Introduction
Aquaporins (AQPs) are water-selective membrane
channel proteins that are expressed in numerous epithelial and
endothelial cells of fluid transporting tissues, including the
kidneys, eyes and lungs, where rapid regulated transport of water
is required (1,2). To date, 13 AQPs have been identified
in mammals, which can be subdivided into two groups based on their
permeability: Seven AQPs are highly selective to the passage of
water (AQP-1, AQP-2, AQP-4 and AQP-5), while five AQPs (AQP-3,
AQP-7, AQP-8, AQP-9 and AQP-10) are termed aquaglyceroporins due to
their ability to transport glycerol and even larger solutes
(3).
AQP-1 is a 28 kD membrane-spanning polypeptide that
was initially identified in red blood cells and renal tubules
(4). Previous studies have
revealed that AQP-1 is widely expressed in a variety of tissues,
including the kidney tubules, microvascular endothelium, salivary
glands and ciliary epithelium (5,6).
Saadoun et al revealed that angiogenesis and endothelial
cell migration were impaired in AQP-1-null mice, which demonstrated
that AQPs play an important role in angiogenesis and the spread of
tumors (7). Humans with AQP-1
mutations exhibit a urinary concentrating defect in the kidneys,
indicating that AQP-1 is associated with renal function (8,9).
Approximately 20% of patients with acute
pancreatitis develop severe acute pancreatitis (SAP), which has a
mortality rate of ~30% (10,11).
Acute lung injury (ALI) occurs as a consequence of markedly
increased endothelial and epithelial permeability, with protein
leakage into the alveolar space and interstitial tissues, leading
to decreased gas exchange (12,13).
SAP is closely associated with ALI (14); the pathogenesis of SAP-associated
ALI focuses on the excessive release of cytokines and inflammatory
mediators, including interleukin (IL)-1β, IL-6, IL-8 and tumor
necrosis factor-α (TNF-α) (15,16).
A previous study demonstrated that the expression levels of AQP-1
and AQP-5 decreased in lungs with pulmonary edema following viral
infection (17).
Qin Yin Tang (QYT), a formula used in Chinese
medicine, has demonstrated efficiency in reducing the mortality
rate in the clinical treatment of ALI following SAP; however, the
associated mechanisms remain unclear. The aim of present study was
to investigate the effect of QYT on the expression of AQP-1
following the induction of SAP in the lungs.
Materials and methods
Animals
Male Wistar rats (weight, 200–240 g; age, 6 weeks)
were purchased from the Animal Center of Dalian Medical University
(Dalian, China). The Animal Research and Care Committee of Dalian
Medical University (Dalian, China) approved the experimental
procedures, and all animal experiments were performed under
approved procedures by the Institutional Animal Use and Care
Committee.
Experimental process
QYT was provided by the Department of Traditional
Chinese Medicine of the First Affiliated Hospital of Dalian Medical
University. The compound was comprised of the following herbs: 15 g
Herba Artemisiae Scopariae, 15 g Gardenia, 15 g
Rheum officinale Baill, 9 g sodium sulfate, 9
g Costus root, 9 g Radix bupleuri, 9 g Rhizoma
corydalis, 9 g Radix paeoniae Alba, 10 g Radix Glycyrrhizae,
9 g Angelica sinensis, 10 g Flos Lonicerae and 12 g Fructus
Forsythia. All herbs were boiled in 300 ml water for 15 min
to obtain the QYT solution.
A total of 32 rats were randomly divided into four
groups, which included the SHAM, ALI, dexamethasone (DEX) and QYT
groups. In clinical practise, DEX is used to alleviate the severity
of pulmonary edema in pancreatitis. In the present study, it was
used as a positive control to observe the therapeutic effect of
QYT. The bile-pancreatic duct underwent retrograde infusion with
sodium deoxycholate (15 mg/kg) to produce SAP-associated ALI in the
ALI, DEX and QYT groups. The surgery was performed on the rats in
the SHAM group without sodium deoxycholate. DEX (2 mg/kg) or QYT (2
ml/100 g) were administered through the femoral vein immediately
following the induction of SAP in the DEX and QYT groups. Following
treatment, the mice were euthanized by decapitation.
Blood and lung tissue were collected at 4, 8 and 12
h following surgery. The lung wet/dry ratio, as well as the levels
of blood gases, serum amylase and TNF-α, were analyzed. In
addition, the mRNA expression of AQP-1 in the lung tissue was
detected by quantitative polymerase chain reaction (qPCR), while
protein expression of AQP-1 was detected by immunohistochemistry
and western blot analysis.
Lung wet/dry ratio analysis
Left lung samples were excised, weighed and baked at
60°C for 24 h to obtain the dry weights. The ratio of wet to dry
weight (W/D) was used as an indicator of pulmonary edema.
