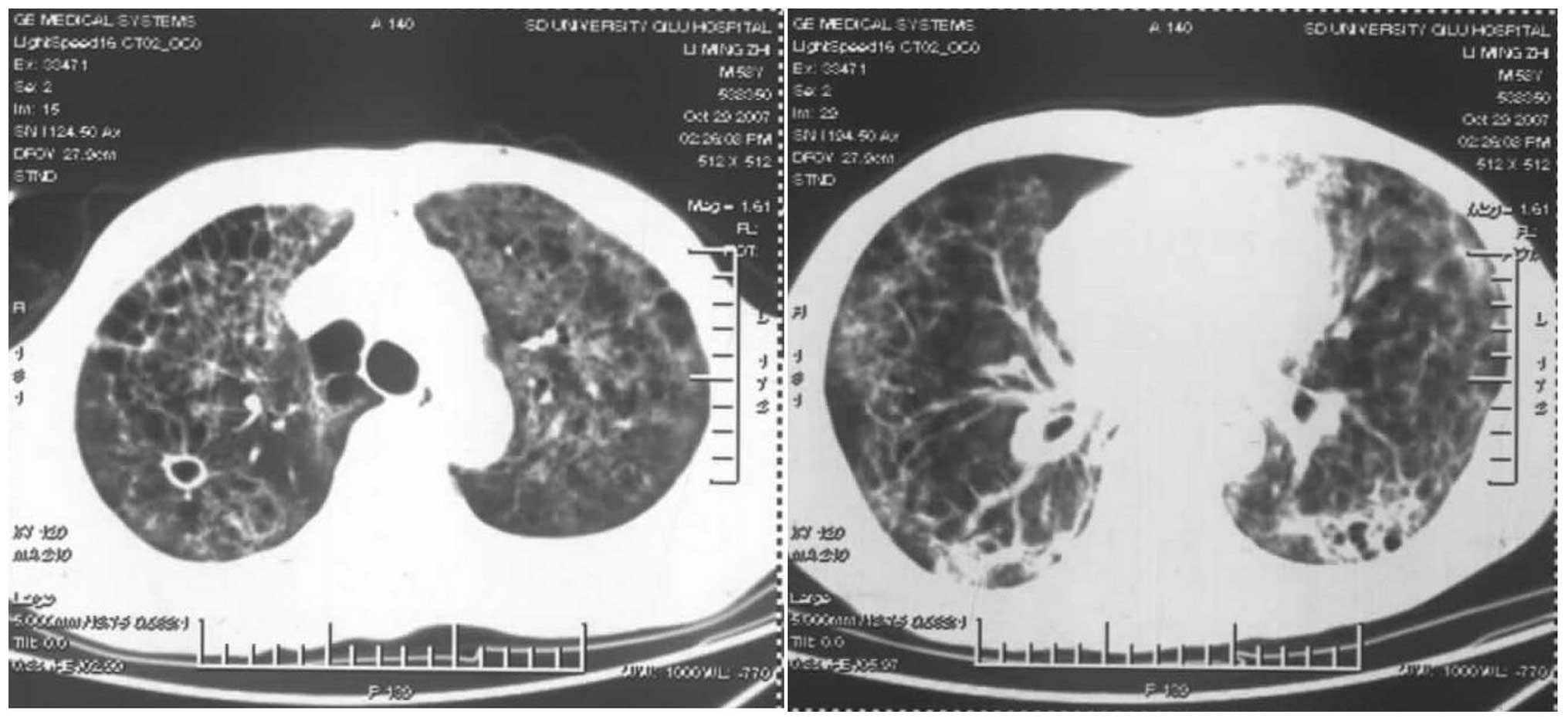
mmol/l. The results of the repeated chest CT scan (SOMATOM Emotion
16-slice; Siemens Healthcare, Erlangen, Germany), performed on the
21st day (seven days after admission), showed that the bilateral
lungs had changed to exhibit diffuse reticular or patchy shadows,
with nodular shadows on the pulmonary hilar region and cyst shadows
on the right upper lobe (Fig. 6);
therefore, the use of vancomycin was ceased. On the 23rd day after
the disease onset, pleural fiber deposition in the visceral layer,
wide pleural adhesions and nodules on the lung surface were
observed in the open lung biopsy taken from the right middle lobe
tissues.
