Introduction
Currently, sentinel lymph node biopsy (SNB) has
become standard practice to avoid axillary lymph node dissection
(ALND) in patients with clinically node-negative breast cancer
(1). Breast cancer lymphatic
drainage patterns are fairly predictable, with the vast majority of
lesions exhibiting primary drainage to axillary lymph nodes
(2). In particular, it has been
reported that the majority of drained sentinel nodes (SN) are in
the level I nodes in the axilla (3). However, there are certain studies
mentioning the potential to drain to an SN in level II and III
nodes or an SN outside the axilla (3).
Single-photon emission computed tomography/computed
tomography (SPECT/CT) shows the exact anatomical location of SN
(4). The first large study on
SPECT/CT in breast cancer reported an improved preoperative
localization of hot nodes (5).
Subsequent studies confirmed the value of SPECT/CT for this purpose
(6,7). Depicting SNs that are not visible on
conventional images is helpful. There is a consensus that the
axilla is the main basin for lymphatic drainage from the breast
(8). However, SPECT/CT exposes
additional sites of lymphatic drainage, including extra-axillary,
which are not depicted by conventional scans. Depiction of the
exact location of extra-axillary nodes facilitates the planning and
execution of the surgery (9).
There are certain atypical patterns, in which SNs are depicted in
extra-axilla, such as the infra or superior clavicular regions,
internal mammary nodes and other organs. In such an atypical
pattern, it may sometimes become difficult to decide the extent of
SNB. Thus, how atypical hot nodes are involved in lymphatic
metastasis was investigated in the present study, particularly in
level II (nodes lying behind the pectoralis major muscle in the
axilla) to III (nodes lying above the pectoralis minor muscle in
the infraclavicular region) regions.
Performing biopsies of SNs located in level II or
III may be difficult with a small wound approach, in contrast to
SNB in level I, which may normally be performed easily. National
Comprehensive Cancer Network guidelines recommend that in the
absence of gross disease in level II nodes, lymph node dissection
should include tissue inferior to the axillary vein from level I to