Histopathological analysis
The left lower lobe was excised and inflated with
10% formaldehyde solution for 24 h. Following fixation, the lung
tissue was embedded in paraffin and divided into several 5-μm
sections for hematoxylin and eosin staining. A total of 10 sections
were selected randomly for analysis.
Following the induction of SAP-associated ALI for 8
h, histopathology was reviewed in a blind manner using a modified
histological scoring system, as previously described (8). The identifiable pathological results
were scored on a scale of 0–4 as follows: 1, alveolar congestion;
2, hemorrhage; 3, leukocyte infiltration or aggregation of
neutrophils in the air space or vessel wall and; 4, thickness of
the alveolar wall. A score of 0 represented normal lungs; 1
represented mild ALI (<25% lung involvement); 2 represented
moderate ALI (25–50% lung involvement); 3 represented severe ALI
(50–75% lung involvement) and; 4 represented extremely severe ALI
(>75% lung involvement) (18).
An overall score of ALI was obtained based on the summation of all
the scores, and the mean ± standard deviation was generated from
the cohort of lung samples (three sections from each lung, eight
lungs per group) at each time point to generate a cumulative
histological ALI score.
Serum amylase, arterial blood and TNF-α
analysis
Amylase activity in the serum was determined by an
automatic biochemistry analyzer (Hitachi 917; Boehringer Mannheim,
Tokyo, Japan). Arterial blood samples were obtained from the
ventral aorta of the rats. Blood gas analyses were performed using
the i-STAT Portable Clinical analyzer (i-STAT Corporation, Windsor,
NJ, USA). In addition, the TNF-α concentration in the supernatants
was measured using a TNF-α assay kit (Nanjing Jincheng Corp.,
Nanjing, China), following the manufacturers’ instructions, and the
measurements were expressed in nmol/mg.
qPCR analysis of AQP-1
Tissue samples of the right lung were homogenized in
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
for total RNA isolation. cDNA was produced by reverse transcription
using an RT kit (Takara Bio, Inc., Shiga, Japan), according to the
manufacturer’s instructions. β-actin served as the internal
control. PCR amplification of AQP-1 and β-actin was performed with
SYBR Green I Taq Master mix (Promega Corporation, Madison,
WI, USA), with cDNA synthesized from the tissues. The primers used
were as follows: AQP-1 forward, 5′-ATGGCCAGCGAAATCAAGAAG-3′, and
reverse, 5′-GATATCATCAGCATCCAGGTC-3′; β-actin forward,
5′-GATATCGCTGCGCTCGTCGTC-3′, and reverse,
5′-CATGAGGTAGTCTGTCAGGTC-3′. Amplification conditions were one
cycle of 5 min at 94°C, 30 cycles of 40 sec at 94°C, 40 sec at the
annealing temperature of 55°C and 10 sec at 72°C, followed by one
cycle of 72°C for 5 min.
Immunohistochemical analysis of
AQP-1
Lung sections were stained using the
streptavidin-peroxidase-biotin immunohistochemical technique.
Experiments were performed following the manufacturer’s
instructions (SABC kit; Boster Biological Tech Ltd., Wuhan, China).
The sections were dewaxed in xylene, cultured in 3% hydrogen
peroxide to eliminate intrinsic peroxidase and quenched in normal
goat serum for 30 min. The sections were subsequently incubated
with an anti-AQP-1 antibody (0.25 mg/ml; rabbit polyclonal;
Proteintech, Chicago, IL, USA) overnight at 4°C. Phosphate-buffered
saline served as the control. A biotinylated goat anti-rabbit
(1:2,000; Vector Laboratories, Burlingame, CA, USA) secondary
antibody was added, and the samples were incubated with an
avidin-biotin complex (Vector Laboratories) for 30 min at room
temperature. 3,3′-Diaminobenzidine was used for color development
and hematoxylin was used for counter staining. Three representative
sections from each rat were used to calculate the average staining
degree for image analysis.