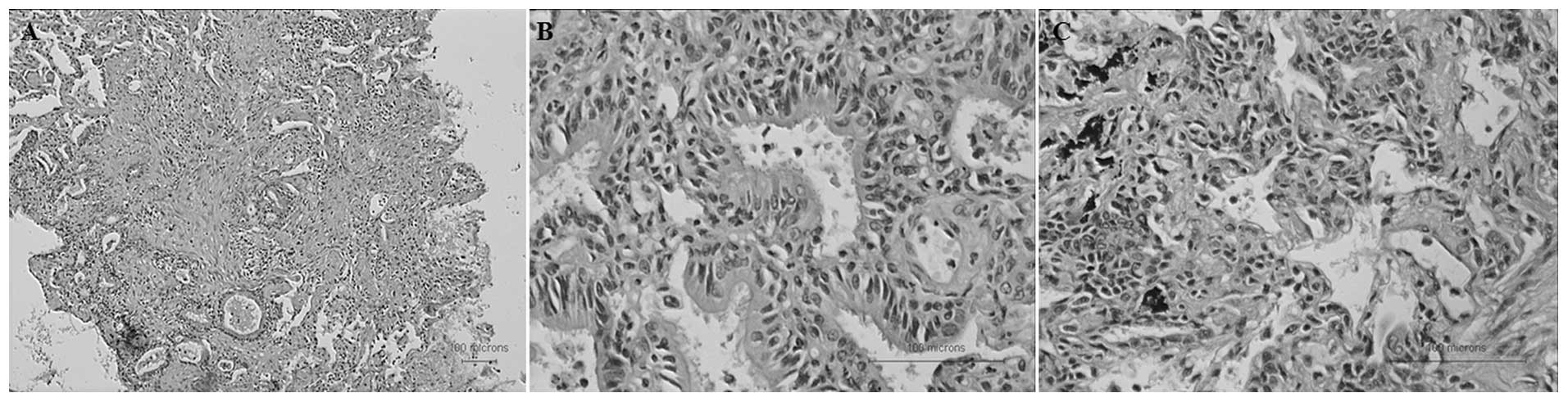
On the 28th day after the disease onset, the
pathological reports showed fibrosis in the bronchi, bronchioles
and surrounding lung tissues, and that bronchitis and bronchiolitis
were also apparent. Bronchial smooth muscle hyperplasia,
bronchiolar metaplasia, focal bronchiolar epithelial hyperplasia
and squamous alveolar type II epithelial cell proliferation were
also observed (Fig. 7). No virus
inclusion bodies and trophozoite of Pneumocystis carinii
were observed in the specimens. The disease was diagnosed to be
acute UC plus ILD and airway disease by combining the clinical,
imaging and pathological features. Every other day, 200 mg
cyclophosphamide was intravenously administered, combined with a γ
globulin intravenous injection once daily (20 g/day). On the 33rd
day after the disease onset, which was the fifth day after
cyclophosphamide treatment, the symptoms of dry cough and difficult
breathing were significantly reduced, and the patient could carry
out daily activities to a slight extent. The blood gas analysis
(conducted in conditions without oxygen) revealed the following
results: pH, 7.485; PaO2, 72 mmHg; PaCO2,
37.1 mmHg; and HCO3− levels, 28 mmol/l. The
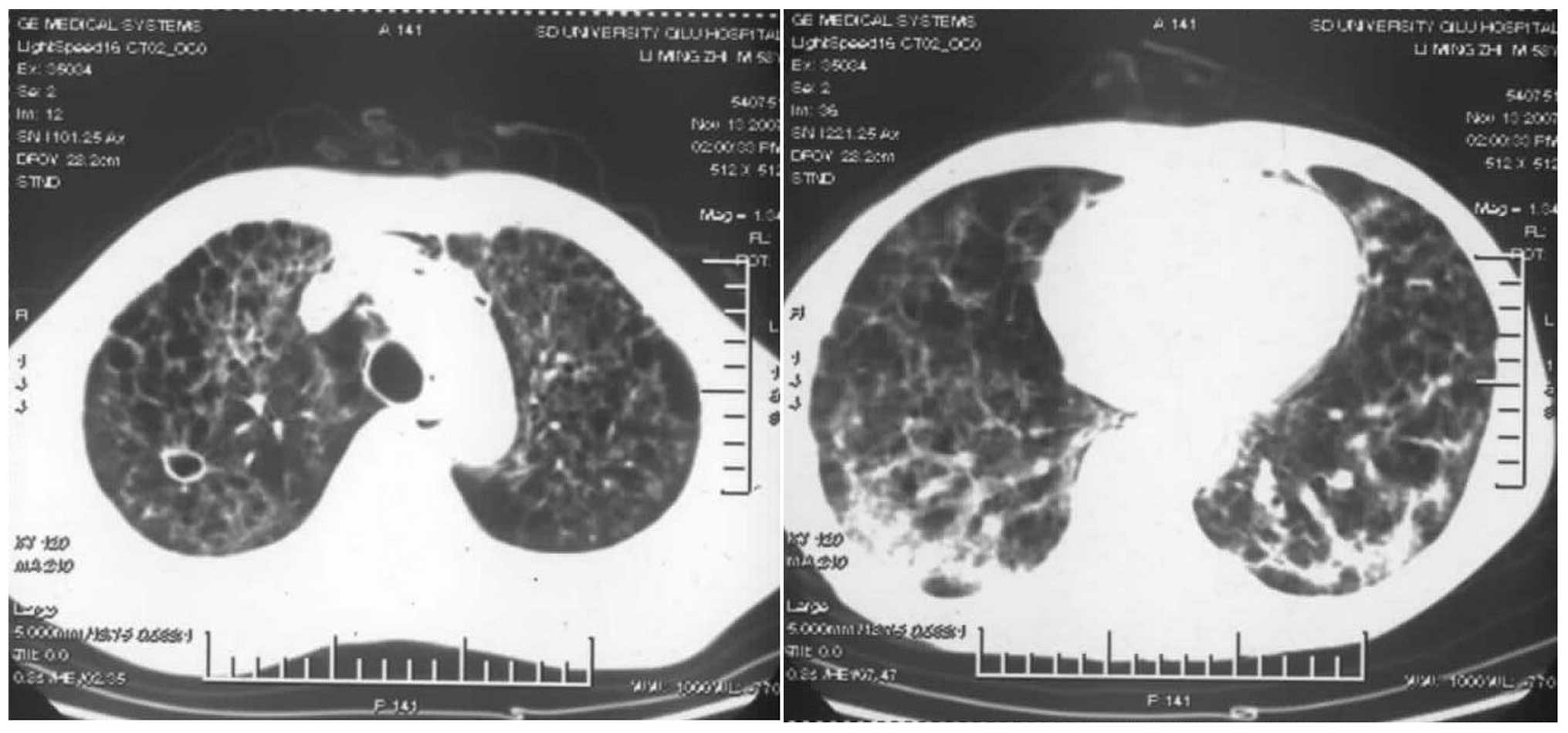
repeated CT scan showed that the patchy and reticular nodules in
the bilateral lungs had become smaller and the cystic wall had
thinned (Fig. 8). On the 35th day
after the disease onset, the condition of the patient was
significantly improved and he was discharged back to the local
hospital for continued administration of cyclophosphamide for 21
days (a total of 2.6 g), as the UC and lungs were in a stable