II, but regarding SNB, the sampling range is not mentioned in
certain terms (10). In the
process of SNB, where in particular nodes located in level II/III
are much deeper than level I in the axilla, more widespread surgery
may be unavoidable and it may take more time to complete SNB. In
fact, SNB may be possible by forcing through the same wound into
level II, in contrast to in level III. When multiple SNs were
detected in the radioisotope method, the hottest node did not show
metastasis. Thus, it is necessary to resolve a clinical question of
how extensive the SNB can be performed, including level II/III in
the axilla. Consequently, metastasis in level II/III was
investigated, depending on whether hot nodes were depicted in level
II/III on SPECT/CT.
Material and methods
Breast cancer patients
Between June 2012 and August 2013, lymphatic flow
was studied using planar lymphoscintigraphy and SPECT/CT in 92
clinical stage 0-IIA breast cancer patients. The axillary lymph
nodes were classified by surgical description of ALND (level I,
below and lateral to the pectoralis minor muscle; level II, behind
the pectoralis minor muscle; and level III, above the pectoralis
minor muscle) (3). The patients
were divided into two groups: With or without hot nodes in level
II/III on SPECT/CT, regardless of whether they were included in
level I or not, and the existence of metastasis in level II/III was
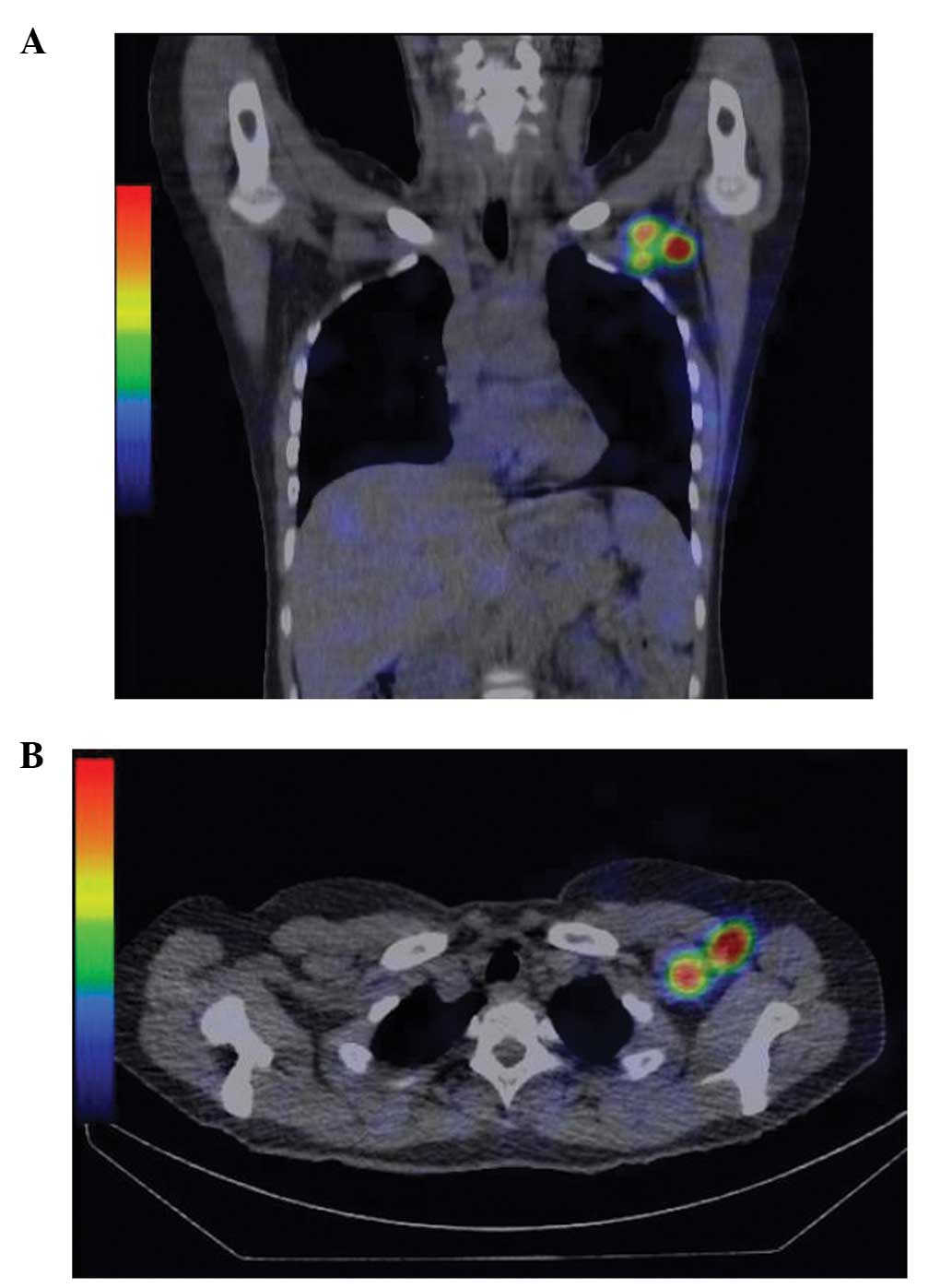
investigated as shown in Fig. 1.
Hot nodes were observed in level II/III on SPECT/CT in 11 patients
(12.0%) and no hot nodes were observed in 81 patients (88.0%). For
identification of the risk factors, SNB-positive frequencies were
evaluated through histology, estrogen receptor, progesterone
receptor, human epidermal growth factor receptor type 2, nuclear
grade, lymphatic invasion (ly), vascular invasion (v) and the
existence of at least one hot node in level II/III, as observed on
the SPECT/CT.
By contrast, SNB was revealed positive for
metastasis in 12 patients (13.0%) and ALND was performed on all 12
patients. These 12 patients, on whom ALND was performed, were
divided into two groups: With or without hot nodes in level II/III.
The nodes in level II/III were harvested by ALND and the existence
of metastasis was proven pathologically.
