Introduction
Breast cancer is one of the most common cancers in
females. The incidence of breast cancer ranks among the top two
cancers in Chinese females, and is a serious threat to health
(1). Lymphatic metastasis often
occurs at the early stage of breast cancer, which is one of the
prognosis factors and is key to determining the clinical stage and
for guiding the selection of breast cancer treatment (2). Axillary lymph node dissection (ALND)
in combination with pharmacology examination (99mTc-dextran
lymphoscintigraphy) has been considered to be the most accurate
method for evaluating lymphatic metastasis; however, ALND usually
leads to a series of short- or long-term complications, such as
wound infection, hematoma formation, pain and limitation of
shoulder activity (3). Moreover,
ALND is not significant in the diagnosis of early-stage breast
cancer patients who are axillary lymph node-negative, but may
seriously affect the patient’s quality of life (4). In the 1990s, the concept of sentinel
lymph nodes (SLNs) was introduced into clinical practice. A number
of studies have indicated that sentinel lymph node biopsy (SLNB)
can predict axillary lymph node metastasis accurately (5,6).
In the treatment of breast cancer, SLNB is quite
significant for the reduction of the upper extremity complications
of patients and for the prediction of axillary lymph node state,
and has gradually become an integral part of the comprehensive
treatment of breast cancer. The identification and location of SLNs
are the key findings of successful SLNB. Currently, the methods
used for the identification and location of SLN include
lymphoscintigraphy, blue dye methods, or a combination of the two
methods.
This study retrospectively evaluated the accuracy of
99mTc-dextran (DX) lymphoscintigraphy for the
identification of SLN location in 235 consecutive cases of breast
cancer in female patients, and analyzed relevant factors affecting
the success of imaging.
Materials and methods
Patients
In this study, 235 consecutive cases of breast
cancer in female patients diagnosed at the Affiliated Cancer
Hospital of Guangxi Medical University (Nanning, China) from
January 2009 to December 2012 were collected as the experimental
subjects. All patients received lymphoscintigraphy prior to radical
mastectomy at the Department of Nuclear Medicine of the Affiliated
Cancer Hospital of Guangxi Medical University. The case inclusion
criteria included the conditions as follows: i) female patients,
ii) preoperative fine needle aspiration or biopsy and
intraoperative frozen pathology verified breast cancer and iii)
clinical stage T1–T2 phase patients. The case exclusion criteria
were as follows (cases with any of the following were excluded from
this study): i) patients receiving ipsilateral axillary trauma or
previous surgery, ii) patients receiving ipsilateral breast cancer
surgery, iii) pregnant or lactating patients and iv) patients with
short-term relapse after radical mastectomy. Patients provided
signed informed consent. Prior written and informed consent was
obtained from every patient and the study was approved by the
ethics review board of the Affiliated Tumor Hospital of Guangxi
Medical University.
Equipment and materials
99mTc-DX and lyophilized dextran
conjugate were provided by Beijing Senke Pharmaceutical Co., Ltd.
(Beijing, China). The radiochemical purity by paper chromatography
analysis was >90% and the marking rate was >95%, with a
particle size of 50–200 nm. A molybdenum-technetium generator was
provided by Beijing Atom Hi-Tech Co., Ltd. (Beijing, China).
Single-photon emission computed tomography (SPECT) was achieved
using a dual-head Discovery VH SPECT scanner purchased from (GE
Healthcare, Pittsburgh, PA, USA), which was configured with a
low-energy high-resolution collimator. The sensitive ray energy
range of the Europrobe hand-held γ detector (Eurorad, Eckbolsheim,
France) was 100–1,000 keV, and 1% methylene blue (MB) was provided
by Beijing Yongkang Pharmaceutical Factory (Beijing, China).
Preoperative lymphoscintigraphy for SLN
location
Within 14–17 h prior to conducting the surgery,
99mTc-DX 37–74 MBq (1–2 mCi)/0.5–1.0 ml, was injected at
sites including the subcutaneous area the tumor site, the mammary
areola and the area surrounding the biopsy residual cavity.
