Introduction
Postoperative acute rejection (AR) in clinical liver
transplantation is a major cause of early allograft dysfunction and
acute function failure. A number of studies suggest that the
inhibitory effect of gadolinium chloride (GdCl3) against
Kupffer cell (KCs) activation shows potential as a protective
intervention in rat models of in vivo hepatic reperfusion
injury and isolated perfused livers (1–3). It
has also been shown that treatment of liver ischemia-reperfusion
injury with GdCl3 reduces the mortality rate, attenuates
neutrophil infiltration and decreases myeloperoxidase activity,
improves hepatic function, reduces platelet accumulation in cold
perfused livers, and prevents apoptosis of sinusoidal endothelial
cells (4). Reduced free radical
formation, lipid peroxidation, and parenchymal necrosis following
GdCl3 administration have also been reported (5). In addition, treatment with
GdCl3 diminishes the production of reactive oxygen
species and the liberation of inflammatory mediators and inhibits
the expression of adhesion molecules (6).
Thus, the identification of a treatment that is able
to specifically inhibit AR and induce immune tolerance is urgent
and essential. The activation of donor KCs is closely correlated
with the occurrence of AR with intense phagocytosis, high
expression levels of membranous molecules, clearly demonstrated
antigen presentation, and the secretion of numerous cytokines that
participate in the immune reaction (7–9).
Therefore, by blocking the immune activity of KCs, AR may
effectively be prevented and inhibited following surgery. In the
present study, GdCl3 was used to inhibit the immune
function of KCs and the depressant function of this treatment on
liver transplantation AR was investigated, with the aim of
investigating the underlying mechanism and providing experimental
evidence for the successful inhibition of postoperative AR in
clinical liver transplantation.
Materials and methods
Experimental animals and treatment
Male Lewis (LEW) and Brown Norway (BN) rats (210–250
g), used as donors and recipients, respectively, were purchased
from the animal research center of Chongqing Medical University
(Chongqing, China). All the animals were housed in the animal care
facility and received humane care in accordance with the National
Institutes of Health guidelines for animal research and the legal
requirements in China. The study was approved by the Ethics
committee of the Second Affiliated Hospital of Chongqing Medical
University (Chongqing, China). All the rats were randomly divided
into three groups. The control group comprised BN rats (n=10) that
underwent exploratory laparotomy. The GdCl3 group (liver
transplantation with GdCl3 pretreatment group) used LEW
(n=15) and BN rats (n=15) and 2 g/l GdCl3 solution (7
mg/kg of body weight; Sigma-Aldrich, St. Louis, MO, USA) was
injected via the vena caudalis into the donor with two days of
continuous administration. On the third day, the donor liver was
transplanted to the recipient. The saline group (liver
transplantation with normal saline pretreatment group) used LEW
(n=15) and BN rats (n=15), and all the operative procedures were
just as in the GdCl3 group, with the exception of using
an identical volume of normal saline instead of GdCl3
solution.
Construction of models of liver
transplantation
Orthotopic liver transplantations were performed
from the LEW to BN rats using Kamada’s method with a few
modifications (10). The
infrahepatic vena cava and the portal vein were linked with cuffs.
The suprahepatic vena cava was inosculated with suture. A stent
tube of the common bile duct was inserted into the common bile duct
and the opening of the stent tube was left outside the body and
used for collecting bile.
Analysis of plasma liver function markers
and histopathological changes
The recipients were humanely sacrificed for
histological inspection seven days post-surgery. The liver tissues
were fixed in 100 g/l neutral formalin solution, embedded in
paraffin wax and the sections were stained with hematoxylin and
eosin to assess the morphological changes. The blood of rats was
obtained through the caudal vein. Plasma liver function markers,
specifically, serum alanine aminotransferase (ALT), aspartate
transaminase (AST) and total bilirubin (TB), were measured with an
automatic biochemical meter (Beckman CX7; Beckman Coulter, Brea,
CA, USA).
Reverse transcription polymerase chain
reaction (RT-PCR) for cytokine mRNA analysis
RNA was extracted from the liver tissue with a
TRIzol reagent kit (Life Technologies, Carlsbad, CA, USA). RT-PCR
was performed using an RT-PCR kit (Roche, Los Angeles, CA, USA).
