Introduction
The circulatory system is the first functional
system formed during mammalian embryogenesis, beginning with the
aggregation of hemangioblasts, the common precursors of endothelial
and hematopoietic cells, shortly following gastrulation. The
hemangioblasts differentiate into several distinct cell lineages
that form the network of blood vessels, the heart, circulating
blood cells and supporting tissues. A number of molecules and
signal transduction pathways are temporally and quantitatively
regulated to maintain normal cardiovascular development (1,2).
These include vascular endothelial growth factor (VEGF), basic
fibroblast growth factor (bFGF), the angiopoietins/Tie pathway, the
ephrins/Eph pathway and the DSL/Notch pathway. All of these
pathways have been studied in vivo using traditional
knockout, knock-in and transgenic methods. In the majority of
cases, these gene mutations induce embryonic lethality between E9.5
to E11.5 (3–5). However, the embryonic lethal
phenotypes are similar and suggest that disruption to any of these
pathways results in defects in the remodeling and maturation of the
vasculature (6,7). The embryonic lethal phenotypes hinder
the investigation of the function of these genes in later
development, including possible roles in the determination of
vessel identity, hematopoiesis, endothelial-mesenchymal transition
and pericyte recruitment.
A number of techniques have been developed to
achieve cell type-/tissue-specific transgene expression. The first
established inducible system was the lac repressor system
regulated by isopropyl-β-D-thiogalactopyranoside (IPTG) (8,9).
Currently, the Cre/loxP and tetracycline systems are the
most widely used systems to control gene expression. Cre
recombinase is a bacteriophage enzyme that recognizes two
34-base-pair loxP sites and excises the DNA between them
(10,11). Conditional gene deletion and
expression strategies have been developed by placing loxP
sites around an essential part of a gene of interest or separating
an ectopic promoter from the coding region of a gene by
loxP-flanked stop sequences (12–14).
In these configurations, the gene alteration is silent prior to the
excision of the loxP sites. Following the introduction of
Cre recombinase under a tissue-specific promoter, the
loxP-flanked sequence is excised and gene inactivation or
transgene expression occurs specifically in the tissues where Cre
is expressed. While the Cre/loxP system provides spatial
control of gene alteration, the tetracycline-inducible system
provides temporal regulation. Under this system, a fusion protein
composed of the VP16 transactivator and either the tetracycline
repressor (tTA) or reversed tetracycline repressor (rtTA) can be
inhibited (tet-off) or activated (tet-on) by tetracycline,
respectively (15,16). Therefore, the addition or
withdrawal of tetracycline controls the activity of the
transactivator, which then regulates the expression of a given gene
from a promoter containing the tet-operator (tetO) sequences.
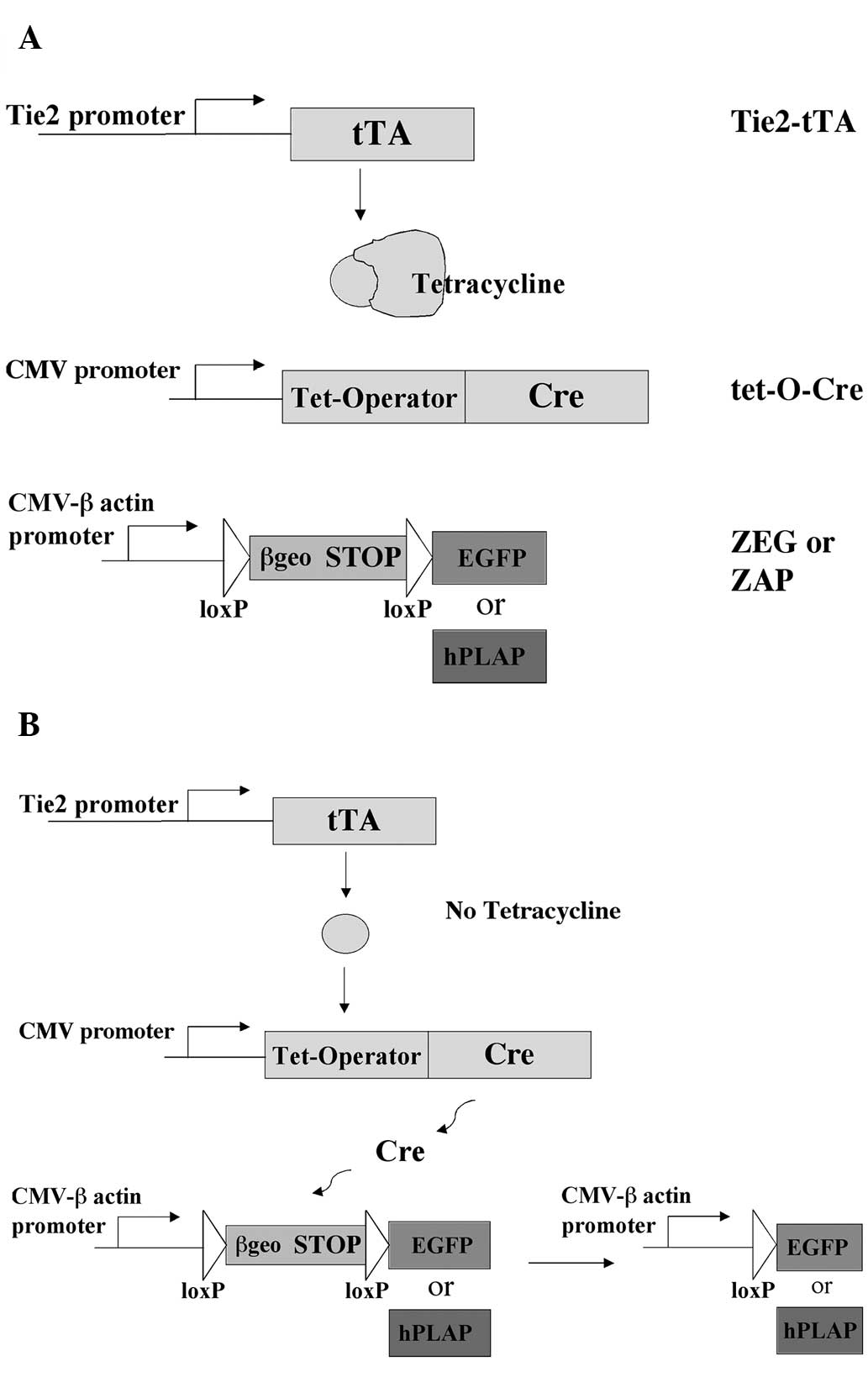
In the present study, a strategy was developed in
which the Cre/loxP and tet-off systems were combined to
allow for spatial and temporal regulation of transgene expression
in vivo. In this system, three transgenes are utilized. The
first transgene drives the expression of a tetracycline-controlled