SPECT/CT method and surgery
The SPECT/CT method and surgical procedure is as
mentioned: A 2-day protocol was used with lymphoscintigraphy and
SPECT/CT on the day 1 and the surgery occurring on day 2. This was
followed by either mastectomy or a partial breast resection. SNB
were performed during the same surgery. The day before surgery,
99mtechnetium-phytic acid was injected into two sites,
at peritumor and around the nipple areola complex, subdermally and
intradermally regarding depth in each site. The total radioactive
dose was 74 MBq. SPECT acquisition (matrix 128*128, 24
sec/frame) was performed using 6° angular step, and a total
acquisition time of 16 min and 7 sec (SPECT: 13 min 57 sec + CT: 2
min 10 sec). Static imaging in the supine position with
simultaneous transmission scanning was performed 3 h after
injection (Discovery NM/CT670; GE Healthcare, LLC, Princeton, NJ,
USA). Following correction for attenuation and scatter, SPECT and
CT axial 4.4-mm slices were generated and fused (Fujifilm RI Pharma
Co., Ltd., Tokyo, Japan). Subsequently, SN mapping was performed to
reveal hot nodes depicted by SPECT/CT (Fig. 1).
On day 2, radioisotope uptake in each lymph node was
measured by a γ-probe using Neo-2000 (NeoProbe, Dublin, OH, USA)
during surgery. Simultaneously, indigo carmine solution was
injected similar to the 99mtechnetium-phytic acid
injection. Blue nodes were found in the axillary sentinel lymph
nodes intraoperatively. SNs were extracted and immediately examined
by pathologists during the surgeries. When metastatic cancer was
positively proven in the SN, ALND was performed, essentially up as
far as level II, excluding isolated tumor cells.
Statistical analysis
Statistical analysis between the two groups (with or
without hot nodes in level II/III) was performed by the
χ2 test. Univariate and multivariate logistic regression
analysis was used for identification of SNB positive for metastasis
risk factors. Using the χ2 test, a statistical analysis
was undertaken between the two groups with or without hot nodes in
level II/III, in 12 patients on whom ALND performed, the same as
above. All the statistical analyses as mentioned above were
performed with the JMP 10 software program (JMP 10.0.0 for
Macintosh; SAS Institute, Inc., Cary, NC, USA).
Results
Patient characteristics
SPECT/CT was performed in 92 clinical stage 0-IIA
breast cancer patients and the patient characteristics and sentinel
node results are presented in Table
I. The mean age was 59.9 years (35–81 years). A total of 43
patients had right breast cancer and 49 patients had left. The mean
number of sampling lymph nodes in SNB was 1.75. And the mean number
of hot nodes visualized on SPECT/CT was 1.49. Regarding SPECT/CT,
all the patients had at least one hot node in level I, and there
were no patients with SN in the internal mammary chain. In
addition, regarding a group composed of 12 patients that had hot
nodes depicted in level II/III on SPECT/CT, 10 had hot nodes in the
two regions (level I and II), and two patients had hot nodes in all
three regions (level I, II and III).
 | Table IDistribution of clinical and
pathological characteristics among patients revealed pathologically
metastasis in SN. |
Table I
Distribution of clinical and
pathological characteristics among patients revealed pathologically
metastasis in SN.