condition. Prednisone (40 mg/day) was administered orally for three
months subsequent to ceasing the use of cyclophosphamide. The UC
relapsed when the prednisone dosage was reduced to 25 mg/day, but
not the coughing, breathing difficulties or the other pulmonary
symptoms. The UC then remitted following the administration of
5-aminosalicylic acid (0.5 g/day orally, four times/day). Telephone
follow-up lasted for five years, during which time the UC relapsed
six times and the patient was admitted and treated in local
hospitals. The pulmonary condition remained stable without
recurrence, but the patient eventually succumbed on November 21,
2012 due to gastrointestinal bleeding.
Discussion
UC may be accompanied by a variety of pulmonary
complications including ILD, but the complication of acute ILD is
rare in clinical settings. Two patients with acute UC plus ILD
reported in the literature succumbed due to ineffective
glucocorticoid treatment (3,4). In
the case of UC accompanied by acute ILD that was diagnosed and
treated by the author, the disease remitted following
administration of cyclophosphamide combined with γ globulin. To
further understand the clinical diagnosis and treatment features of
UC accompanied by acute ILD, cases with a pathologically based
diagnosis were analyzed retrospectively according to the English
literature identified by a search through PubMed.
The keywords ‘Colitis, Ulcerative/complications’ and
‘Lung Diseases, Interstitial’ and ‘Colitis,
Ulcerative/complications’ and ‘Pulmonary fibrosis’ were used to
search PubMed. Studies not published in English and those in which
the disease was caused by drug-induced factors were excluded.