transactivator (tTA) under the control of the Tie2 promoter, which
allows selective transgene expression only in endothelial and
hematopoietic cells. The second transgene expresses the Cre
recombinase gene from a minimal promoter containing tet-operator
(tetO) sequences (17). The third
transgene is the ZEG or ZAP reporter, which has a strong global
promoter separated from an enhanced green fluorescence protein
(EGFP) or human placental alkaline phosphatase (hPLAP) reporter
gene by a loxP-flanked stop sequence. The stop sequence
contains a lacZ-neomycin resistance fusion (β-geo) gene with
translation and transcription stops (13,18).
With the combination of these transgenes, Cre expression does not
occur in the presence of tetracycline and consequently the reporter
genes are not expressed. At any point during embryonic development
or adulthood, Cre expression can be activated through the removal
of tetracycline, leading to Cre excision and expression of the
reporter genes.
The advantage of combining the
tetracycline-inducible and Cre/loxP systems is that once Cre is
activated, it will excise the target transgene and make a permanent
genetic alteration. The reporter gene will be expressed in the
Cre-expressing cells and in all the progeny of that cell, even if
the inducing condition is removed. Thus a limited number of cells
can be marked for reporter gene expression for applications such as
mosaic analysis. This also represents a valuable approach to the
modelling of cancer, where a limited number of cells in an organ
undergo the critical oncogenic mutations and those cells then go on
to form a tumor. In developing cancer treatments and testing them,
this type of model may be useful to ascertain the effectiveness of
a treatment at eliminating the cancerous cells. By contrast, in
systems where only an inducing agent, for example tetracycline or
tamoxifen, is used, expression of the target transgene requires the
continuous application of the inducing condition, and that
continuous application leads to an endless supply of oncogene
expressing cells; thus, it is difficult to determine whether a
treatment eliminates the oncogenic cells.
Materials and methods
Transgenic mice
The Tie2-tTA mice (provided by Dr Andras Nagy;
Lunenfeld-Tanenbaum Research Institute, Toronto, Canada) express
the tTA in endothelial and hematopoietic cells under the control of
a 2.1 kb Tie2 promoter fragment and a 10 kb fragment carrying an
enhancer present in the first intron of Tie-2 (19). The tet-O-Cre mice (also provided by
Dr Andras Nagy), carry the Cre coding sequence downstream of a
minimal cytomegalovirus (CMV) promoter and tetracycline operator.
ZEG and ZAP reporter mice were constructed in our laboratory, as
previously described (13,18). The mice have a strong
CMV-enhancer/chicken β-actin promoter followed by a
loxP-flanked stop sequence consisting of a
lacZ-neomycin resistance fusion (β-geo™) gene and three
polyadenylation sequences. The loxP-flanked stop is followed
by hPLAP in ZAP mice and EGFP in ZEG mice. The Animal Care
Committee of Sunnybrook Research Institute (Toronto, Canada)
approved all animal experiments carried out in the present
study.
Mouse genotyping
The ZEG and ZAP mice were genotyped by lacZ
staining of ear biopsies as previously described (13,18).