The cDNA produced was used for the amplification of interferon
(IFN)-γ, IL-2, IL-4, IL-10 and β-actin, respectively (primers were
made by the Shanghai Biochemical Products Factory, Shanghai,
China). Specific primer sequences of IL-10, IL-2, IL-4, IFN-γ and
β-actin were as follows: IL-10, forward: 5′-CCA AGC TTA TCG GAA
ATG-3′, and reverse: 5′-CAC TTG TAA ATC TTT CTT CGGG-3′; IL-2,
forward: 5′-TAG TGG CTG TCG AGA AGC TGC3′, and reverse: 5′-GGC GTC
TTT CAT AGA CAG G-3′; IL-4, forward: 5′-CAT GGT CCG AGA TGT GCA ACT
GGC-3′, and reverse: 5′-CGG GCT CAG CAA CTC CAG C-3′; IFN-γ,
forward: 5′-CCA CGA GGA ATT CTA CGC CCT GGGC-3′, and reverse:
5′-AAG CTT GGG GAA CAG GTA GG-3′; β-actin, forward: 5′-CAT TGT GAT
GGA CTC CGG AG-3′, and reverse: 5′-CTG CCG GTC CAG TAG TATA-3′. The
PCR conditions were 30 cycles of denaturation at 94°C for 60 sec,
annealing at 58°C for 60 sec and extension at 72°C for 60 sec, and
finally an extension at 72°C for 7 min. Agarose gel electrophoresis
was used to separate the products of PCR. Ethidium bromide
staining, a gelatin imaging system and figure analysis system
(GelDoc 2000; Bio-Rad, Hercules, CA, USA) were also used for
observing and semiquantitatively counting their relative
quantities, expressed as relative optical density (ROD) with
normalization to β-actin.
ELISA for analysis of cytokines in
bile
On the seventh day after transplantation, bile was
collected in order to measure the expression levels of IFN-γ and
IL-4 with an ELISA reagent kit (Beijing Dingguo Changsheng Biotech
Co. Ltd., Beijing, China), following the manufacturer’s
instructions.
Isolation of KCs
KCs were isolated using collagenase digestion and
differential centrifugation using Percoll. KCs were collected and
cultured in plates with RPMI-1640 solution at 37°C in the presence
of 5% CO2. Non-adherent cells were removed after 6 h by
replacing the buffer. The KCs were regulated to a density of
1×106 cells/well. The purity and viability of the cells
were >90 and >95%, respectively. Next, the morphological
characteristics of the KCs were observed under a phase contrast
microscope (BX51; Olympus, Tokyo, Japan).
Detection of nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) in KCs
For NF-κB activity analysis, KCs were harvested and
lysed at 24 h after liver transplantation, and nuclear proteins
were extracted using an Active Motif Nuclear Extract kit (Active
Motif, Carlsbad, CA, USA). The relative activity of NF-κB was
represented by the optical density value of colorimetric analysis.
The DNA-binding activity of NF-κB was determined by ELISA using the
TransAM® NF-κB p65 Family kit (Active Motif), according
to the manufacturer’s instructions. In brief, nuclear extract was
transferred into a 96-well plate coated with NF-κB p65 consensus
oligonucleotides. Subsequently, the NF-κB p65 protein bound to the
target sequence was detected by primary rabbit anti-rat p65
antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and a
goat anti-rabbit horseradish peroxidase-conjugated secondary
antibody (Santa Cruz Biotechnology Inc.). Absorbance was quantified
by spectrophotometry (DR 5000 spectrophotometer, Hach Company,
Indiana, USA) at 595 nm as a relative measure of protein-bound
NF-κB p65.
Membranous molecules on KCs
A monoclonal antibody against major
histocompatibility complex (MHC)-II, cluster of differentiation
(CD)80 or CD86 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA)
combined with fluorescein isothiocyanate was added to the liquid
suspension of KCs that was acquired previously and the mixture was
incubated for 30 min. A flow cytometer (FC500 MPL; Beckman Coulter)
was used to assay the positive cells and the mean fluorescence
intensity.
Data analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed with SPSS software,
version 9.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance
with Fisher’s protected least significant difference post hoc
analysis and Student’s test was used to identify significant
differences between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Survival condition of postoperative
rats
In the initial two days following surgery, all rats
in the GdCl3 and saline groups survived, and no
difference in the quality of life was identified. From the third
day following surgery, differences appeared between the groups.
Food intake and energy levels gradually recovered to pre-operative
levels in the GdCl3 group; however, in the saline group,
these representations deteriorated. From the seventh day following
surgery, the difference became increasingly evident. All the rats
in the GdCl3 group survived and their general condition
recovered to nearly normal levels. However, in the saline group,
the rats presented clear occurrence of ascites, jaundice and even
mortality. After the first month following surgery, the survival
rates in the GdCl3 and saline groups were 86 and 47%,
respectively, and the difference was statistically significant
(P<0.01; Table I).
 | Table ISurvival rates of postoperative rats
in the control, GdCl3 and saline groups. |
Table I
Survival rates of postoperative rats
in the control, GdCl3 and saline groups.