Lymphoscintigraphy was performed following the injection at 15 min,
30 min, 1 h and 2 h, and at 1 h prior to surgery the next day. The
SPECT acquisition conditions were set as matrix, 256×256; 1-fold
magnification; peak energy, 140 keV; window width, 20%; and frame
count, 5×105. A freshly prepared
99mTcO4 point source was used as the location
source, which included an active point source and four fixed-point
sources located at the supraclavicular fossa, xiphoid, and
bilateral areola. A collective focus of radioactive density, with
the exception of that at the injection sites, was considered as
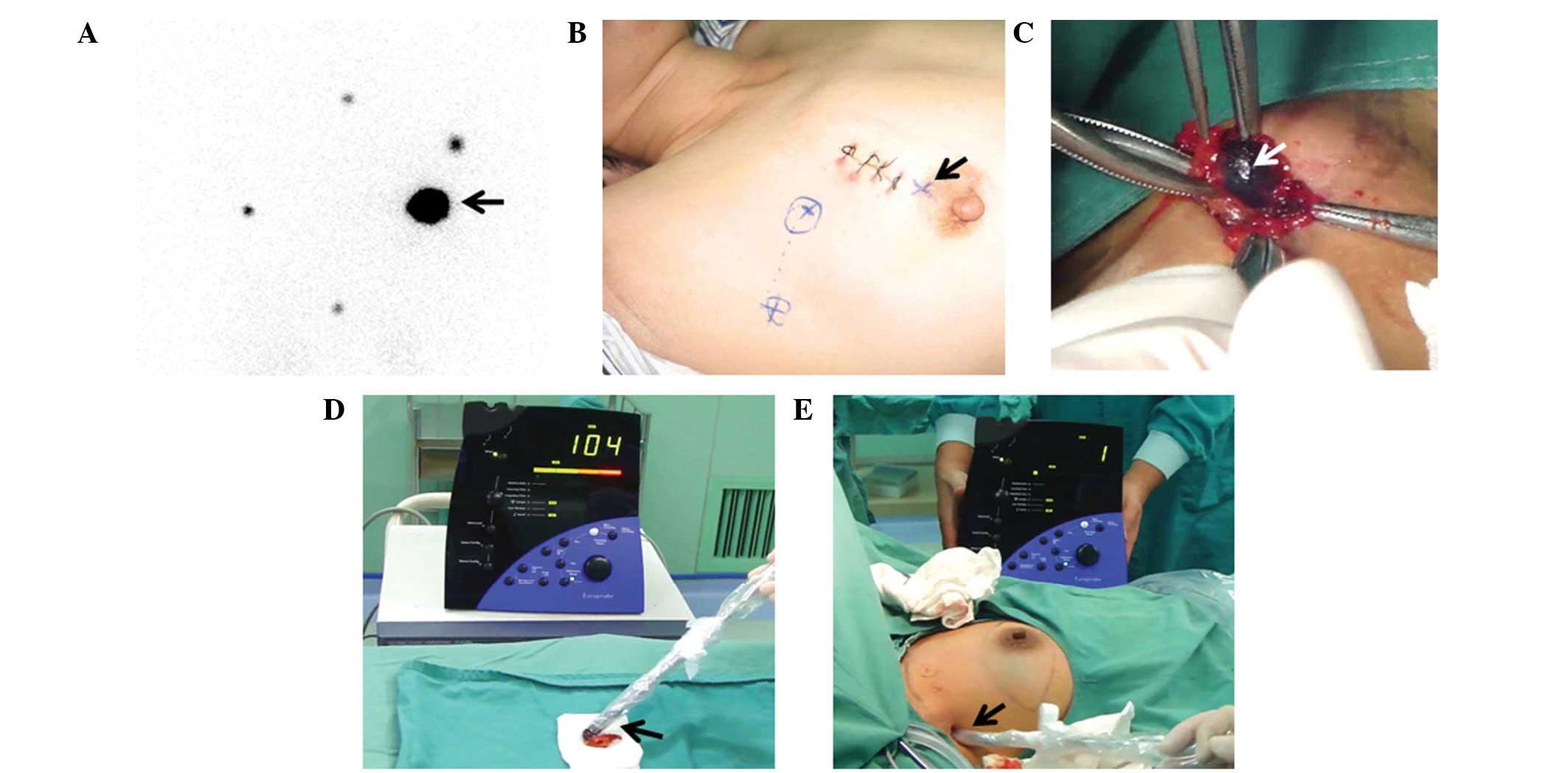
positive lymph node imaging (Fig.