Tie2-tTA and tet-O-Cre mice were genotyped using PCR using genomic
DNA isolated from mouse ear biopsies. Genomic DNA was extracted
from the biopsies using a REDExtract-N-Amp™ Tissue PCR
kit (Sigma-Aldrich, St. Louis, MO, USA) following the
manufacturer’s instructions. For each sample analyzed, ~100 ng of
DNA was amplified using a S1000™ Thermal Cycler
(Bio-Rad, Hercules, CA, USA). The primers used were as follows: Cre
forward, 5′-AATTTACTGACC GTACACCA-3′; Cre reverse,
5′-CGCCGCATAACCAGT GAAAC-3′; tTA forward, 5′-CTCACTTTTGCCCTTTAG
AA-3′; tTA reverse, 5′-GCTGTACGCGGACCCACTTT-3′. The thermal-cycle
program was as follows: 94°C for 5 min (1 cycle), 94°C for 30 min,
55°C for 1 min, 72°C for 1 min (30 cycles), 72°C for 5 min (1
cycle).
Alkaline phosphatase (AP) staining
AP staining was performed as previously described
(14). Briefly, pregnant females
were euthanized by cervical dislocation and the uterus was removed
and washed in cold phosphate-buffered saline (PBS). Embryos were
dissected out of the uterus and parts of the yolk sacs were removed
for genotyping, whereas the embryos were fixed in lacZ fix
solution (0.2% glutaraldehyde, 50 mM EGTA, pH 7.3, 100 mM
MgCl2 in 100 mM sodium phosphate, 0.02% NP-40 and 0.01%
sodium deoxycholate, pH 7.3) for 15 min. For the cross sections,
the slides were prepared as described below. Embryos or slides were
then washed in PBS and endogenous APs were inactivated by
incubation in PBS at 70–75°C for 30 min. Following washing in AP
buffer (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 10 mM
MgCl2) for 10 min, the samples were stained with AP
staining solution [(100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 50 mM
MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, 337 mg/ml
nitroblue tetrazolium salt (Roche Diagnostics, Basel, Switzerland),
and 175 mg/ml 5-bromo-4-chloro-3-indolyl phosphate, toluidinium
salt (Roche Diagnostics)]. The staining reaction was allowed to
proceed for 10–30 min at room temperature. The samples were then
washed in PBS and stored at 4°C.
Immunohistochemistry
The tissue samples were dissected in cold PBS and
fixed in 4% paraformaldehyde overnight, washed in PBS three times
for 15 min and cryoprotected in 15% sucrose in PBS for 1 h at 4°C
followed by 30% sucrose in PBS overnight at 4°C. The samples were
then incubated in Tissue-Tek OCT (Sakura Finetek USA Inc, Torrance,
CA, USA) at 4°C for 4 h prior to embedding in OCT over dry ice. The
frozen blocks were cryosectioned at 7 μm, placed onto
L-polylysine-coated slides (Thermo Fisher Scientific, Waltham, MA,
USA), and dried for 1–4 h at room temperature prior to storage at
−20°C. For immunostaining, the sections were quenched sequentially
in 3% hydrogen peroxide and blocked with diluted 10% normal goat
serum (for EGFP) or 5% normal rabbit serum [for
platelet-endothelial cell adhesion molecule (PECAM)]. Slides were
then incubated at 4°C overnight with the primary antibodies, which
comprised anti-PECAM-1 monoclonal antibody (1:100; BD Pharmingen,
San Diego, CA, USA) and anti-GFP monoclonal (3E6) antibody
(1:2,000; Molecular Probes, Eugene, OR, USA). The next day, the
slides were exposed for 30 min to biotinylated secondary antibodies
including rabbit anti-rat or goat anti-rabbit (1:200; Vector
Laboratories, Inc., Burlingame, CA, USA). The peroxidase activities
were visualized using streptavidin-horseradish peroxidase together
with the diaminobenzidine detection system (Vector Laboratories,
Inc.). Finally, slides were washed and counterstained with
hematoxylin (Surgipath; Leica Microsystems, Wetzlar, Germany).
Images of slides were captured by photography using a Leica DFC300
camera with Leica FireCam 120 program.
Flow cytometry
Single cell suspensions were prepared by
dissociating tissues in fluorescence-activated cell sorting (FACS)
buffer (PBS with Ca2+/Mg2+, 0.1%
NaN3 and 5% FCS) and filtering through a 40-μm cell
filter. Spleen samples were treated with freshly prepared red blood
cell lysis buffer (9:1 mix of 0.83% NH4Cl and 1 m
Tris-HCl pH 7.65) and refiltered. Samples were resuspended in FACS
buffer supplemented with 1 mg/ml of propidium iodide. Appropriate
isotype controls were included with each experiment. Fluorescence
of EGFP was detected on the FL-1 channel of a FACSCalibur
instrument (BD Biosciences, Franklin Lakes, NJ, USA). Results were
then analyzed using FlowJo (TreeStar Inc., San Carlos, CA,
USA).