| Postoperative
survival rates, % |
|---|
|
|
|---|
| Group | 1 day | 2 days | 3 days | 4 days | 5 days | 7 days | 1 month |
|---|
| Control | 100 | 100 | 100 | 100 | 100 | 100 | 99 |
| GdCl3 | 100 | 100 | 100 | 100 | 100 | 100 | 86a |
| Saline | 100 | 100 | 93 | 87 | 87 | 80 | 47 |
Histopathological changes of liver
Under a light microscope, the hepatocytes in the
GdCl3 group presented moderate vacuolar degeneration and
edema and a small number of inflammatory cells were observed to be
infiltrating the portal area. However, in the saline group, the
hepatocytes presented severe edema and large-area necrosis and
large quantities of infiltrating inflammatory cells (not
shown).
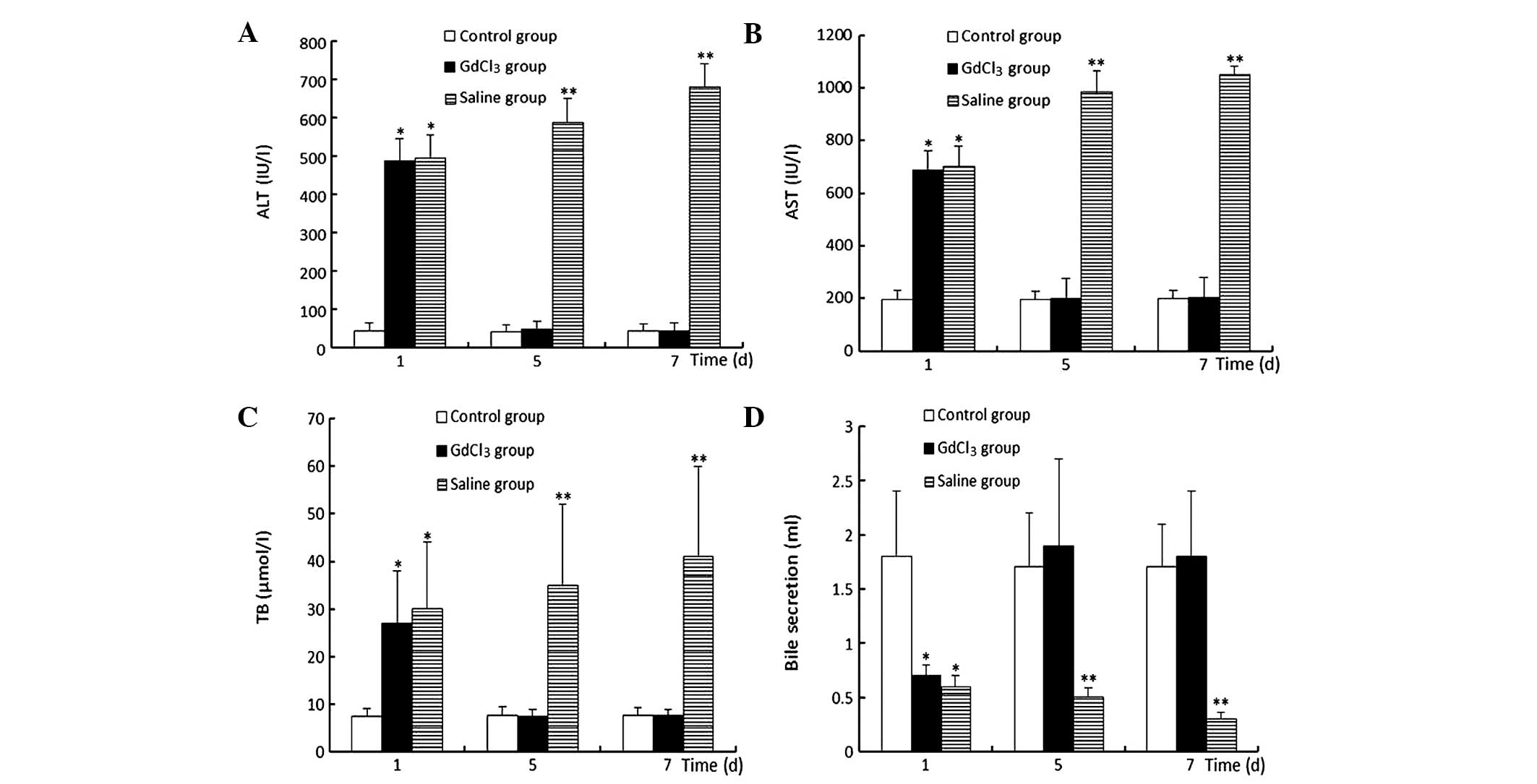
Plasma liver function markers
In the GdCl3 group, on the first day
following surgery, the ALT, AST and TB levels were clearly elevated
and the volume of bile secreted was decreased compared with those
in the control group (P<0.05). Notably, from the second day
after surgery, the aforementioned markers gradually recovered and
by the fifth day after surgery, the markers were restored to normal
levels. However, in the saline group, the aforementioned liver
function markers were progressively aggravated, increasing to
maximum levels on the seventh day following surgery (Fig. 1).