Western blot analysis of AQP-1
Homogenized lung tissue was lysed on ice and the
cellular plasma proteins were extracted with a protein extraction
kit (Pierce Biotechnology, Inc., Rockford, IL, USA), according to
the manufacturer’s instructions. Protein concentrations were
determined by a Coomassie Brilliant Blue dye-binding assay. Samples
(100 μg) were analyzed by SDS-PAGE and electrotransferred to a
polyvinylidene diflouride (PVDF) membrane (Millipore Corporation,
Billerica, MA, USA). The membrane was blocked for 1 h at room
temperature with 5% skimmed milk in Tris-buffered saline with
Tween-20 [TBST; 50 mM Tris-HCl (pH 7.4), 150 mM NaCl and 0.1%
Tween-20]. The membrane was incubated overnight at 4°C with a
rabbit anti-rat polyclonal antibody against AQP-1 (1:200 dilution)
in TBST. Following washing with TBST, the PVDF membrane was
incubated with a biotin-conjugated anti-rabbit antibody (GE
Healthcare, Tokyo, Japan), and diluted to 1:2,000 in TBST at room
temperature for 1 h. The bound antibody was detected by enhanced
chemiluminescence (Amersham, GE Healthcare, Pittsburgh, PA, USA)
and semi-quantitatively analyzed by densitometry with a Science Lab
99 Image Gauge System (Fujifilm, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed by one-way analysis of variance followed by the
post hoc Bonferroni test. Pearson’s correlation analysis was used
to determine the association between AQP-1 expression and the
degree of lung edema. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pathological changes in the lungs of all
the groups
Following the induction of ALI in the rats, evident
swelling and slight hemorrhage was observed in the pancreas. After
8 h, the rats in the ALI group exhibited areas of spotty or patchy
necrosis in the pancreatic tissue. In the DEX and QYT groups,
pancreatic necrosis and pulmonary edema were significantly
alleviated compared with the rats in the ALI group. The pancreas
and lungs of the rats in the SHAM group did not exhibit any marked
pathomorphological changes. In the ALI group, evident inflammatory
cell infiltration was observed in the interstitium of the lung
under a microscope, with notable interstitial hyperemia and edema,
a thickened septum and bullae formation through alveolar dilation.
The inflammation was attenuated significantly in the DEX and QYT
groups when compared with the ALI group. No inflammatory response
was observed in SHAM group. The histopathological scores of all the
groups are shown in Table I.
 | Table IHistopathological scores in the ALI
and SHAM groups. |
Table I
Histopathological scores in the ALI
and SHAM groups.
| Group | Cases, n | 4 h | 8 h | 12 h |
|---|
| SHAM | 8 | 0.33±0.58 | 0.34±0.46 | 0.41±0.58 |
| ALI | 8 | 1.50±0.84a | 1.90±0.74a | 2.08±0.64a |
| DEX | 8 | 0.96±0.62b | 0.87±0.48b | 0.91±0.70b |
| QYT | 8 | 0.98±0.43b | 0.89±0.37b | 0.92±0.54b |
Decreased W/D ratio and increased
arterial blood gases in the lungs following QYT treatment
The W/D ratio, a parameter of pulmonary edema, was
higher in the ALI group (P<0.01) when compared with the SHAM
group (Table II). In addition,
the W/D ratio decreased in the DEX and QYT groups when compared
with the ALI group (P<0.05); no statistically significant
difference was observed in the ratio between the QYT and DEX
groups. Furthermore, marked hypoxemia existed in the ALI group, as
compared with the SHAM group (P<0.01). In the DEX and QYT
groups, the partial pressure of oxygen was significantly higher
(P<0.05) compared with the ALI group (Table III).
 | Table IILung wet/dry ratio comparison in each
group following treatment. |
Table II
Lung wet/dry ratio comparison in each
group following treatment.
| Group | Cases, n | 4 h | 8 h | 12 h |
|---|
| SHAM | 8 | 5.76±0.45 | 6.38±0.52 | 6.09±0.28 |
| ALI | 8 | 10.12±0.68a | 11.56±0.79a | 12.49±0.61a |
| DEX | 8 | 7.45±0.32b | 7.03±0.56b | 6.76±0.41b |
| QYT | 8 | 7.46±0.29b | 7.10±0.38b | 6.69±0.35b |
 | Table IIIArterial blood gas (mmHg) in each
group of rats at different time points. |
Table III
Arterial blood gas (mmHg) in each
group of rats at different time points.
| Group | Cases, n | 4 h | 8 h | 12 h |
|---|
| SHAM | 8 | 12.6±0.5 | 11.3±0.7 | 11.8±0.4 |
| ALI | 8 | 8.7±0.8a | 7.9±1.1a | 7.4±0.8a |
| DEX | 8 | 10.6±0.46b | 9.5±0.54b | 9.2±0.35b |
| QYT | 8 | 11.2±0.34b | 9.7±0.45b | 9.3±0.37b |
Decreased levels of serum TNF-α and
amylase in the QYT group
Serum sample analysis results revealed that the
serum levels of TNF-α in the ALI group were significantly higher
(P<0.01) compared with those in the SHAM group at the different
time points. The serum level of TNF-α in the DEX and QYT groups was
significantly lower (P<0.05) compared with the ALI group. No
statistically significant difference in the levels of TNF-α were
observed between the DEX and QYT groups (Table IV). When compared with the SHAM
group, the levels of serum amylase were significantly higher
(P<0.01) in the ALI group, while the levels of serum amylase in
the DEX and QYT groups were significantly lower (P<0.05)
compared with the ALI group (Table
V).