Results
Tie2-tTA/tet-O-Cre transgene combination
activates Cre excision in endothelial and hematopoietic cells
The efficiency of Cre excision activated by the
Tie2-tTA/tet-O-Cre transgene combination was analyzed by
maintaining mice in the absence of tetracycline (Fig. 1B). ZAP was initially used as the
reporter mouse line (13,20). Tet-O-Cre male mice were bred with
Tie2-tTA/ZAP female mice and embryos were collected at E8.5, 9.5,
10.5 and 11.5. The embryos and yolk sacs of the entire litter were
stained for AP activity. Part of the yolk sac was also genotyped by
PCR to identify triple transgenic embryos. At E8.5, no staining was
observed in the Tie2-tTA/tet-O-Cre/ZAP embryos and yolk sac. At
E9.5, a substantial number of cells positively stained for AP were
observed in the yolk sac and the heart (data not shown). At E10.5,
the majority of the yolk sac stained for AP (Fig. 2B) and AP positive vessels were also
observed within the cranium and somites (Fig. 2B). At E11.5 the yolk sac strongly
stained for AP, while the vessels in the embryos were already deep
beneath the tissue at this stage and were not visible clearly.
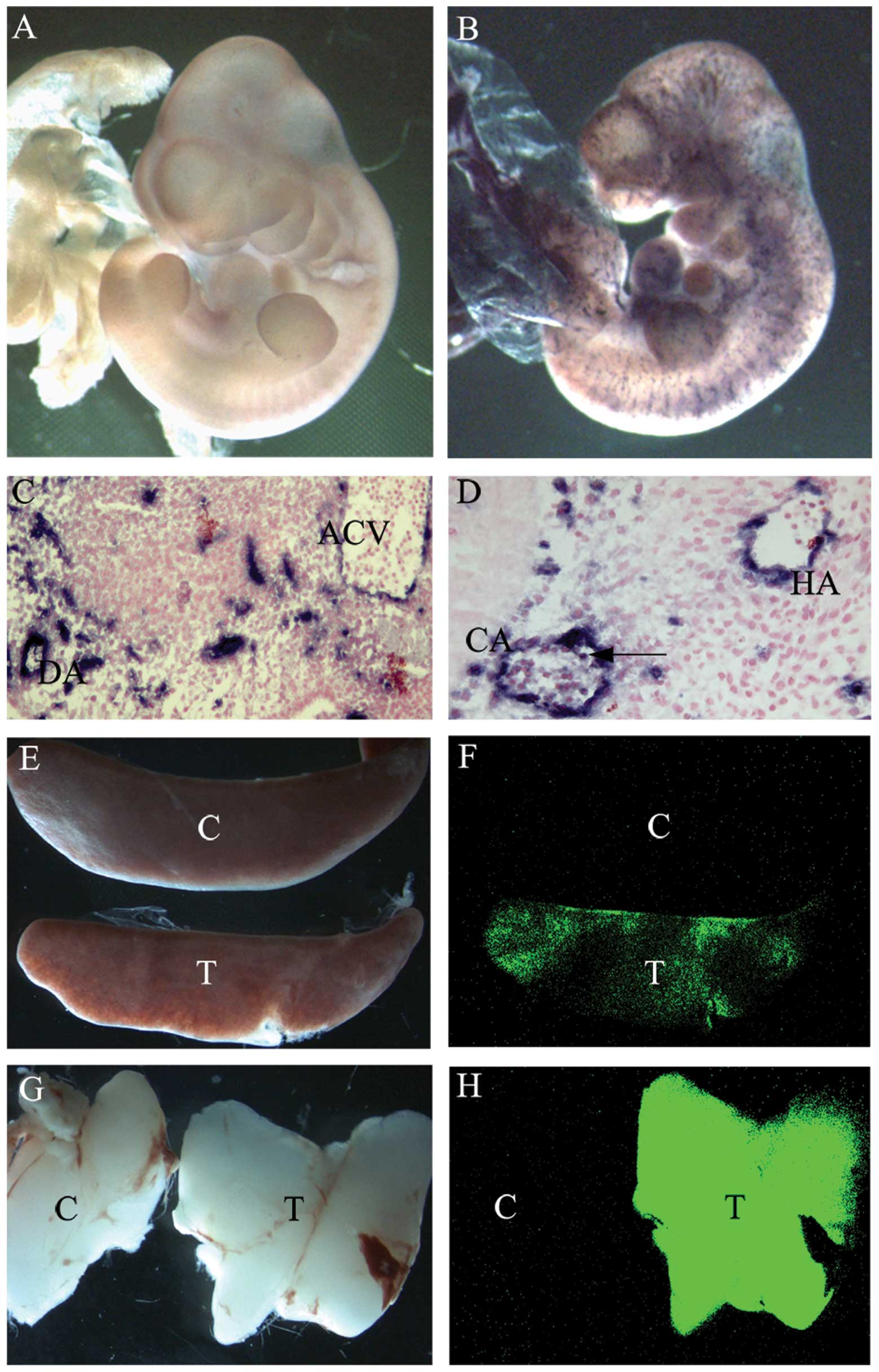 | Figure 2Measurement of Cre excision in the
absence of tetracycline treatment. (A and B) Whole-mount AP
staining of (A) E10.5 control embryos and (B) E10.5
Tie2-tTA/tet-O-Cre/ZAP embryos; (C and D) AP staining of sections
of E11.5 Tie2-tTA/tet-O-Cre/ZAP embryos. Arrows indicate AP
positive hematopoietic cells. (E–H) EGFP fluorescence. (E) Bright
field view of spleens from control and Tie2-tTA/tet-O-Cre/ZEG mice
and (F) the same spleens under GFP light. The spleen from a control
mouse is dark, whilst the spleen from a triple transgenic mouse
exhibits green fluorescence. (G) Bright field view of thymuses from
control and Tie2-tTA/tet-O-Cre/ZEG mice and (H) the same spleens
under GFP light. The thymus from a control mouse is dark, whilst
the thymus from a Tie2-tTA/tet-O-Cre/ZEG mouse exhibitis green
fluorescence. AP, alkaline phosphatase; DA, dorsal aorta; ACV,
anterior cardinal vein; CA, cortical artery; HV, head vein; C,
control mice; T, triple transgenic Tie2-tTA/tet-O-Cre/ZEG mice;
EGFP, enhanced green fluorescence protein. |
The embryos were frozen and transversely sectioned
to observe the AP staining within the embryo. Virtually all
endothelial cells of Tie2-tTA/tet-O-Cre/ZAP embryos in sections
showed positive AP staining at E11.5 (Fig. 2C and D). The endogenous Tie2
receptor is expressed not only in endothelial cells, but also in
hematopoietic cells, which aggregate and adhere to the endothelial
cells of arteries (21). It was
observed that a fraction of hematopoietic cells were positively
stained for AP on E11.5 within the arteries, whereas hematopoietic
cells in the veins were not stained (Fig. 2C and D). Similar observations have