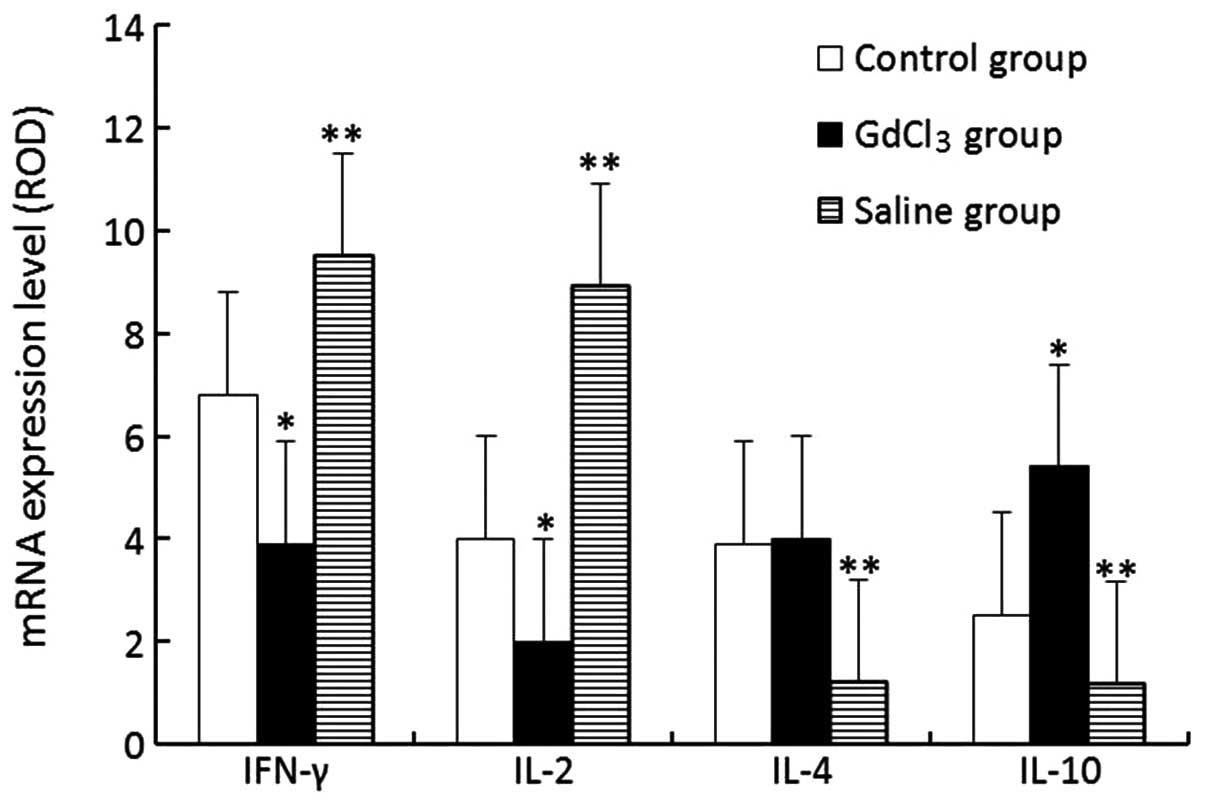
RT-PCR assay of hepatocellular
cytokines
The expression levels of IFN-γ and IL-2 mRNA in the
GdCl3 group were evidently lower compared with those in
the control group (P<0.05); however, the IL-10 level in the
GdCl3 group was higher than that in the control group
(P<0.05) and the IL-4 levels showed no differences between the
GdCl3 and control groups. The results for the saline
group displayed an opposite trend to those in the GdCl3
group (Fig. 2).