1A). Active point sources between the probe and imaging area
helped to determine the surface location of the collective focus of
density and were marked on the body surface (Fig. 1B).
Intraoperative blue dye methods and ‘hot
spot’ detection
As shown in Fig.
1C, during the surgery, the patient was maintained in a supine
position and 1% MB was administered by subcutaneous injection.
After 5 min, SLNB was performed along the blue-stained lymph
vessels to detect the stained lymph nodes. At the same time, the
handheld γ detector was used to detect hot nodules, which were
those having a count 10-fold higher than the basal count. The
counts of cold nodules were 10% of those of the hot nodules, and
the counts of warm nodules were between those of the hot and cold
nodules (Fig. 1D and E).
SLN treatment and axillary treatment
methods
All the blue-stained lymph nodes and warm nodules
resected during the surgery were considered as SLNs and were sent
for rapid frozen section pathological biopsy. If the results
suggested that SLN metastasis had occurred, routine ALND was
carried out. If no metastasis was identified, the surgeon decided
whether the patient could be treated with ALND. However, the lymph
node detection was considered to have failed if no blue-stained
lymph nodes or hot nodules were detected, and if this occurred, the
patients were treated with ALND.
Evaluation criteria
The location accuracy of lymphoscintigraphy was
evaluated on the basis of the criteria for evaluating SLNB
(7). The following formulae were
applied: i) γ-probe detection rate (%) = (SLN-positive cases/SLN
cases detected) ×100; ii) sensitivity = (imaging-positive cases/SLN
metastasis cases); iii) false negative rate (%) = (imaging-false
negative cases/SLN metastasis cases) × 100; iv) specificity (%) =
(imaging-true negative cases/SLN-negative cases + SLN-false
positive cases) ×100; and v) positive predictive value =
imaging-true positive cases/(SLN-true positive cases + SLN-false
positive cases).
Statistical analysis
SPSS statistical software, version 15.0 (SPSS, Inc.,
Chicago, IL, USA) was used to analyze the data, which were compared
using the χ2 test. Differences were considered to be
statistically significant when P<0.05.
Results
Lymphoscintigraphy
The clinical characteristics of the patients are
shown in Table I. The ages of the
enrolled patients ranged from 24 to 77 years old, with a median age
of 45 years. There were 63 cases of patients who were menopausal,
and 172 cases that were premenopausal. There were 140 patients that
had tumors located in the upper outer quadrant, while the remaining
95 cases had tumors in other quadrants. A tumor size ≤2 cm was
found in 123 cases, and there were 112 cases with tumors >2 cm
but ≤5 cm. Moreover, there were 16 cases of ductal carcinomas in
situ, 185 cases of invasive ductal carcinomas, 12 cases of
invasive lobular carcinoma and 22 cases of other types, including
squamous cell carcinoma and mucous adenocarcinoma.
 | Table IClinical characteristics of patients
and the associated lymphoscintigraphy results. |
Table I
Clinical characteristics of patients
and the associated lymphoscintigraphy results.