been previously described for Tie2-Cre/CAG-CAT-Z embryos (22).
While AP expression of Tie2-tTA/tet-O-Cre/ZAP
embryos was restricted at E8.5 and E9.5, Tek-tTA/Tet-O-LacZ
embryos showed lacZ expression beginning at E8.5 (Tek is a
Tie2 promoter) (23). This
supports previous observations that Cre excision and resultant
reporter gene expression may not occur until 1–2 days after Cre
expression is initiated (20).
ZEG reporter mice were used to analyze the
expression within adult tissues since the second reporter EGFP may
be visualized in live animals and cells either directly or using
flow cytometry (18,20). Tie2-tTA/ZEG mice were bred with
tet-O-Cre mice and triple transgenic Tie2-tTA/tet-O-Cre/ZEG pups
were obtained, which displayed light green fluorescence at birth,
particularly in the ears and the tail (data not shown). These pups
were later confirmed to be triple transgenic using PCR and
lacZ staining of ear biopsies (lacZ is expressed from
the non-excised allele of the ZEG transgene). Although tTA has been
reported to be toxic in mammalian cells (15), the triple transgenic embryos did
not exhibit any obvious phenotype. All of the triple transgenic
mice showed GFP expression at birth and in hematopoietic organs.
Hematopoietic tissues, including the thymus and spleen, were also
GFP fluorescent (Fig. 2F and H).
In frozen sections of highly vascularized tissues, including the
lung and kidney, blood vessel endothelial cells also exhibited
green fluorescence (data not shown). These results confirm that the
Tie2-tTA/tet-O-Cre transgenes mediate Cre excision in endothelial
and hematopoietic cells.
Cre excision is delayed by maintenance on
doxycycline and activated by doxycycline withdrawal
Once it was established that the triple transgenic
system produced reporter gene expression in endothelial and
hematopoietic cells in the absence of tetracycline, it was then
determined whether reporter gene expression would be inhibited in
the presence of tetracycline. It was hypothesized that tetracycline
would inhibit tTA activation of the tet-O-Cre transgene; thus, Cre
would not be expressed, and consequently the AP and EGFP reporter
genes would not be expressed (Fig.
1A).
Breeding pairs of tet-O-Cre males and Tie2-tTA/ZEG
females were maintained on 0.1 mg/ml doxycycline supplied in the
drinking water from the time they were mated. At this level,
doxycycline, a tetracycline analogue, is sub-therapeutic but
sufficient to repress tTA (23).
Following doxycycline treatment, breeding pairs did not produce any
pups exhibiting green fluorescence, although Tie2-tTA/tet-O-Cre/ZEG
triple transgenic pups (identified by PCR genotyping) were observed
in a normal Mendelian ratio. The thymus, spleen, kidney and liver
of these newborn triple transgenic pups did not show any GFP
fluorescence, indicating that tTA was repressed by doxycycline.