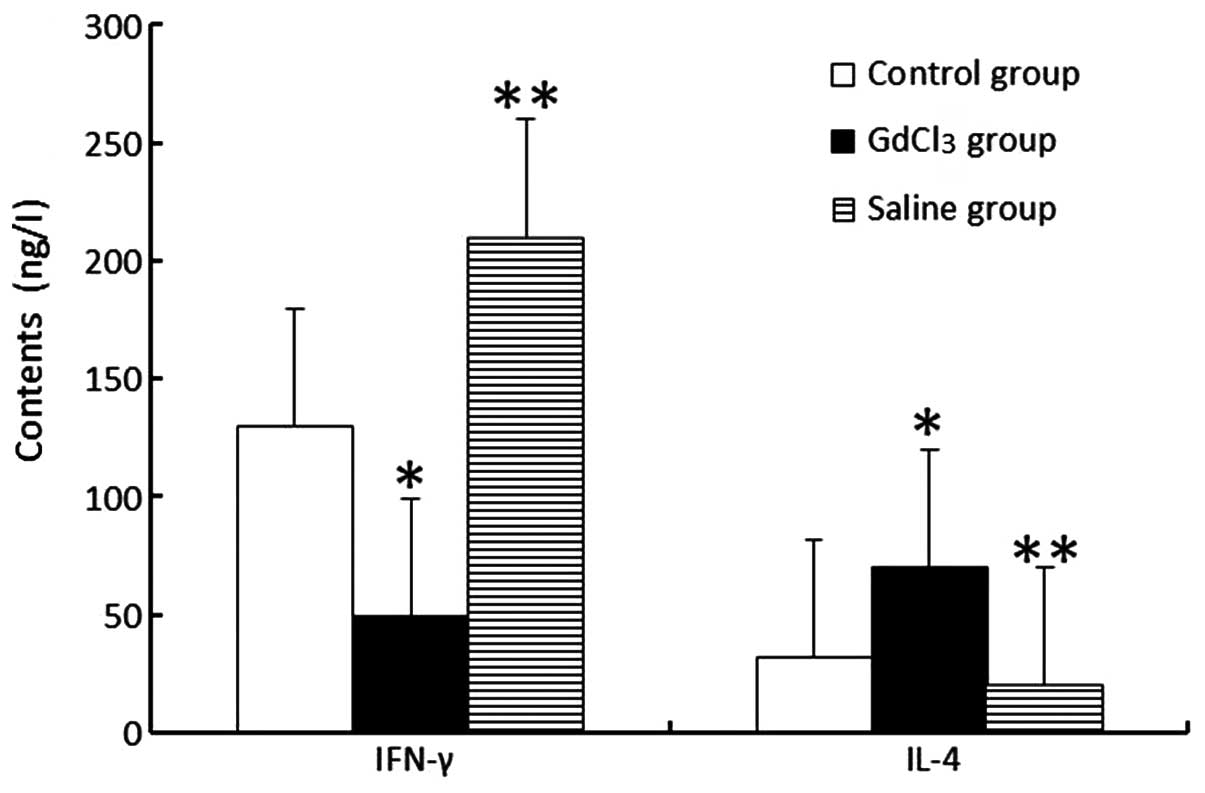
ELISA analytical results of cytokines in
bile
In terms of the ELISA analysis of bile, the
concentration of IFN-γ in the GdCl3 group was markedly
lower compared with that in the control group (P<0.05). However,
the IL-4 level in the GdCl3 group increased compared
with that in the control group (P<0.05). The changes observed in
the saline groups were completely opposite to those in the
GdCl3 group (Fig.
3).
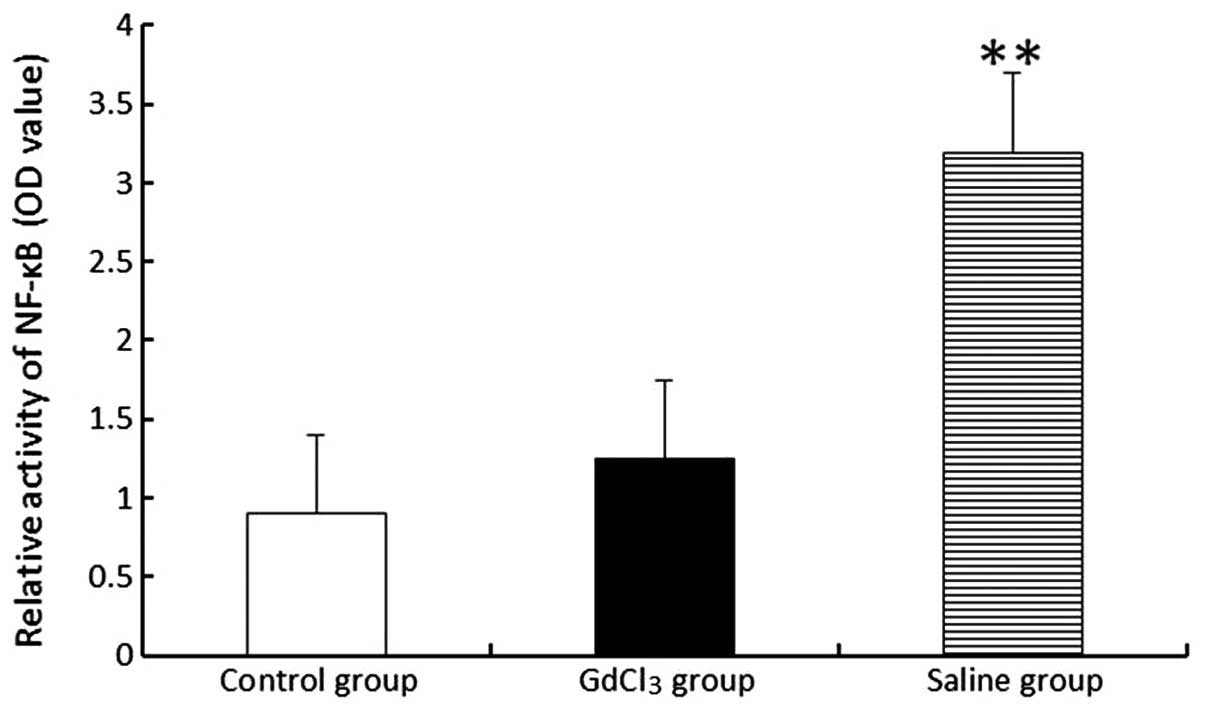
NF-κB activity
The activity of NF-κB was not revealed to differ