 | Table IVSerum levels of tumor necrosis
factor-α in each group (nmol/mg). |
Table IV
Serum levels of tumor necrosis
factor-α in each group (nmol/mg).
| Group | Cases, n | 4 h | 8 h | 12 h |
|---|
| SHAM | 8 | 71.25±5.25 | 74.31±6.43 | 78.28±5.26 |
| ALI | 8 |
143.47±29.00a |
234.20±13.23a |
273.86±14.21a |
| DEX | 8 | 98.32±27.00b |
110.48±32.20b |
108.72±27.42b |
| QYT | 8 | 98.98±23.45b |
112.34±31.97b |
109.67±26.44b |
 | Table VSerum amylase levels in each group
(U/L). |
Table V
Serum amylase levels in each group
(U/L).
| Group | Cases, n | 4 h | 8 h | 12 h |
|---|
| SHAM | 8 | 953±88 | 1123±52 | 978±79 |
| ALI | 8 | 4068±361a | 4452±348a | 4101±432a |
| DEX | 8 | 1231±135b | 1312±42b | 1128±216b |
| QYT | 8 | 1154±141 | 1298±56 | 1084±178 |
Increased AQP-1 mRNA and protein
expression in the QYT treatment group
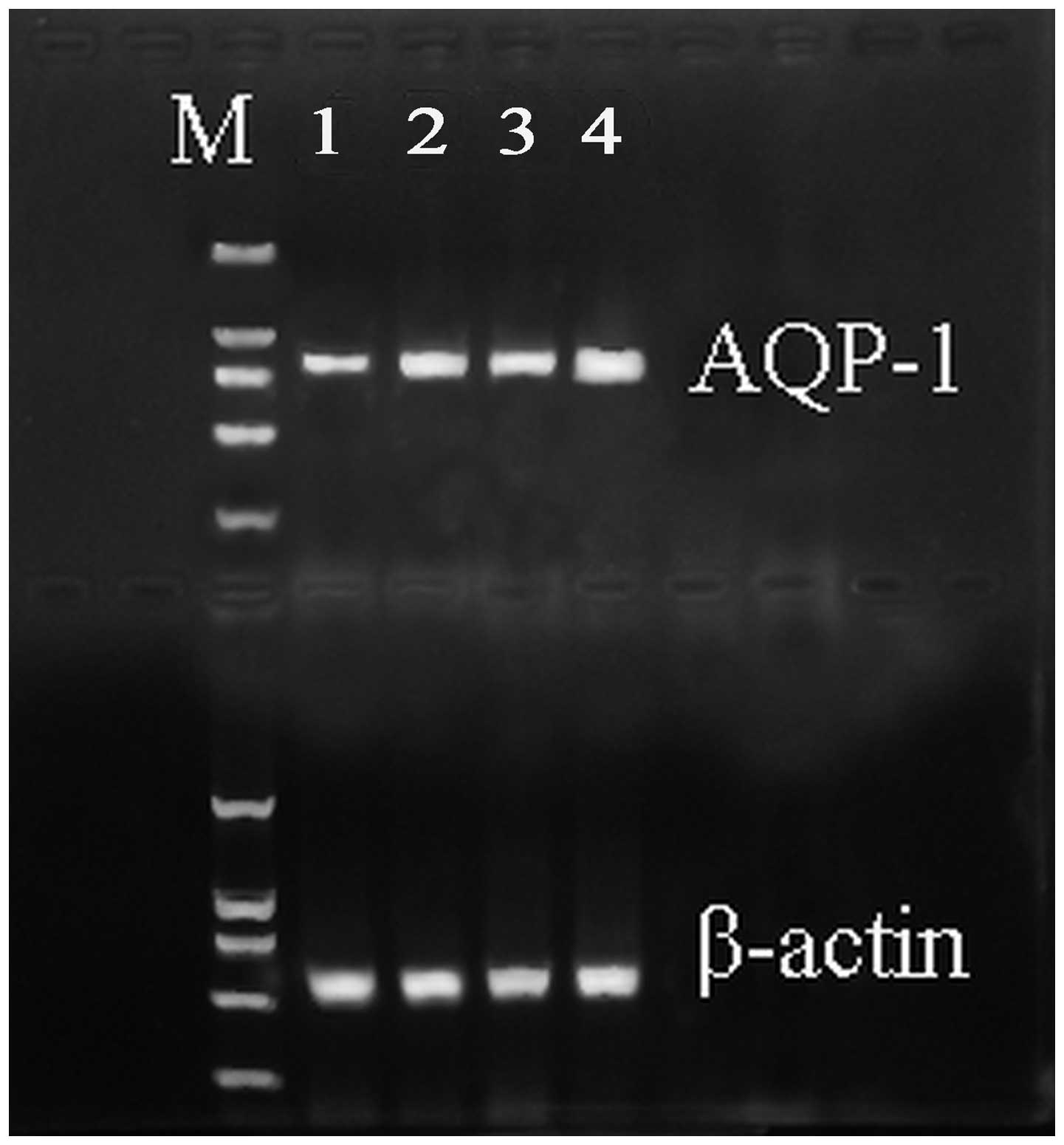
qPCR analysis was performed to detect the expression
level of AQP-1 mRNA in the four groups. The mRNA expression level
of AQP-1 significantly decreased in the ALI group when compared
with the SHAM group (Fig. 1). In
addition, the expression levels were upregulated in the DEX and QYT
groups (P<0.01) when compared with the ALI group.