In some litters, doxycycline was withdrawn two days
after the pups were born and pups were sacrificed after 28 days in
order to determine whether Cre excision and subsequent EGFP
expression could be obtained by postnatal doxycycline withdrawal
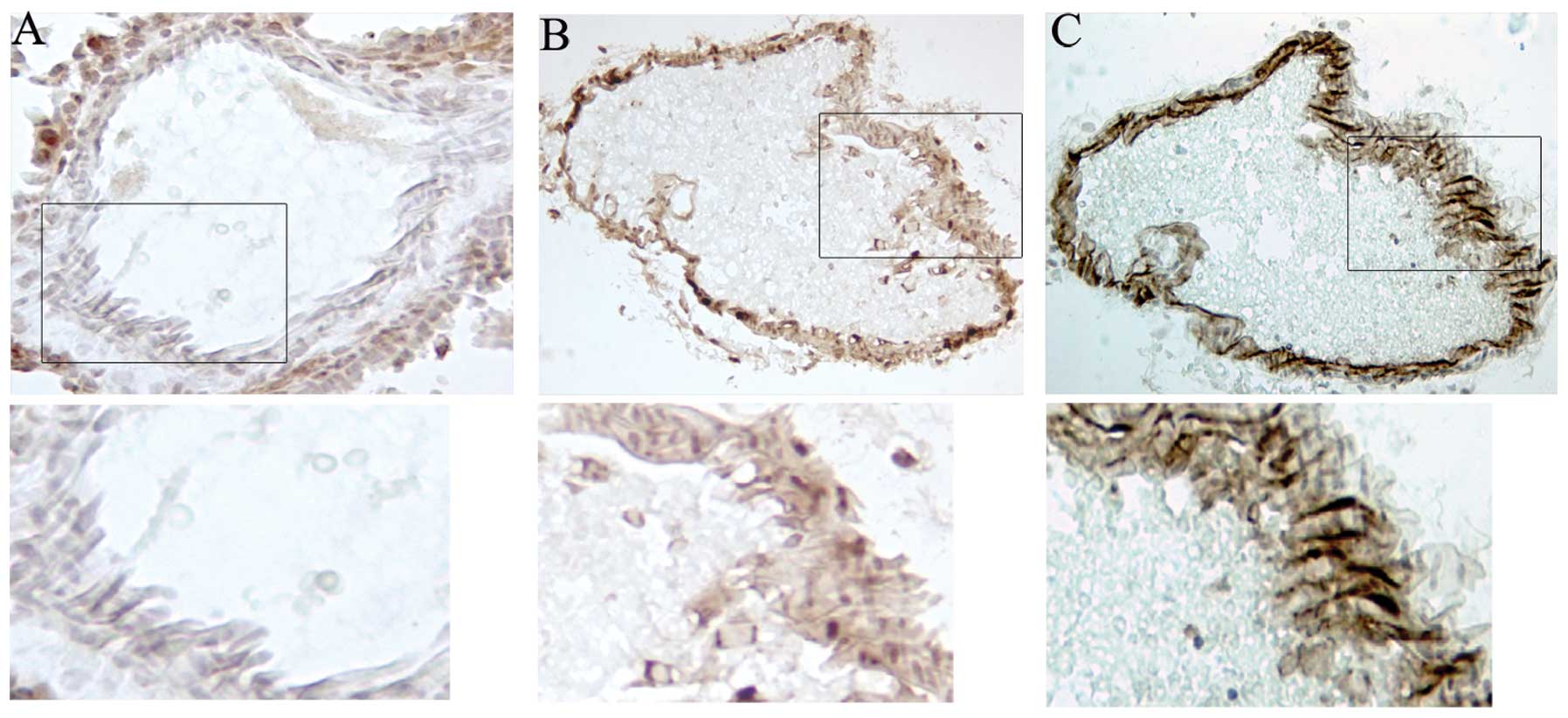
(Fig. 1B). Mosaic expression of
EGFP was observed in endothelial cells in various organs, as
demonstrated by comparing immunostaining for EGFP and the
endothelial marker PECAM-1 staining in serial sections of liver
(Fig. 3B and C). With removal of
doxycycline, Tie2-tTA/tet-O-Cre/ZEG triple transgenic thymus showed
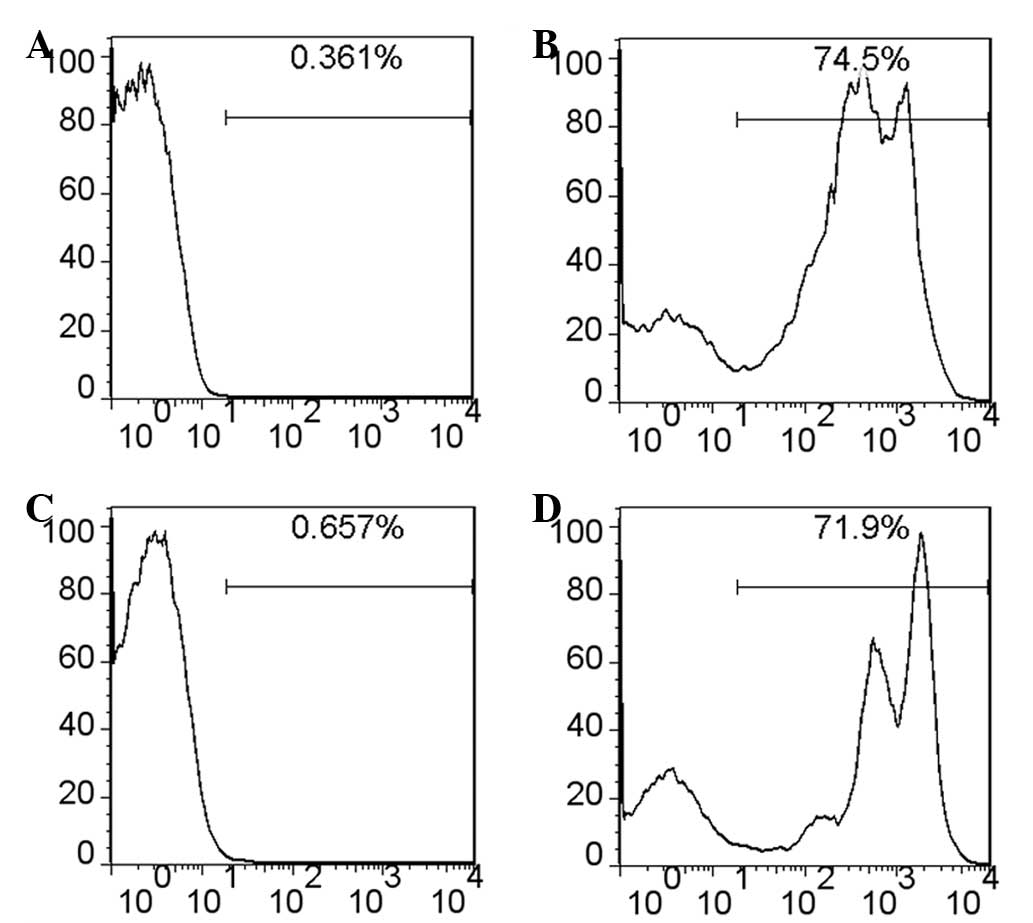
green fluorescence and EGFP+ cells were detected in the
spleen and thymus using flow cytometry (Fig. 4B and D). Therefore, removal of
doxycycline efficiently led to Cre expression and excision and
consequent expression of the EGFP reporter.
Discussion
Recently, a number of studies have attempted to
characterize the genes involved in pathological processes of adult
vasculature (24–26). The Cre conditional system combined
with the inducible tTA system used in the present study enables the
deletion of a gene or expression of a mutated gene at any time
point during the lifespan of the mice. In particular, it allows
studies of gene functions at stages of development after which
deletion or misexpression is normally lethal. For example, deletion
of the Notch1 receptor has been found to be embryonic lethal at
E9.5, thus limiting the understanding of its role during early
embryogenesis (3,6). Using this system, this problem may be
overcome, and the Notch1 receptor can be deleted after E10.5 to
study its potential function in adult angiogenesis, which has not
been possible to investigate directly by gene targeting
studies.
Unlike endothelial cells, which remain stable in the
majority of adult organs, the hematopoietic system is a developing
system (27,28). Mutation of a specific gene at
different ages can result in different types of diseases or
prognosis (29–31). By controlling the temporal
activation of transgene expression, this inducible system may be
useful in order to investigate gene function in hematopoiesis,
leukemogenesis and lymphomagenesis, in particular
disease-associated genes where genetic modification results in