between the GdCl3 (1.25±0.63) and control (0.91±0.62)
groups. However, NF-κB demonstrated an increased activity level in
the saline (3.21±0.65) group compared with those in the control and
GdCl3 groups (P<0.01; Fig. 4).
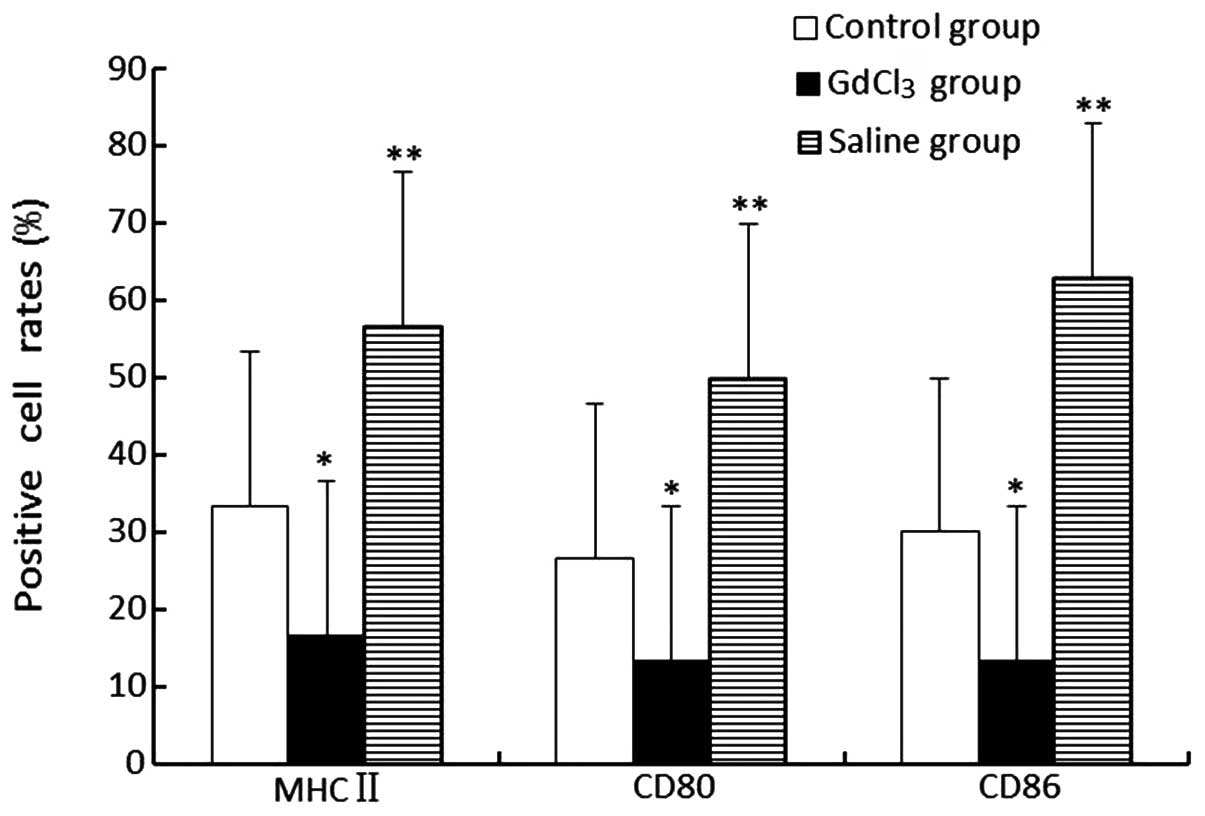
Expression levels of membranous molecules
on KCs
The expression levels of MHC-II, CD80 and CD86 in
the GdCl3 group were revealed to be lower compared with
those in the control and saline groups (P<0.05). Additionally,
the saline group highly expressed these three molecules (P<0.01;
Fig. 5).