According to the pathological diagnosis, 24 cases with UC
complicated by ILD (six females and 10 males; age range, 13–70
years; mean age, 43±15.35 years) were selected. In eight cases the
gender and age were not reported, including two cases with acute
ILD insensitive to glucocorticoid therapy. Of these two cases, one
patient with idiopathic interstitial pneumonia (4) succumbed 75 days after the disease
onset due to opportunistic infections in the lungs, and one patient
with the usual type of acute interstitial pneumonia and acute
exacerbation (3) succumbed three
months after the disease onset. The keywords ‘Colitis,
Ulcerative/complications’ and ‘Bronchitis’, ‘Colitis,
Ulcerative/complications’ and ‘Bronchiolitis’ and ‘Colitis,
Ulcerative/complications’ and ‘Lung Diseases, Obstructive’ were
then used to search PubMed. Studies not published in English and
those in which the disease was caused by drug-induced factors were
excluded. According to the pathological diagnosis, 33 cases of UC
with concurrent airway disease were selected, including two cases
(9,10) in which pathology showed airway
inflammation, bronchial fibrosis and bronchial stenosis.
Twenty-four cases of pathologically diagnosed UC
complicated by ILD retrieved in this study were assessed for
certain characteristics, including the association between UC
activity and the onset of ILD and the time and duration of the
ILD. Among the 24 cases, 10 (11–13)
did not report the order of the ILD and UC incidences. In 12 cases
(12/14, 85.71%) the ILD occurred 1–15 years after the diagnosis of
UC (3,4,7–9,14–18);
this included only five cases in which the ILD occurred during
active UC (5/12, 41.67%) (3,4,14,15).
In two cases it was reported that the ILD occurred prior to the UC
(17,19); in one of these cases (19) the ILD occurred immediately
subsequent to the UC relapse, indicating that UC activity may be
associated with the incidence of ILD.
Inducing factors were also examined, including
whether the UC colectomy induced the ILD. Only four out of the 24
cases mentioned colectomy, including two cases (2/4, 50%) in which
the ILD occurred subsequent to the colon resection (3,18),
suggesting that the colectomy may be a stimulus for the occurrence
of UC complicated by ILD. The two cases in which the ILD occurred
prior to the colectomy were in different situations. McKee et
al (7) reported one case in
which the ILD occurred in the UC stable phase 10 years after the
onset of the UC. Colectomy was then performed due to the recurrence
of the UC; however, the ILD then continued to progress until
mortality, suggesting that the colectomy may have aggravated the
existing ILD condition. However, Isenberg et al (8) reported a case with a contrasting
condition; this patient had been diagnosed with UC for 15 years and
exhibited lung infiltrate in the bilateral lungs during the UC
stable period, but had no pulmonary symptoms. The open lung biopsy
diagnosis revealed diffuse vasculitis and interstitial pneumonia.
Without the use of corticosteroids, the chest radiograph on the
10th day after colectomy showed pulmonary infiltrate absorption,
and the chest radiograph then returned to normal.
Another inducing factor assessed was smoking
history. This was not reported in 17 cases (17/24, 70.8%), and only
two of the remaining seven cases (2/7, 28.6%) (3,14)
had a smoking history. Smoking history has therefore not been
determined to be a predisposing factor of ILD.
The clinical manifestations of the cases were next
assessed. With regard to the clinical symptoms, seven patients
exhibited dry cough (7/24, 29.2%) (7,9,13,16–19);
two, sputum (2/24, 8.3%) (15,18);
nine, shortness of breath and difficulty breathing (9/24, 37.5%)
(3,7,9,13,14,17–19);
four, pleuritic chest pain (4/24, 16.7%) (7,9,14,16);
four, fever (4/24, 16.7%) (4,15,16,18);
one, night sweats (1/24, 4.2%) (16); two, fatigue (2/24, 8.3%) (3,13);
two, weight loss (2/24, 8.3%) (13,15);
one, arthralgia (1/24, 4.2%) (16); and 10, no respiratory symptoms
(10/24, 41.7%) (8,12,14).
Symptoms of dry cough, shortness of breath and difficulty breathing
were the most common in clinical settings. When UC is accompanied
by acute ILD, precautions should therefore be taken to avoid the
aggravation of progressive acute dyspnea (3). Patients with UC but without
respiratory symptoms should also not be disregarded (12). With regard to the clinical signs
assessed in the cases, one patient exhibited low lung breath sounds
(1/24, 4.2%) (18); three, moist
rales (3/24, 12.5%) (3,7,19);
two, crepitus (2/24, 8.3%) (13,17);
one, expiratory wheezing (1/24, 4.2%) (13); and one, clubbing (1/24, 4.2%)
(19). With regard to lung
function, restrictive pulmonary dysfunction was mentioned in four
out of the 24 cases (3,7,9,13).