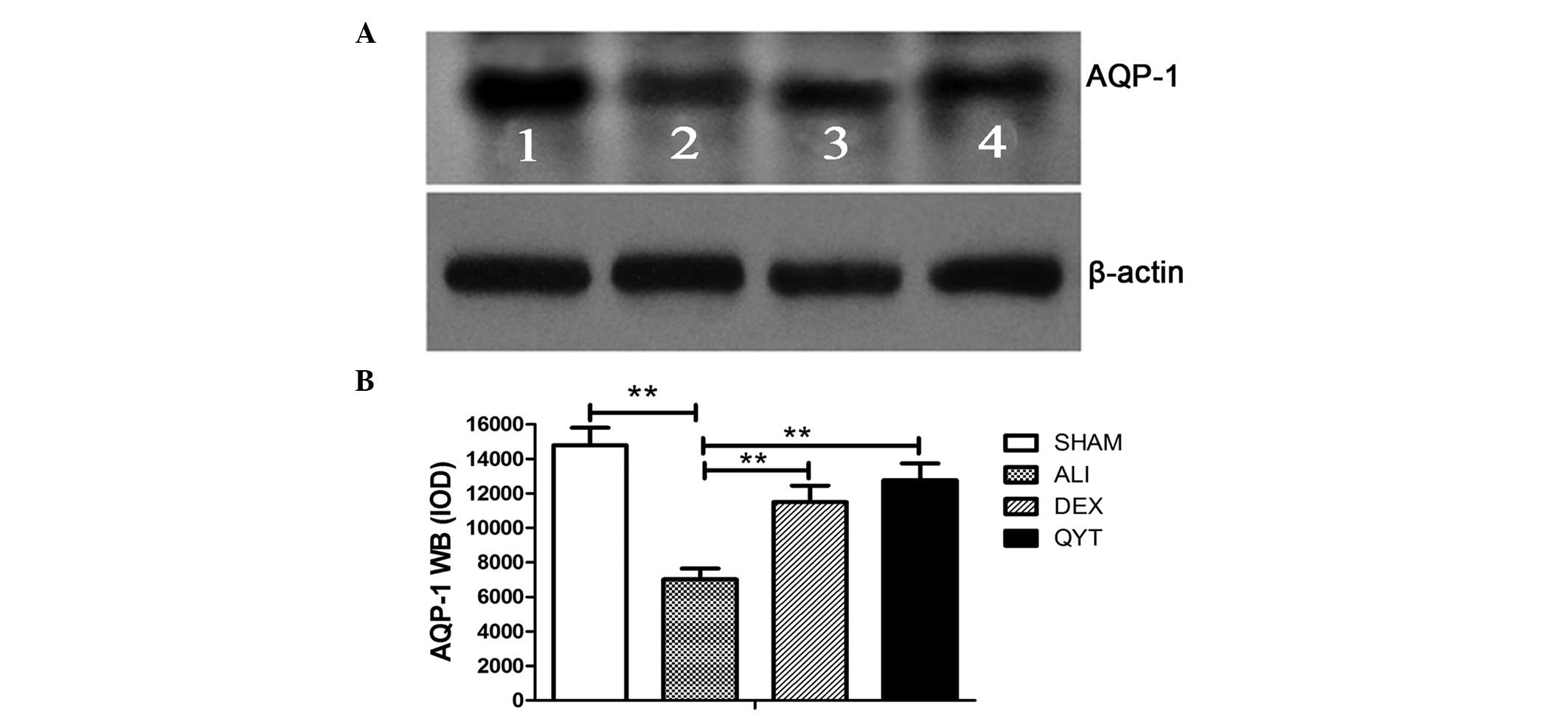
To determine whether the upregulation in the mRNA
expression level of lung AQP-1 was consistent with protein
expression, western blot analysis was performed to assess the
protein expression levels of AQP-1 in the DEX and QYT groups.