| Patient
characteristics | Total, n (n=92) | Pathologically
positive for SNB, n (n=12) | Pathologically
negative for SNB, n (n=80) | P-value |
|---|
| Age, years |
| Mean (range) | 59.9 | 57.5 (35–76) | 60.3 (35–81) | NS |
| SD | 12.4 | 11.9 | 12.5 | |
| Main region of
cancer |
| A | 27 | 1 | 26 | NS |
| B | 5 | 0 | 5 | |
| C | 38 | 6 | 32 | |
| D | 17 | 3 | 14 | |
| E | 5 | 2 | 3 | |
| ER |
| Positive | 63 | 8 | 55 | NS |
| Negative | 24 | 4 | 20 | |
| Unknown | 5 | 0 | 5 | |
| PgR |
| Positive | 51 | 6 | 45 | NS |
| Negative | 36 | 6 | 30 | |
| Unknown | 5 | 0 | 5 | |
| HER2 |
| Positive | 17 | 2 | 15 | NS |
| Negative | 68 | 10 | 58 | |
| Unknown | 7 | 0 | 7 | |
| Nuclear grade |
| 1 | 36 | 4 | 32 | NS |
| 2 | 25 | 3 | 22 | |
| 3 | 24 | 5 | 19 | |
| Unknown | 7 | 0 | 7 | |
| ly |
| Positive | 15 | 8 | 7 | P<0.0001 |
| Negative | 70 | 4 | 66 | |
| Unknown | 7 | 0 | 7 | |
| v |
| Positive | 6 | 0 | 6 | NS |
| Negative | 69 | 12 | 67 | |
| Unknown | 7 | 0 | 7 | |
| Ki67, % |
| Mean | 25.3 | 41.2 | 22.8 | P=0.031 |
| SD | 24.9 | 32.9 | 22.7 | |
| Tumor size, cm |
| Mean | 1.86 | 2.58 | 1.74 | NS |
| SD | 1.38 | 1.29 | 1.37 | |
| Pathology |
| DCIS | 20 | 0 | 20 | NS |
| IDC | 60 | 9 | 51 | |
| Special type | 12 | 3 | 9 | |
| Hot nodes in level
II/III |
| Positive | 11 | 4 | 7 | P=0.014 |
| Negative | 81 | 8 | 73 | |
In patients with lymph flow, there was an SN in a
single node field in 90 patients (97.8%) and two node fields in two
patients (2.2%). In one patient, hot nodes were depicted in two
node fields; axilla and liver, and in another patient the two node
fields were depicted in the axilla and supraclavicular region.
Pathological lymph node metastasis was observed with
a significantly higher frequency in the group with hot nodes
depicted in level II/III than in the non-level II/III (Table I, P=0.014). In other
clinical-pathological factors, there was a significant difference
in ly (P<0.0001) and Ki67 (P=0.031), as shown in Table I.
In univariate analyses, hot nodes in level II/III,
ly and Ki67 were all significantly associated with pathological SN
metastasis (Table II, left
column). In addition, using multivariate analysis, hot nodes in
level II/III and ly were independent factors associated with
metastasis pathologically found in axillary lymph nodes (Table II, right column). Hot nodes
depicted in level II/III in SPECT/CT may be important preoperative
information, as SN metastasis may be more strongly suspected.
 | Table IIUnivariate and multivariate logistic
regression model of clinico-pathological factors and odds of
metastasis pathologically found in axillary lymph node. |
Table II
Univariate and multivariate logistic
regression model of clinico-pathological factors and odds of
metastasis pathologically found in axillary lymph node.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value |
|---|
| Ki67 | 0.11 | 0.83–9.28 | 0.031 | 0.65 | 0.047–10.77 | 0.75 |
| Ly | 4.78 | 4.78–87.74 | <0.0001 | 24.78 | 4.77–197.40 | 0.00004 |
| Hot node in Level
II/III | 5.21 | 1.166–21.68 | 0.024 | 8.89 | 1.129–88.91 | 0.042 |
By contrast, 12 SNB-positive patients on whom ALND
was performed were investigated. In four of the 12 patients, the
hot nodes were observed in level II/III on SPECT/CT, and no hot
nodes in level II/III in eight patients, as shown in Table III. Of these four, there were two
with hot nodes in level II with metastatic nodes pathologically
evident in the same lesion (level II) and no metastasis in level
III. However, there was pathologically no metastasis in level
II/III among the eight patients who had no hot nodes in level
II/III. Statistically there were no significant differences in the
existence of metastatic nodes in level II/III (P=0.053). Notably,
there were two cases in which metastasis was pathologically proven
in SN at level II (not level III), where hot nodes were depicted in
the same site (level II) on SPECT/CT.