embryonic lethality.
In the present study, mosaic reporter gene
expression occurred in endothelial and hematopoietic tissues in
adult triple transgenic mice following doxycycline withdrawal. This
may result from incomplete Cre activity, transgene silencing and/or
reduced activation of the Tie2 promoter in adults. Mosaic
expression has been observed in the majority of
tetracycline-regulated models and also in other inducible systems
(20,32). However, gene functional studies can
be conducted by comparison with appropriate controls and
quantitative studies may be achieved through a variety of
techniques.
In conclusion, it was demonstrated in the present
study that a tetracycline-regulated Cre/loxP system may be
used to conditionally express transgenes in endothelial and
hematopoietic cells. The combination of the Cre/loxP and
tetracycline-inducible systems to activate transgene expression can
be used in other tissues by incorporating different promoters to
drive the expression of tTA or rtTA (17,32).
The application of this system may improve our ability to bypass
embryonic lethal phenotypes and study gene function in adult mice.
Furthermore, it may provide valuable animal models for therapeutic
development.
Acknowledgements
This study was supported by a grant from the Heart
and Stroke Foundation of Canada. The authors are grateful to Dr.
Andras Nagy for providing tet-O-Cre mice and to the support from
the Taishan Scholar Program of Shandong Province (Ju Liu).
References
|
1
|
Rossant J and Howard L: Signaling pathways
in vascular development. Annu Rev Cell Dev Biol. 18:541–573. 2002.
View Article : Google Scholar
|
|
2
|
Alva JA and Iruela-Arispe ML: Notch
signaling in vascular morphogenesis. Curr Opin Hematol. 11:278–283.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Limbourg FP, Takeshita K, Radtke F, et al:
Essential role of endothelial Notch1 in angiogenesis. Circulation.
111:1826–1832. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krebs LT, Shutter JR, Tanigaki K, et al:
Haploinsufficient lethality and formation of arteriovenous
malformations in Notch pathway mutants. Genes Dev. 18:2469–2473.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duarte A, Hirashima M, Benedito R, et al:
Dosage-sensitive requirement for mouse Dll4 in artery development.