Western blot analysis demonstrated a significant increase in the
protein expression levels of AQP-1 in the DEX and QYT groups when
compared with the ALI group. Densitometric analysis of the 28-kD
AQP-1 band revealed a significant increase in the protein
expression levels of AQP-1 in the SHAM group when compared with the
ALI group. The protein expression levels of AQP-1 were lower in the
ALI group, but higher in the DEX and QYT groups, when compared with
the SHAM group (P<0.05; Fig.
2).
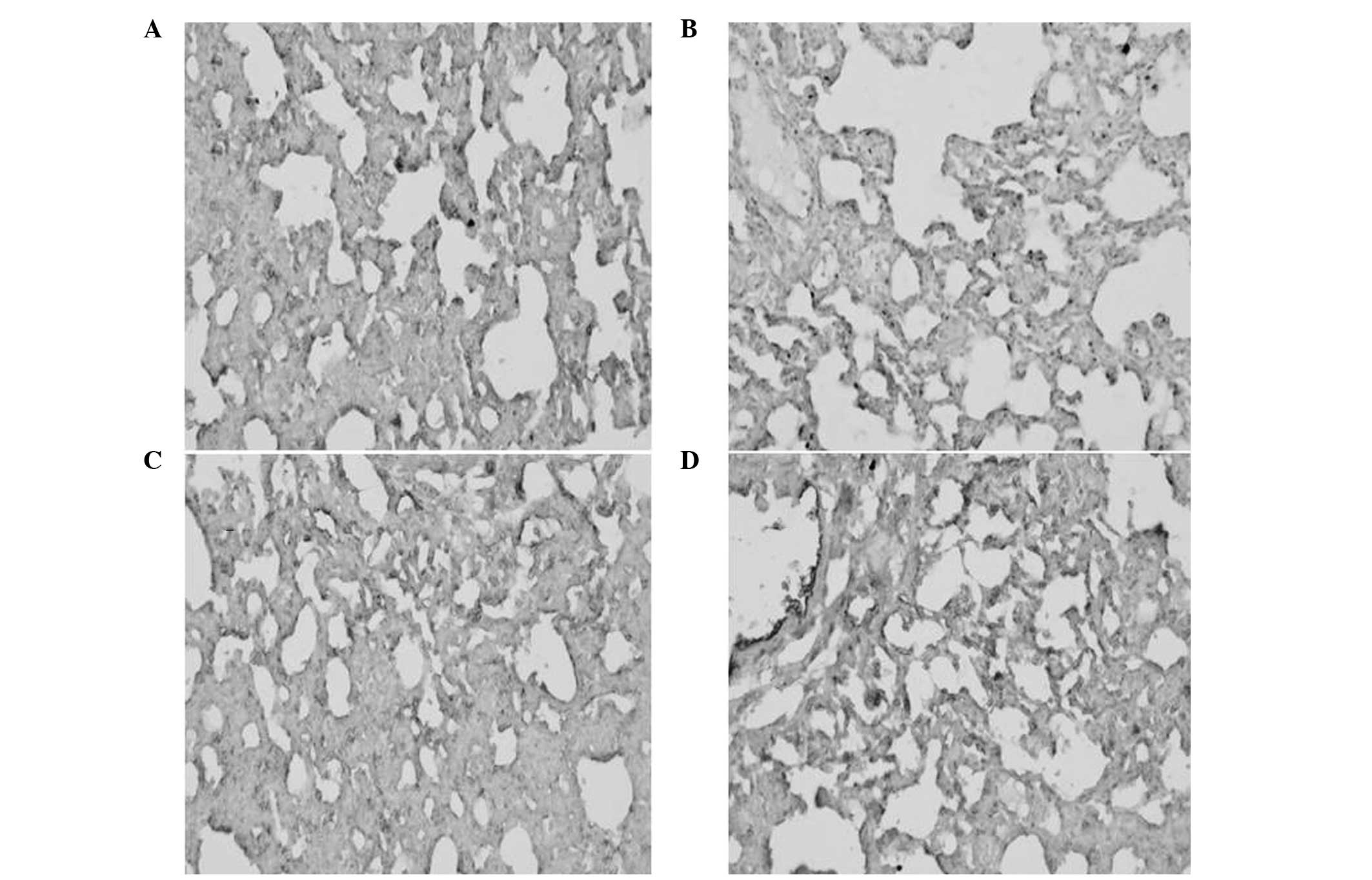
Immunohistochemical analysis of the AQP-1
protein
As shown in Fig. 3,
the percentage of positive AQP-1 protein expression was
significantly lower in the ALI group when compared with the SHAM
group (P<0.01). By contrast, the percentage of positive AQP-1
protein expression was higher in the DEX and QYT groups when
compared with the ALI group (P<0.01; Fig. 3). Consistent with western blot
analysis results, these observations indicate that QYT treatment
effectively upregulated AQP-1 protein expression.