 | Table IIIDistribution of the deepest
metastatic lymph node examined pathologically among patients
revealing hot nodes positive verses negative by SPECT/CT
analysis. |
Table III
Distribution of the deepest
metastatic lymph node examined pathologically among patients
revealing hot nodes positive verses negative by SPECT/CT
analysis.
| Pathological
findings | Metastasis in level
I only, n | Metastasis up as
far as level II, n | Total, n | P-value |
|---|
| SPECT/CT
findings |
| Hot nodes were
detected in level II/III | 2 | 2 | 4 | |
| No hot nodes in
level II/III | 8 | 0 | 8 | 0.053 |
| Total | | | 12 | |
Discussion
SPECT/CT reveals various lymphatic drainages,
including axilla, infra or superior clavicular region internal
mammary nodes and other organs, while the axilla is the main basin
for lymphatic drainage from the breast (4,8,9).
These atypical patterns may sometimes make it difficult to decide
the extent of SNB required.
Although axillary drainage is the principal
lymphatic path of the breast, the breast lymphatic vessels drain to
infraclavicular systems less frequently (8,11).
The present study experienced SNs that could not be found in
low-axilla where they typically exist, but were in a deeper area
than observable through the surgeon’s sensation. Therefore, a
hypothesis was developed that it may be useful in the process of
intraoperative SNB to determine whether there are SNs in level
II/III of axilla or not. By contrast, if lymphatic basin of
extra-axillary areas is detected, this information may be helpful
when considering systematic therapies. Uren et al (3) reported that at least one SN was
observed in the axilla in the majority of patients, 96.7%.
Additionally, the study described that an SN was observed at level
II in the axilla in 10% of patients and an SLN at level III was
observed in 2% of patients, using a peritumoral injection study. In
the present study, there were hot nodes in at least one node in the
axilla in all 92 patients, and a second hot node in level II/III in
11 patients (12.0%), indicating that this result is in accordance
with a previous study (3).
Irregular locations of SNs have been reported,
including in levels II/III or outside the axillary (12,13).
Intradermal injections of tracer have been accepted to demonstrate
the low frequency of accumulation in the internal mammary
lymphatics (14). In the present
results, no hot nodes were detected in internal mammary lymphatics,
regardless of studies describing internal mammary lymphatic basins
(15). Peritumoral and sub-areolar
injection is preferable for the identification of the SNB (14,16),
and peritumoral injections demonstrated a significantly lower
proportion of patients who showed drainage to the internal mammary
nodes (8). The two sites injection
method; peritumor and around the nipple areola complex, was a large
part of this study (77.2%). However, of note is the clinical
inefficacy of the internal mammary node dissection as reported by
Veronesi et al (17),
indicating that there could be numerous problems surrounding the
validity of internal mammary SNB (18). Thus, the present study focused on
level I or II/III of axilla, with the exception of internal mammary
chain.
With regards to the uncommon locations of SN, it was
noted that a hot spot in the liver was observed in one case in the
study, which may be explained as a direct lymphatic drainage route
through the sub-diaphragmatic lymphatic plexus and abdominal wall
vessels (Gerota’s para-mammary route) (19). Alternatively, Tanis et al
(8) reported that the blockade of
normal lymph flow can also cause drainage in a retrograde direction
to the liver through the internal mammary chain. This atypical
lymphatic basin could be depicted by SPECT/CT prior to surgery,
therefore these irregular lymphatic basins may be an important
source of information for determining a treatment strategy or
surveillance following surgery, rather than during surgery
(4).
In the present study, multivariable analysis
indicated that the detection of hot nodes in level II/III on
SPECT/CT and ly were significantly correlated with pathological
metastasis in SN. Therefore, the present study demonstrated that
the patients with a hot node in level II/III, as depicted by
SPECT/CT, may have the risk of lymph node metastasis in the axilla.
One clinical question regarding SNB in axilla would be how
extensive are plural SNs that should be selected for SNB
examination in those patients with SNs in level II/III in addition
to in level I, and this has been largely unaddressed previously and
remains unresolved to the best of our knowledge. With regards to
this, the present study may provide evidence.