| Clinical
characteristics | Lymphoscintigraphy
positive, n (%) | Lymphoscintigraphy
negative, n (%) | χ2 | P-value |
|---|
| Patient age
(years) |
| ≤30 | 5 (83.3) | 1 (16.7) | 0.054 | 0.973 |
| >30, ≤50 | 133 (85.8) | 22 (14.2) | | |
| >50 | 64 (86.5) | 10 (13.5) | | |
| Menstrual status |
| Menopausal | 52 (82.5) | 11 (17.5) | 0.491 | 0.483 |
| Premenopausal | 150 (87.2) | 22 (12.8) | | |
| Tumor size |
| T1: ≤2 cm | 108 (87.8) | 15 (12.2) | 0.730 | 0.393 |
| T2: >2 cm, ≤5
cm | 94 (83.9) | 18 (16.1) | | |
| Tumor location |
| Upper outer
quadrant | 116 (82.6) | 24 (17.4) | 2.158 | 0.141 |
| Other quadrants | 86 (90.5) | 9 (9.5) | | |
| Preoperative
biopsy |
| Yes | 136 (83.4) | 27 (16.6) | 2.162 | 0.141 |
| No | 66 (91.7) | 6 (8.3) | | |
| Neoadjuvant
chemotherapy |
| Yes | 19 (95.0) | 1 (5.0) | 0.775 | 0.378 |
| No | 183 (85.1) | 32 (14.9) | | |
| Type of tumor |
| Intraductal
carcinoma | 14 (87.5) | 2 (12.5) | 2.178 | 0.536 |
| Invasive ductal
carcinoma | 157 (84.5) | 28 (15.5) | | |
| Invasive lobular
carcinoma | 12 (100) | 0 (0) | | |
| Others | 19 (86.4) | 3 (13.6) | | |
In this study, 202 patients among the 235 patients
showed positive results in lymphoscintigraphy imaging. The
detection rate was 86.0% (202/235). Moreover, 191 cases of these
202 patients were detected to have ‘hot nodules’ or ‘warm nodules’.
In addition, there were 11 cases of patients in which no SLNs were
detected by lymphoscintigraphy prior to surgery or by γ-probe
methods during the surgery, but in which SLNs were detected using
blue dye methods. The detecting coincidence rate was 94.6%
(191/202) for lymphoscintigraphy in combination with the γ-probe
method. The successfulness of lymphoscintigraphy was identified to
have no association with the age of the patient, menstrual status,
tumor location, tumor size, pathological type, preoperative biopsy
and neoadjuvant chemotherapy (P>0.05; Table I).
Comparison of lymphoscintigraphy results
with lymph node pathology results
A comparison of the results showed that there was
one patient for which lymphoscintigraphic imaging gave a positive
result prior to surgery, but in which blue stained lymph nodes or
‘hot spots’ were not observed during the surgery, while the
pathology results following ALND found that lymph node metastasis
had occurred (1/11). Moreover, there were 11 patients for whom
negative results were obtained by lymphoscintigraphy prior to
surgery, but blue-stained lymph nodes were detected during surgery.
Intraoperative frozen section pathology and postoperative routine
pathology confirmed that metastasis to the blue-stained lymph node
had not occurred in these 11 patients, nor to the lymph nodes
obtained from ALND. A further five patients (5/33) received
negative lymphoscintigraphy imaging results and were not found to
have SLNs during the surgery; however, following ALND, pathology
results indicated that axillary lymph node metastasis had
occurred.
Lymph node pathology results and axillary
treatment
Pathology results demonstrated that there were 43
cases of SLN-positive patients and 159 cases of SLN-negative
patients, with 17 cases that were axillary node-positive and 215
cases that were axillary node-negative. A total of 55 patients
received only SLN resection, and the intraoperative frozen
pathology biopsy showed that all these 55 cases were SLN-negative.
Thus, they were not treated with ALND. A total of 180 cases
received ALND, and pathology results suggested that 23 of them were
SLN-positive but axillary node-negative.