Genes Dev. 18:2474–2478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krebs LT, Xue Y, Norton CR, et al: Notch
signaling is essential for vascular morphogenesis in mice. Genes
Dev. 14:1343–1352. 2000.PubMed/NCBI
|
|
7
|
Uyttendaele H, Ho J, Rossant J and
Kitajewski J: Vascular patterning defects associated with
expression of activated Notch4 in embryonic endothelium. Proc Natl
Acad Sci USA. 98:5643–5648. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baim SB, Labow MA, Levine AJ and Shenk T:
A chimeric mammalian transactivator based on the lac repressor that
is regulated by temperature and isopropyl
β-D-thiogalactopyranoside. Proc Natl Acad Sci USA. 88:5072–5076.
1991.PubMed/NCBI
|
|
9
|
Wyborski DL and Short JM: Analysis of
inducers of the E.coli lac repressor system in mammalian
cells and whole animals. Nucleic Acids Res. 19:4647–4653.
1991.PubMed/NCBI
|
|
10
|
Plück A: Conditional mutagenesis in mice:
the Cre/loxP recombination system. Int J Exp Pathol. 77:269–278.
1996.PubMed/NCBI
|
|
11
|
Kos CH: Cre/loxP system for generating
tissue-specific knockout mouse models. Nutr Rev. 62:243–246.
2004.PubMed/NCBI
|
|
12
|
Lewandoski M: Conditional control of gene
expression in the mouse. Nat Rev Genet. 2:743–755. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lobe CG, Koop KE, Kreppner W, et al: Z/AP,
a double reporter for Cre-mediated recombination. Dev Biol.
208:281–292. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J and Lobe CG: Cre-conditional
expression of constitutively active Notch1 in transgenic mice.
Genesis. 45:259–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gossen M and Bujard H: Tight control of
gene expression in mammalian cells by tetracycline-responsive
promoters. Proc Natl Acad Sci USA. 89:5547–5551. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gossen M, Freundlieb S, Bender G, et al:
Transcriptional activation by tetracyclines in mammalian cells.
Science. 268:1766–1769. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
St-Onge L, Furth PA and Gruss P: Temporal
control of the Cre recombinase in transgenic mice by a tetracycline
responsive promoter. Nucleic Acids Res. 24:3875–3877. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Novak A, Guo C, Yang W, Nagy A and Lobe
CG: Z/EG, a double reporter transgenic mouse line that expresses
enhanced green fluorescent protein upon Cre-mediated recombination.
genesis. 28:147–155. 2000. View Article : Google Scholar
|
|
19
|
Schlaeger TM, Bartunkova S, Lawitts JA, et
al: Uniform vascular-endothelial-cell-specific gene expression in
both embryonic and adult transgenic mice. Proc Natl Acad Sci USA.
94:3058–3063. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo C, Yang W and Lobe CG: A Cre
recombinase transgene with mosaic, widespread tamoxifen-inducible
action. Genesis. 32:8–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takakura N, Huang XL, Naruse T, et al:
Critical role of the TIE2 endothelial cell receptor in the
development of definitive hematopoiesis. Immunity. 9:677–686. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kisanuki YY, Hammer RE, Miyazaki J, et al:
Tie2-Cre transgenic mice: a new model for endothelial cell-lineage
analysis in vivo. Dev Biol. 230:230–242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sarao R and Dumont DJ: Conditional
transgene expression in endothelial cells. Transgenic Res.
7:421–427. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rammos C, Hendgen-Cotta UB, Deenen R, et
al: Age-related vascular gene expression profiling in mice. Mech
Ageing Dev. 135:15–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stan RV, Tse D, Deharvengt SJ, et al: The
diaphragms of fenestrated endothelia: gatekeepers of vascular
permeability and blood composition. Dev Cell. 23:1203–1218. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Carson-Walter EB, Cooper A, et al:
Vascular gene expression patterns are conserved in primary and
metastatic brain tumors. J Neurooncol. 99:13–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Steinman RA: Cell cycle regulators and
hematopoiesis. Oncogene. 21:3403–3413. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith C: Hematopoietic stem cells and
hematopoiesis. Cancer Control. 10:9–16. 2003.PubMed/NCBI
|
|
29
|
Tang J, Shao W, Dorak MT, et al: Positive
and negative associations of human leukocyte antigen variants with
the onset and prognosis of adult glioblastoma multiforme. Cancer
Epidemiol Biomarkers Prev. 14:2040–2044. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dorak MT, Burnett AK and Worwood M: HFE
gene mutations in susceptibility to childhood leukemia: HuGE
review. Genet Med. 7:159–168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brumpt C, Delabesse E, Beldjord K, et al:
The incidence of clonal T-cell receptor rearrangements in B-cell
precursor acute lymphoblastic leukemia varies with age and
genotype. Blood. 96:2254–2261. 2000.PubMed/NCBI
|
|
32
|
Belteki G, Haigh J, Kabacs N, et al:
Conditional and inducible transgene expression in mice through the
combinatorial use of Cre-mediated recombination and tetracycline
induction. Nucleic Acids Res. 33:e512005. View Article : Google Scholar : PubMed/NCBI
|