Where open lung biopsy or thoracoscopy was visible by eye, two
cases (8,16) of UC concurrent with ILD had pleural
fibrosis and pleural adhesions and one case (7) had diffuse cystic lung lesions,
suggesting that the lung lesions were diverse in cases of UC
accompanied by ILD. The assessment of pathogenesis revealed that 22
cases (22/24, 91.7%) had chronic disease. Only two cases had acute
disease (2/24, 8.3%) (3,4); however, the disease progressed
rapidly and severely in these two cases and the patients eventually
succumbed.
The evaluation of drug treatment revealed that
glucocorticoids were used in 13 cases (3,4,7,9,13,14,16–19)
and not in one case (8). Drug
treatment was not reported in 10 cases. Out of the 11 chronic cases
using glucocorticoids, the drugs were revealed to be effective in
10 cases (10/11, 90.9%) and ineffective in only one case (1/11,
9.1%) (7). By contrast, high-dose
corticosteroid therapy was ineffective in the two cases with acute
onset (including one case with concurrent opportunistic infections)
(3,4), suggesting that glucocorticoids remain
the preferred effective drug for cases of chronic disease, but that
they should be selected with caution and vigilance for pulmonary
opportunistic infections in cases of acute onset disease.
Cyclophosphamide and γ globulin are typically used in the treatment
for rheumatic diseases in clinical practice, but were not
administered in the retrieved 24 cases of UC concurrent with
ILD.
With regard to the prognosis of the cases, only one
out of the 22 cases with chronic onset disease (7) (1/22, 4.5%) succumbed, while both of
the two cases with acute onset disease succumbed (3,4). UC
accompanied by acute ILD therefore requires early diagnosis for
effective measures to be taken. The assessment of follow-up times
indicated that, in addition to three cases of mortality during
hospitalization, 10 cases were not followed-up. Out of the 11 cases
where follow-up was conducted, the duration ranged between four
weeks (9) and six years (16), with an average of 21±21.9
months.
Imaging findings from chest X-ray or CT scans
revealed that one patient exhibited diffuse ground-glass shadows
(1/24, 4.2%) (3); three, multiple
pulmonary infiltrate shadows (3/24, 12.5%) (8,9,17);
three, diffuse interstitial fibrosis (3/24, 12.5%) (7,13,18);
three grid-like shadows (3/24, 12.5%) (3,13,19);
one, alveolar shadows (1/24, 4.2%) (3); three, traction bronchiectasis (3/24,
12.5%) (3,7,14);
three, multiple nodules (3/24, 12.5%) (14–16);
one, pulmonary consolidation (1/24, 4.2%) (9); one, air bronchogram (1/24, 4.2)
(15); two, cavity disease (2/24,
8.3) (14,15); and one, pleural effusion (1/24,
4.2%) (9). Diffuse interstitial
fibrosis, multiple nodules, reticular patterns, honeycombing,
traction bronchiectasis and a number of other symptoms were common
and showed that the ILD lesions were not in the early stage. The
diffuse ground-glass shadows indicated that the pulmonary lesions
were in the acute exudative phase (3); therefore, precautions against acute
ILD must be taken if these shadows are identified.
Following the assessment of clinical manifestations,
the histological types and the methodology used to obtain the
pathological specimens were examined. Firstly, the pathological
types of the cases were determined. Among the 24 cases, eight
patients exhibited alveolar septal fibrosis (8/24, 33.3%) (12); three, fibrosing alveolitis
(16,17,19);
two, diffuse interstitial pulmonary fibrosis (2/24, 8.3%) (17,18);
one, idiopathic interstitial pneumonia (1/24, 4.2%) (4); one, usual interstitial pneumonia with
acute exacerbation (1/24, 4.2%) (3); two, cryptogenic organizing pneumonia
(2/24, 8.3%) (14,16); three, Wegener’s granulomatosis
(3/24, 12.5%) (16,20); two, sarcoidosis (2/24, 8.3%)
(11,15); one, interstitial pneumonia and
diffuse vasculitis (1/24, 4.2%) (8); one, bronchitis and bronchiolitis with
peribronchiolar fibrosis and stenosis (1/24, 4.2%) (9); one, interstitial pneumonia with
necrotizing bronchiolitis and bronchiectasis (1/24, 4.2%) (13); and one, pulmonary interstitial
fibrosis with bronchiectasis and organizing lipid pneumonia (1/24,
4.2%) (7). In the majority of the
24 cases, only one pathological type was apparent (20 cases, 20/24,
83.3%) and there were four cases in which two or more pathological
types were apparent (four cases, 4/24, 16.7%) (7–9,13)
were rare. With regard to the methods used to obtain the
pathological specimens, the majority of the specimens were obtained
using transbronchial lung biopsy (12 cases, 12/24, 50%) (4,12,17,18)
and open lung biopsy (7 cases, 7/24, 29.2%) (7–9,11,13,16,19).