Discussion
KCs, which are the resident macrophages of the
liver, not only exert phagocytosis but also excrete significant
amounts of pro-inflammatory and anti-inflammatory cytokines. The
understanding of the contribution of KCs to the inflammatory
response is likely to help in developing immunological and
pharmacological strategies aimed at attenuating the excessive
cytokine secretion and decreasing immune damage. Although the role
of KCs in directly inducing liver transplantation (LT) tolerance
has been reported (7,11), immune tolerance by inhibition of
KCs has been highlighted in numerous recent studies (11–13).
A number of scholars in China and around the world have
demonstrated that GdCl3 selectively reduces the number
of KCs and their functions in experiments, through an unclear
mechanism (3,4,14).
Based on this point, if GdCl3 was used in a
KC-associated study, the results may be beneficial in explaining
the mechanism of hepatic postoperative acute rejection. In the
present study, the aim was to explore the mechanism of the function
of GdCl3 with regard to KCs and LT rejection and
tolerance, and the following was concluded.
The KCs in the GdCl3 group represented a
non-activated state with decreased apophysis on the cell surface,
lower function of the organelle, and decreased expression of the
MHC-II molecule. These observations indicate that GdCl3
treatment may inhibit or obstruct phagocytosis, absorption, antigen
presentation and the combination capacity of the MHC of KCs with
antigens or extraneous materials. In this way, immune rejection may
be effectively prevented.
Activated T cells are critical in the immune
response, and their activation requires two types of signal
stimulus (from the combination of the T-cell receptor and the
peptide-MHC and from co-stimulated molecules) (15). As identified in the
GdCl3 group, the expression levels of co-stimulated
molecules (CD80 and CD86) on the KCs decreased. A possible
mechanism may be that by downregulating the expression of
co-stimulated molecules on KCs, the activation of T lymphocytes at
the upper stream was prohibited.
The activation of NF-κB plays a controversial role
due to its dual action in the induction of both protective and
pro-inflammatory genes. However, it is generally recognized that
this dichotomy of NF-κB promoting the hepatic inflammatory response
and also protecting against it reflects the expression of
NF-κB-dependent genes in different hepatic cell populations
(16). In the present study, it
was identified that the activation of NF-κB in the group pretreated
with GdCl3 was lower compared with that in the saline
group. Thus, inhibiting the NF-κB activity in KCs may be considered
as a route of inducing immune tolerance.
Th0 is able to differentiate into two subsets of
helper T cells (Th1 and Th2), depending on the cytokine stimulation
and production. Furthermore, the role of KCs in this process cannot
be ignored. It is well known that cytokines produced by these two
subsets play pivotal roles in the modulation of the immune
response. As reported in numerous studies, the Th1 cytokines,
including IFN-γ and IL-2, participate in the promotion of graft
rejection (16–18). By contrast, an increased expression
of the Th2 cytokines, including IL-10 and IL-4, may play a
significant role in inducing an immune tolerance (19–24).
This study also demonstrated that the IL-10 and IL-4 levels in the
group pretreated with GdCl3 were evidently higher
compared with the respective levels in the saline group; however,
the IFN-γ and IL-2 levels in the GdCl3 group were
clearly lower compared with those in the saline group. These
results may indicate that deviation from Th1 to Th2 may be a
mechanism of immune tolerance.
In conclusion, GdCl3 efficiently
inhibited the immunological activity of KCs and suppressed acute
rejection following liver allograft transplantation in rats. In
addition, it may be concluded that the immune tolerance induced by
the inhibitory effect of GdCl3 on KCs was a combined
action requiring the participation of numerous mechanisms. In
further studies, new cytokines and regulative routes could be
identified to explain this effect, and further studies may also be
necessary for understanding the exact mechanism.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (grant no. 81370580).
References
|
1
|
Rüttinger D, Vollmar B, Kempter B and
Messmer K: Failure of Kupffer cell blockade to prevent disseminated
intravascular coagulation in endotoxemic rats despite improved
survival. Langenbecks Arch Surg. 383:75–80. 1998.PubMed/NCBI
|
|
2
|
Vollmar B, Rüttinger D, Wanner GA, et al:
Modulation of Kupffer cell activity by gadolinium chloride in
endotoxemic rats. Shock. 6:434–441. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dimitrios G, George P, Stavros I, et al:
The protective effect of alpha-tocopherol and GdCl3
against hepatic ischemia/reperfusion injury. Surg Today.