Discussion
AQPs play a crucial role in maintaining water
homeostasis and glycerol metabolism. Four members of the AQP
protein family are expressed in the airways and lungs, and are
involved in the pathological course of various types of lung
disease, including pulmonary edema (19). The present study revealed that the
mRNA and protein expression levels of AQP-1 were significantly
downregulated (P<0.01) in rats with ALI induced by SAP, as
compared with the rats in the SHAM control group. SAP induced
aggravated pulmonary edema. Following treatment with DEX and QYT,
AQP-1 expression was significantly upregulated (P<0.01) in the
lungs with alleviative pulmonary edema, as compared with the ALI
group. Previous studies have revealed that inflammatory cytokines
play an important role in the pathogenesis of acute pancreatitis,
with the levels of TNF-α significantly higher in patients with SAP
compared with those with a mild disease at the early stage of acute
attack. These studies demonstrated that TNF-α is an important index
for the severity of SAP (20,21).
A marked negative linear association was observed between the
expression levels of AQP-1 and TNF-α in the present study.
Furthermore, the results demonstrated that TNF-α regulated the
expression of AQP-1 by an unknown mechanism and participated in the
formation of pulmonary edema during the pathophysiological process
of SAP-associated ALI development.
QYT (pancreas clearance soup) is an effective
traditional prescription used for the treatment of SAP, which has
advantages of a low cost and a high therapeutic effect (21). Modern clinical and experimental
studies (22–24) have focused on such components, a
number of which can alleviate the conditions of the patients
independently. The mechanisms of alleviating SAP by QYT include
improving gastrointestinal function, promoting the excretion of
endotoxins and inhibiting the release of inflammatory mediums and
cytokines, in order to prevent organ damage. The current study
confirmed that QYT alleviated the symptoms of ALI induced by SAP.
Furthermore, QYT was shown to regulate the expression levels of
AQP-1. DEX, a non-specific immune inhibitor, inhibits the gene
synthesis of numerous inflammatory mediators and reduces the
inflammatory reaction by increasing the synthesis of
anti-inflammatory proteins; thus, producing a therapeutic effect on
rats with SAP (25,26). A study by Yang et al
(27) demonstrated that DEX and
QYT have a similar therapeutic effect on ALI when QYT was used to
treat SAP. The incidence rates of the two severe complications,
acute respiratory distress syndrome and intestinal paralysis, in
the group treated with QYT were 3.6 and 5.4%, respectively, while
in the control group the incidence rates were 12.7 and 18.2%,
respectively (P<0.05). Furthermore, QYT effectively decreased
the levels of TNF-α, IL-6 and IL-8 in patients with pancreatitis,
indicating that QYT functions by downregulating the expression of
TNF-α. The present study did not investigate the effect of QYT on
the expression of immunoglobulin; thus, clinical studies are
required for further investigation.
In conclusion, the results of the present study
demonstrate that QYT upregulates the synthesis of AQP-1 by
inhibiting inflammatory reactions and reducing the secretion of
TNF-α; thus, alleviating ALI induced by SAP. The higher expression
levels of AQP-1 in the lungs may be one of the pathogenic factors
of ALI induced by SAP. Administration of QYT may reduce the extent
of pulmonary edema by decreasing the expression levels of TNF-α;
therefore, protecting pulmonary function.
Acknowledgements
The study was supported by a grant from the Liaoning
Province Natural Science Foundation (no. 2013023021).
References
|
1
|
Verkman AS: Aquaporins in clinical
medicine. Annu Rev Med. 63:303–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agre P, Bonhivers M and Borgnia MJ: The
aquaporins, blueprints for cellular plumbing systems. J Biol Chem.
273:14659–14662. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benga G: On the definition, nomenclature
and classification of water channel proteins (aquaporins and
relatives). Mol Aspects Med. 33:514–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denker BM, Smith BL, Kuhajda FP and Agre
P: Identification, purification, and partial characterization of a
novel Mr 28,000 integral membrane protein from erythrocytes and
renal tubules. J Biol Chem. 263:15634–15642. 1988.PubMed/NCBI
|
|
5
|
Verkman AS: Mammalian aquaporins: diverse
physiological roles and potential clinical significance. Expert Rev
Mol Med. 10:e132008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mobasheri A and Marples D: Expression of
the AQP-1 water channel in normal human tissues: a semiquantitative
study using tissue microarray technology. Am J Physiol Cell
Physiol. 286:C529–C537. 2004. View Article : Google Scholar
|
|
7
|
Saadoun S, Papadopoulos MC, Hara-Chikuma M
and Verkman AS: Impairment of angiogenesis and cell migration by
targeted aquaporin-1 gene disruption. Nature. 434:786–792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma T, Yang B, Gillespie A, et al: Severely
impaired urinary concentrating ability in transgenic mice lacking
aquaporin-1 water channels. J Biol Chem. 273:4296–4299. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li ZZ, Xing L, Zhao ZZ, et al: Decrease of
renal aquaporins 1–4 is associated with renal function impairment
in pediatric congenital hydronephrosis. World J Pediatr. 8:335–341.