In three cases the specimens were obtained by thoracoscopic biopsy
(3/24, 12.5%) (14,16), one by autopsy (1/24, 4.2%)
(3), and one by mediastinum lymph
node biopsy (1/24, 4.2%) (15). Of
these methods of obtaining specimens in the 24 cases, all
transbronchial lung biopsies were performed prior to 2001 and the
open lung biopsies were conducted prior to 2003. Two of the three
thoracoscopy procedures (2/3, 66.6%) were performed in 2010. The
clinical data of the 24 cases showed that biopsy specimens obtained
from the transbronchial lung biopsy were too small to assess the
fibrosis and degree of inflammation in the lung tissues, but for
light injuries this remains a common screening method used in
clinical practice. Taking specimens from open lung biopsies was
ideal, and showed important value for clarifying the ILD type, but
with heavy injury the diagnosis method was often used only when
other inspection methods were intolerable to patients with severe
ILD (7). The trauma induced by
thoracoscopic surgery was small, and the procedure had similar
diagnostic value to the open lung biopsy; thoracoscopic surgery may
therefore become an important tool for the future diagnosis of
ILD.
In the retrieved cases of UC concurrent with airway
inflammation, the pathology of two cases (9,10)
showed peribronchial fibrosis and bronchial stenosis, with
pathological features similar to those of airway-centered
interstitial fibrosis. Churg et al (21) reported that cases of central airway
fibrosis exhibited a number of common characteristics. Firstly, the
cause of the fibrosis was unclear, although several cases had a
history of inhalation exposure. Secondly, the patients presented
with a chronic cough and progressive dyspnea with restrictive
ventilatory dysfunction, and the chest CT showed bronchial and
vascular fibrosis, and interstitial infiltrates that were primarily
located in the central hilar region. Thirdly, chest computed
tomography demonstrated peribronchovascular fibrosis and
interstitial thickening. In addition, subpleural focal pulmonary
fibrosis was apparent, bronchioles were often narrow and twisted,
without occlusion, and the interstitial airway walls and the
mesenchyme were occasionally infiltrated by chronic inflammatory
cells. Finally, corticosteroids and bronchodilators had poor
effects.
Of the remaining retrieved cases, one patient with
UC described in 1987 by Wilcox et al (10) had progressive exertional dyspnea;
the chest X-ray showed a diffuse prominence of pulmonary vessels,
and lumen stenosis and irregularities caused by submucosal
concentric fibrosis of the respiratory bronchioles appeared in the
open lung biopsy findings. One patient with a 13-year history of UC
presented with dry cough, shortness of breath, pleuritic chest pain
and restrictive pulmonary dysfunction (9). The chest X-ray showed right apical
homogenous shadows and a small pleural effusion on the right, which
developed into the peripheral consolidation in both upper lobes
with air bronchograms, ill defined shadowing in both lower lobes
and considerable loss of volume in both lungs after four weeks. The
open lung biopsy showed acute and chronic bronchial and bronchiolar
inflammatory cell infiltrate with associated peribronchiolar
fibrosis. Furthermore, bronchiole stenosis appeared due to the
concentric submucosal fibrous thickening. These two cases were not
considered to be cryptogenic organizing pneumonia due to a lack of
granulation tissue or fibrous tissue blocking the lumens of the
bronchi.
In the case reported in the present study,
prednisone was used due to the UC relapse, and ILD occurred
following a reduction in the prednisone dosage. 5-aminosalicylic
acid was ceased four months prior to the onset of the ILD, and the
ILD was stabilized when 5-aminosalicylic acid was re-administered
during the UC relapse. Therefore the hypothesis that ILD was caused
by drug factors could be excluded according to this drug medication
history.