36:450–456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sindram D, Porte RJ, Hoffman MR, et al:
Synergism between platelets and leukocytes in inducing endothelial
cell apoptosis in the cold ischemic rat liver: a Kupffer
cell-mediated injury. FASEB J. 15:1230–1232. 2001.PubMed/NCBI
|
|
5
|
Giakoustidis DE, Iliadis S, Tsantilas D,
et al: Blockade of Kupffer cells by gadolinium chloride reduces
lipid peroxidation and protects liver from ischemia/reperfusion
injury. Hepatogastroenterology. 50:1587–1592. 2003.PubMed/NCBI
|
|
6
|
Jahnke C, Mehrabi A, Golling M, et al:
Evaluation of microperfusion disturbances in the transplanted liver
after Kupffer cell destruction using GdCl3: An
experimental porcine study. Transplant Proc. 38:1588–1595. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Liu Z, Liang S, et al: Role of
Kupffer cells in the induction of tolerance of orthotopic liver
transplantation in rats. Liver Transpl. 14:823–836. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Liu H, Liu Z, et al: Blockade of
inducible costimulator pathway to prevent acute rejection in rat
liver transplantation. Am J Surg. 198:244–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie X, Ye Y, Zhou L, et al: Küpffer cells
promote acute rejection via induction of Th17 differentiation in
rat liver allografts. Transplant Proc. 42:3784–3792. 2010.
|
|
10
|
Delrivière L, Gibbs P, Kobayashi E, et al:
Technical details for safer venous and biliary anastomoses for
liver transplantation in the rat. Microsurgery. 18:12–18.
1998.PubMed/NCBI
|
|
11
|
Chen GS and Qi HZ: Effect of Kupffer cells
on immune tolerance in liver transplantation. Asian Pac J Trop Med.
5:970–972. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tiegs G and Lohse AW: Immune tolerance:
what is unique about the liver. J Autoimmun. 34:1–6. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You Q, Cheng L, Kedl RM and Ju C:
Mechanism of T cell tolerance induction by murine hepatic Kupffer
cells. Hepatology. 48:978–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jones C, Badger SA, Hoper M, et al:
Hepatic cytokine response can be modulated using the Kupffer cell
blocker gadolinium chloride in obstructive jaundice. Int J Surg.
11:46–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lorenzo LP, Shatynski KE, Clark S, et al:
Defective thymic progenitor development and mature T-cell responses
in a mouse model for Down syndrome. Immunology. 139:447–458. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Llacuna L, Marí M, Lluis JM, et al:
Reactive oxygen species mediate liver injury through parenchymal
nuclear factor-kappaB inactivation in prolonged
ischemia/reperfusion. Am J Pathol. 174:1776–1785. 2009. View Article : Google Scholar
|
|
17
|
Zhao X, Boenisch O, Yeung M, et al:
Critical role of proinflammatory cytokine IL-6 in allograft
rejection and tolerance. Am J Transplant. 12:90–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Li P and Gao S: Effect of
TGF-beta1 on the expression of IL-12, IL-15, IL-18, IL-4 and IL-10
in heart transplantation rejection in rats. J Huazhong Univ Sci
Technolog Med Sci. 27:643–645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Yan T, Shi LJ, et al: Knockdown of
interleukin-2 by shRNA-mediated RNA interference prolongs liver
allograft survival. J Surg Res. 159:582–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Chen J, Liu Z, et al: Relationship
between TH1/TH2 cytokines and immune tolerance in liver
transplantation in rats. Transplant Proc. 40:2691–2695. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao W, Zeng F, Kang K, et al: Lipoxin A4
attenuates acute rejection via shifting TH1/TH2 cytokine balance in
rat liver transplantation. Transplant Proc. 45:2451–2454. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng JF, Chen F, Liu H, et al: Induction
of immune tolerance by pre-infusion of apoptotic lymphocytes
derived from peripheral blood of donor rats before liver
transplantation. Minerva Chir. 68:183–189. 2013.PubMed/NCBI
|
|
23
|
Hu A, Li Q, Shi H, et al: Donor-derived
bone marrow transfusion produces mixed chimerism and promotes a Th2
shift in Th1/Th2 balance in rat heterotopic small bowel
transplantation. Dig Liver Dis. 44:988–994. 2012. View Article : Google Scholar
|
|
24
|
Lian ZR, Xu YF, Wang XB, et al:
Suppression of histone deacetylase 11 promotes expression of IL-10
in Kupffer cells and induces tolerance following orthotopic liver
transplantation in rats. J Surg Res. 174:359–368. 2012. View Article : Google Scholar : PubMed/NCBI
|