Additionally, in two of the four patients with hot
nodes depicted as SN in level II/III by SPECT/CT, notably there
were metastatic nodes pathologically evident in the same lesion in
these two cases. Specifically, the two patients with hot nodes in
level II had hot nodes present in level I in the axilla also,
resulting in metastatic nodes pathologically evident in the same
lesion (level II). This indicated that there may be a potential
risk for metastasis in level II if hot nodes were observed in level
II on SPECT/CT. Thus, if there were multiple SNs included in level
II, the present data indicates that the SNs in level II should be
sampled aside from SNs in the shallow level of axilla in level I,
and excluding SN sampling in level II should be avoided as much as
possible.
SNB is associated with morbidity less than ALND
(21) and it is a prerequisite for
reducing the requirement for ALND and avoiding the associated
morbidity (22). In the results of
a systemic review to investigate the safety of SNB without ALND of
cN0 breast cancer patients, whilst the loco-regional
recurrence rate was 0.6% for 36 months in no ALND with SNB-negative
patients, the loco-regional recurrence rate increased to 1.7% (95%
CI, 1.0–2.7) in non-ALND with SNB-positive patients indicating a
threefold risk of recurrence (23). Thus, SNB without ALND may be
unacceptable due to an increased risk for loco-regional recurrence
in SNB-positive patients. The standards that followed the St.
Gallen consensus meeting in 2013, ‘ACOSOG-Z011,’ affected numerous
breast cancer surgeons. While the policy of avoiding full axillary
clearance following 1–2 positive SNs was endorsed in situations of
consecutive surgery and radiotherapy (73% YES and 21% NO), the
completion of ALND was decided as safely avoidable in patients with
1–2 positive SNs and mastectomy without radiotherapy and this
remains the large majority view (4% YES and 91% NO) (24,25).
Presently, the prevailing belief is that local control is highly
important with regards to surgery of the axilla, particularly in
situations without application of radiotherapy.
The present data indicated that hot nodes in level
II/III on SPECT/CT may be risk factors of SN metastasis, including
SNs in the same site. In conclusion, a concept of local control in
axilla is gaining recognition in breast cancer, and so it can be
argued that whether there are hot nodes in level II/III in axillary
as determined by SPECT/CT is important information. In particular
support of this, patients observed with hot nodes in level II on
SPECT/CT with metastasis were subsequently confirmed at the same
site by pathological examination. Therefore, the risk of metastasis
SNs in level II/III should be noted aside from those SNs in the
shallow level of the axilla.
References
|
1
|
Noguchi M, et al; ALMANAC Trialists Group.
The changing role of axillary lymph node dissection for breast
cancer. Breast Cancer. 20:41–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goyal A, et al; ALMANAC Trialists Group.
Role of routine preoperative lymphoscintigraphy in sentinel node
biopsy for breast cancer. Eur J Cancer. 41:238–243. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uren RF, et al: SPECT/CT scans allow
precise anatomical location of sentinel lymph nodes in breast
cancer and redefine lymphatic drainage from the breast to the
axilla. Breast. 21:480–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Ploeg IM, et al: The Hybrid
SPECT/CT as an additional lymphatic mapping tool in patients with
breast cancer. World J Surg. 32:1930–1934. 2008.PubMed/NCBI
|
|
5
|
Lerman H, Metser U, Lievshitz G, et al:
Lymphoscintigraphic sentinel node identification in patients with
breast cancer: the role of SPECT-CT. Eur J Nucl Med Mol Imaging.
33:329–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pecking AP, Wartski M, Cluzan RV, et al:
SPECT-CT fusion imaging radionuclide lymphoscintigraphy: Potential
for limb lymphedema assessment and sentinel node detection in
breast cancer. Cancer Treat Res. 135:79–84. 2007. View Article : Google Scholar
|
|
7
|
van der Ploeg IM, Nieweg OE, Kroon BBR, et
al: The yield of SPECT/CT for anatomical lymphatic mapping in
patients with breast cancer. Eur J Nucl Med Mol Imaging.