Several different diseases can induce acute diffuse
pulmonary parenchymal disease, including infectious diseases
(tuberculosis, chlamydia and aspergillosis, and diseases caused by
viruses, Pneumocystis carinii and mycoplasma), diffuse
alveolar hemorrhage, left ventricular dysfunction, hypersensitivity
pneumonitis, eosinophilic pneumonia, diffuse alveolar damage
(histologically shown by transparent films), acute fibrinous and
organizing pneumonia (fibrin and organizing pneumonia observed in
the alveolus) and acute exacerbation of usual interstitial
pneumonia (with pathological features of usual interstitial
pneumonia and diffuse alveolar damage superimposition) (3). In one case of UC, reported by Aydoğdu
et al (1), the patient had
complicated cryptogenic organizing pneumonia due to virus
infection, and the clinical manifestations were acute respiratory
distress and leak syndromes. The case reported in the present study
manifested as an acute course of disease, but the diseases
mentioned above could be excluded based on the patient history,
clinical manifestations, imaging, pathology and other laboratory
tests.
The pathological features of the case reported in
the present study were different from airway-centered fibrosis
(with features of inhalation exposure history and bronchial lumen
stenosis), cryptogenic organizing pneumonia (with features of
granulation tissue or fibrous tissue causing an obstruction in the
bronchiole), respiratory bronchiolitis associated with ILD (with
pathological features of alveolar macrophage accumulation in the
respiratory bronchiole and surrounding alveolus) with mild
interstitial inflammation and fibrosis around the bronchioles
(22). Therefore, these diseases
reported previously could be excluded.
Numerous pathologies, including ILD, airway disease,
lung cysts and pleural fibrosis and adhesions, appeared in the case
reported in this study, unlike the previously reported 24 cases of
UC concurrent with ILD (including the two cases with acute UC plus
ILD). In terms of drug efficacy, the two cases reported in the
literature with acute UC plus ILD eventually succumbed due to
ineffective high-dose glucocorticoid treatment. In the case
reported in this paper, an acute exacerbation also occurred
following high-dose corticosteroid treatment, but the disease
rapidly remitted upon the administration of cyclophosphamide
combined with γ globulin, providing a successful treatment for
severe cases of acute UC plus ILD and airway disease.
The combination of the clinical manifestations,
imaging studies and pathological features led to the case reported
in this study being diagnosed as acute UC plus ILD and airway
disease. Excluding the drug-induced factors, the occurrence of
UC-associated pulmonary complications may be explained by the fact
that the lung, stomach and intestines all originate from
gastrulation and are vulnerable to the impact of the same
auto-antibodies. UC is a systemic disease, and the non-specific
inflammation of the bronchial subepithelial layer and submucous
layer of the colon are similar; therefore, immune disorder may be
the key to its pathogenesis (23).
UC and ILD are associated with circulating immune complexes, which
activate lymphocytes and macrophages, release cytokines, stimulate
mesenchymal cells, including the proliferation and synthesis of
fibroblasts, and release collagen into the lung mesenchyme
(4). The condition in the present
study may have been associated with the explosive increase in
circulating immune complexes following glucocorticoid reduction.
The effective usage of cyclophosphamide combined with γ globulin
treatment in this case may be explained by the quick reduction of
circulating immune complex levels caused by cyclophosphamide and
the avoidance of opportunistic infection occurrence in the lungs
due to γ globulin.
The results of the retrospective analysis for the 24
cases of UC complicated by ILD and the one case reported in this
study indicated that UC activity and colectomy may be associated
with the incidence of ILD and that lung lesions present diversity
when UC is concurrent with ILD, which is rare in clinical settings.
Precautions against UC with acute ILD should be taken when there
are symptoms of dry cough and progressive dyspnea and when the
chest CT exhibits diffuse ground-glass shadows. Thoracoscopic
surgery may become a more effective means of obtaining pathological
specimens. Glucocorticoids remain the first choice drug therapy in
chronic UC concurrent with ILD, but these should be selected
carefully and with vigilance for lung opportunistic infections in
cases of acute UC plus ILD, in which the selection of
cyclophosphamide combined with γ globulin could be considered.
Since the specimens in this study were small, more extensive case
investigations or prospective studies are required.
References
|
1
|
Aydoğdu M, Gürsel G, Özyilmaz E, Akyürek N
and Memış L: A case of ulcerative colitis complicated with
bronchiolitis obliterans organizing pneumonia (BOOP) and air leak
syndrome. Turk J Gastroenterol. 23:590–595. 2012.PubMed/NCBI
|
|
2
|
Ward H, Fisher KL, Waghray R, Wright JL,
Card SE and Cockcroft DW: Constrictive bronchiolitis and ulcerative
colitis. Can Respir J. 6:197–200. 1999.PubMed/NCBI
|
|
3
|
Marten K, Fend F, Hautmann H, Kremer M,
Rummeny EJ and Engelke C: Case report: Fatal acute exacerbation of
usual interstitial pneumonia in ulcerative colitis. Br J Radiol.