2012.
|
|
10
|
Heinrich S, Schafer M, Rousson V and
Clavien PA: Evidence-based treatment of acute pancreatitis: a look
at established paradigms. Ann Surg. 243:154–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Windsor JA and Petrov MS: Acute
pancreatitis reclassified. Gut. 62:4–5. 2013. View Article : Google Scholar
|
|
12
|
Shields CJ, Winter DC and Redmond HP: Lung
injury in acute pancreatitis: mechanisms, prevention, and therapy.
Curr Opin Crit Care. 8:158–163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou MT, Chen CS, Chen BC, Zhang QY and
Andersson R: Acute lung injury and ARDS in acute pancreatitis:
mechanisms and potential intervention. World J Gastroenterol.
16:2094–2099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Surbatović M, Jovanović K, Radaković S and
Filipović N: Pathophysiological aspects of severe acute
pancreatitis-associated lung injury. Srp Arh Celok Lek. 133:76–81.
2005.(In Serbian).
|
|
15
|
Mayer J, Rau B, Gansauge F and Beger HG:
Inflammatory mediators in human acute pancreatitis: clinical and
pathophysiological implications. Gut. 47:546–552. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Neuhöfer P, Song L, et al: IL-6
trans-signaling promotes pancreatitis-associated lung injury and
lethality. J Clin Invest. 123:1019–1031. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Towne JE, Harrod KS, Krane CM and Menon
AG: Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung
after acute viral infection. Am J Respir Cell Mol Biol. 22:34–44.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belperio JA, Keane MP, Burdick MD, et al:
Critical role for CXCR2 and CXCR2 ligands during the pathogenesis
of ventilator-induced lung injury. J Clin Invest. 110:1703–1716.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YW, Bi LT, Hou SP, et al: Reduced
lung water transport rate associated with downregulation of
aquaporin-1 and aquaporin-5 in aged mice. Clin Exp Pharmacol
Physiol. 36:734–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue D, Zhang W, Zhang Y, et al: Adjusting
effects of baicalin for nuclear factor-kappaB and tumor necrosis
factor-alpha on rats with caerulein-induced acute pancreatitis.
Mediators Inflamm. 2006:262952006.PubMed/NCBI
|
|
21
|
Li ZL, Wu CT, Lu LR, Zhu XF and Xiong DX:
Traditional Chinese medicine Qing Yi Tang alleviates oxygen free
radical injury in acute necrotizing pancreatits. World J
Gastroenterol. 4:357–359. 1998.PubMed/NCBI
|
|
22
|
Zhang XP, Shi Y and Zhang L: Progress in
the study of therapeutic effects of traditional Chinese medicine
and extracts in treating severe acute pancreatitis. JOP. 8:704–714.
2007.PubMed/NCBI
|
|
23
|
Zhao YQ, Liu XH, Ito T and Qian JM:
Protective effects of rhubarb on experimental severe acute
pancreatitis. World J Gastroenterol. 10:1005–1009. 2004.PubMed/NCBI
|
|
24
|
Zhang MJ, Zhang GL, Yuan WB, Ni J and
Huang LF: Treatment of abdominal compartment syndrome in severe
acute pancreatitis patients with traditional Chinese medicine.
World J Gastroenterol. 14:3574–3578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugiyama Y, Kato S, Abe M, Mitsufuji S and
Takeuchi K: Different effects of dexamethasone and the nitric oxide
synthase inhibitor L-NAME on caerulein-induced rat acute
pancreatitis, depending on the severity. Inflammopharmacology.
13:291–301. 2005. View Article : Google Scholar
|
|
26
|
Kandil E, Lin YY, Bluth MH, et al:
Dexamethasone mediates protection against acute pancreatitis via
upregulation of pancreatitis-associated proteins. World J
Gastroenterol. 12:6806–6811. 2006.PubMed/NCBI
|
|
27
|
Yang DY, Duan SB and Aili JT: Effect of
QYT in treating severe acute pancreatitis and its impacts on blood
level of tumor necrosis factor-alpha, interleukin-6 and
inteleukin-8. Zhongguo Zhong Xi Yi Jie He Za Zhi. 29:1122–1124.
2009.(In Chinese).
|