36:903–909. 2009.
|
|
8
|
Tanis PJ, et al: Anatomy and physiology of
lymphatic drainage of the breast from the perspective of sentinel
node biopsy. J Am Coll Surg. 192:399–409. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vermeeren L, et al: SPECT/CT for
preoperative sentinel node localization. J Surg Oncol. 101:184–190.
2010.PubMed/NCBI
|
|
10
|
National Comprehensive Cancer Network.
NCCN Guidelines for Treatment of Cancer by Site, Version 3. 2014,
Breast Cancer. http://www.nccn.org/professionals/physician_gls/f_guidelines.aspuri.
Accessed 11 Jan 2014
|
|
11
|
Stavros AT, Rapp CL and Parker SH:
Evaluation of regional lymph nodes in breast cancer patients.
Breast Ultrasound. 1st edition. Lippincott Williams & Wilkins;
Philadelphia: pp. 834–876. 2004
|
|
12
|
Brouwer OR, et al: Lymphoscintigraphy and
SPECT/CT in multicentric and multifocal breast cancer: does each
tumour have a separate drainage pattern? Results of a Dutch
multicentre study (MULTISENT). Eur J Nucl Med Mol Imaging.
39:1137–1143. 2012. View Article : Google Scholar
|
|
13
|
Suami H, et al: The lymphatic anatomy of
the breast and its implications for sentinel lymph node biopsy: a
human cadaver study. Ann Surg Oncol. 15:863–871. 2008. View Article : Google Scholar
|
|
14
|
Tanis PJ, et al: Impact of non-axillary
sentinel node biopsy on staging and treatment of breast cancer
patients. Br J Cancer. 87:705–710. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anan K, et al: Double mapping with
subareolar blue dye and peritumoral green dye injections decreases
the false-negative rate of dye-only sentinel node biopsy for early
breast cancer: 2-site injection is more accurate than 1-site
injection. Surgery. 139:624–629. 2006. View Article : Google Scholar
|
|
16
|
Veronesi U and Valagussa P: Inefficacy of
internal mammary nodes dissection in breast cancer surgery. Cancer.
47:170–175. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noguchi M: Internal mammary sentinel node
biopsy for breast cancer: is it practicable and relevant? (Review).
Oncol Rep. 9:461–468. 2002.PubMed/NCBI
|
|
18
|
Fregnani JHTG and Macéa JR: Drenaje
Linfático de la Mama. desde la Teoría a la Práctica Quirúrgica
Lymphatic Drainage of the Breast: from Theory to Surgical Practice.
Int J Morphol. 27:873–878. 2009.
|
|
19
|
Haagensen CD: Lymphatics of the breast.
The Lymphatics in Cancer. WB Saunders Company; Philadelphia, PA:
pp. 300–387. 1972
|
|
20
|
De Gournay E, et al: Impact of sentinel
node biopsy on long-term quality of life in breast cancer patients.
Br J Cancer. 1–9. 2013.
|
|
21
|
Lyman GH, et al; American Society of
Clinical Oncology. American Society of Clinical Oncology guideline
recommendations for sentinel lymph node biopsy in early-stage
breast cancer. J Clin Oncol. 23:7703–7720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pepels MJ, et al: Safety of avoiding
routine use of axillary dissection in early stage breast cancer: a
systematic review. Breast Cancer Res Treat. 125:301–313. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giuliano AE, et al: Locoregional
recurrence after sentinel lymph node dissection with or without
axillary dissection in patients with sentinel lymph node
metastases: the American College of Surgeons Oncology Group Z0011
randomized trial. Ann Surg. 252:426–433. 2010.
|
|
24
|
Harbeck N, et al: St. Gallen 2013: brief
preliminary summary of the consensus discussion. Breast Care
(Basel). 8:102–109. 2013. View Article : Google Scholar : PubMed/NCBI
|