78:762–766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chikano S, Sawada K, Ohnishi K, Fukunaga
K, Tanaka J and Shimoyama T: Interstitial pneumonia accompanying
ulcerative colitis. Intern Med. 40:883–886. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Janssen WJ, Bierig LN, Beuther DA and
Miller YE: Stridor in a 47-year-old man with inflammatory bowel
disease. Chest. 129:1100–1106. 2006.PubMed/NCBI
|
|
6
|
Talwar A, Kunst H, Ngatchu T and Trotter
S: A case presentation of a pulmonary complication of ulcerative
colitis. BMJ Case Rep. 2013:pii: bcr0220125806. 2013.PubMed/NCBI
|
|
7
|
McKee AL, Rajapaksa A, Kalish PE and
Pitchumoni CS: Severe interstitial pulmonary fibrosis in a patient
with chronic ulcerative colitis. Am J Gastroenterol. 78:86–89.
1983.PubMed/NCBI
|
|
8
|
Isenberg JI, Goldstein H, Korn AR, Ozeran
RS and Rosen V: Pulmonary vasculitis - an uncommon complication of
ulcerative colitis. Report of a case. N Engl J Med. 279:1376–1377.
1968. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hilling GA, Robertson DA, Chalmers AH and
Rigby HS: Unusual pulmonary complication of ulcerative colitis with
a rapid response to corticosteroids: case report. Gut. 35:847–848.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilcox P, Miller R, Miller G, Heath J,
Nelems B, Muller N and Ostrow D: Airway involvement in ulcerative
colitis. Chest. 92:18–22. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vaiphei K, Gupta N, Sinha SK, Nagi B and
Singh K: Association of ulcerative colitis with pulmonary
sarcoidosis, subcutaneous lipomatosis and appendiceal
adenocarcinoma. Indian J Gastroenterol. 22:193–194. 2003.PubMed/NCBI
|
|
12
|
Karadag F, Ozhan MH, Akçiçek E, Günel O,
Alper H and Veral A: Is it possible to detect ulcerative
colitis-related respiratory syndrome early? Respirology. 6:341–346.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazer BD, Eigen H, Gelfand EW and Brugman
SM: Remission of interstitial lung disease following therapy of
associated ulcerative colitis. Pediatr Pulmonol. 15:55–59. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basseri B, Enayati P, Marchevsky A and
Papadakis KA: Pulmonary manifestations of inflammatory bowel
disease: case presentations and review. J Crohns Colitis.
4:390–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nilubol N, Taub PJ, Venturero M, Lichtiger
S and Bauer JJ: Ulcerative colitis and sarcoidosis. Mt Sinai J Med.
68:400–402. 2001.
|
|
16
|
Stebbing J, Askin F, Fishman E and Stone
J: Pulmonary manifestations of ulcerative colitis mimicking
Wegener’s granulomatosis. J Rheumatol. 26:1617–1621.
1999.PubMed/NCBI
|
|
17
|
Shneerson JM: Steroid-responsive
alveolitis associated with ulcerative colitis. Chest. 101:585–586.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balestra DJ, Balestra ST and Wasson JH:
Ulcerative colitis and steroid-responsive, diffuse interstitial
lung disease. A trial of N = 1. JAMA. 260:62–64. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pérez de Llano LA, Lancho A, Soilán del
Cerro JL and López-Róses L: Cryptogenic fibrosing alveolitis
predating ulcerative colitis. Am J Gastroenterol. 91:822–823.
1996.PubMed/NCBI
|
|
20
|
Lebas E, Gielen S, Nguyen M, Ghaye B,
Bartsch P and Belaiche J: Acute colitis in Wegener’s disease: a
case report. Rev Med Liege. 61:163–168. 2006.(In French).
|
|
21
|
Churg A, Myers J, Suarez T, Gaxiola M,
Estrada A, Mejia M and Selman M: Airway-centered interstitial
fibrosis: a distinct form of aggressive diffuse lung disease. Am J
Surg Pathol. 28:62–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wells AU, Nicholson AG, Hansell DM and du
Bois RM: Respiratory bronchiolitis-associated interstitial lung
disease. Semin Respir Crit Care Med. 24:585–594. 2003. View Article : Google Scholar
|
|
23
|
Camus P, Piard F, Ashcroft T, Gal AA and
Colby TV: The lung in inflammatory bowel disease. Medicine
(Baltimore). 72:151–183. 1993. View Article : Google Scholar : PubMed/NCBI
|