Evaluation of the positioning accuracy of
lymphoscintigraphy
Based on the evaluation criteria, the SLNB results
obtained using 99mTc-DX lymphoscintigraphy were
analyzed. The detection rate of the γ probe in patients that were
positive by lymphoscintigraphy was 94.6% (191/202), and the false
negative rate for predicting axillary status was 13.3% (4/30).
Moreover, the sensitivity was 90.7% (39/43), the specificity was
23.4% (45/192) and the positive predictive value was 13.5%
(21/155). These results suggest that the use of the γ-probe method
in lymphoscintigraphy can localize SLN accurately for SLNB, but is
not suitable for use in the determination or prediction of axillary
lymph node metastasis.
Discussion
In the present study, lymphoscintigraphic imaging in
combination with γ-probe analysis had an SLN detection rate of
94.6% (191/202), and the false negative rate for predicting
axillary status was 13.3% (4/30). Moreover, the sensitivity was
90.7% (39/43), the specificity was 23.4% (45/192), and the positive
predictive value was 13.5% (21/155). Due to the role of macrophage
phagocytosis in lymph nodes, lymphoscintigraphic tracers were
retained within the SLNs to enable the radiographic imaging. The
lymph node contents as well as the distribution, shape, size and
functional status of lymphatic vessels may be observed by
lymphoscintigraphic imaging (8–11).
Thus, this method can diagnose the lymph node metastasis of
malignant tumors, and also determine pathological changes in the
lymphatic system caused by a benign condition (12–14).
The detection rate of lymphoscintigraphy is ~90–97%,
which is similar to that reported in the majority of literature
(15,16), indicating that the use of a γ probe
in lymphoscintigraphy may provide accurate SLN localization for
SLNB. However, the SLN imaging of the patients conducted using
lymphoscintigraphy is not fully developed. Statistical analysis
demonstrated that the success of lymphoscintigraphy had no
correlation with patient age, menstrual status, tumor location,
tumor size, pathological type, preoperative biopsy and neoadjuvant
chemotherapy (P>0.05). However, relevant factors include the
injection site and the degree of lymph node invasion (17,18).
The enrolled patients were all treated with the same
radioactive tracers with the same volume of injection dose and the
same imaging conditions so that the success rate of
lymphoscintigraphy was less affected by these factors (19–21).
99mTc-DX lymphoscintigraphy is able to accurately locate
breast SLNs to guide breast SLN location with the use of a γ probe
and SLNB (22–24). However, individualized treatment
for patients should be considered in order to improve the success
rate of imaging and better guide SLNB.
Acknowledgements
This study was supported as a Guangxi Health
Department project (grant no. Z2007179).
References
|
1
|
Mohan A and Ponnusankar S: Newer Therapies
for the Treatment of Metastatic Breast Cancer: a Clinical Update.
Indian J Pharm Sci. 75:251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lucci A, Hall CS, Lodhi AK, et al:
Circulating tumour cells in non-metastatic breast cancer: a
prospective study. Lancet Oncol. 13:688–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valente SA, Levine GM, Silverstein MJ, et
al: Accuracy of predicting axillary lymph node positivity by
physical examination, mammography, ultrasonography, and magnetic
resonance imaging. Ann Surg Oncol. 19:1825–1830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scaranelo AM, Eiada R, Jacks LM, Kulkarni
SR and Crystal P: Accuracy of unenhanced MR imaging in the
detection of axillary lymph node metastasis: study of
reproducibility and reliability. Radiology. 262:425–434. 2012.
View Article : Google Scholar
|
|
5
|
Hung T, Piris A, Lobo A, et al: Sentinel
lymph node metastasis is not predictive of poor outcome in patients
with problematic spitzoid melanocytic tumors. Hum Pathol. 44:87–94.
2013. View Article : Google Scholar
|
|
6
|
McMasters M, Tuttle M, Carlson J, et al:
Sentinel lymph node biopsy for breast cancer: A suitable
alternative to routine axillary dissection in multi-institutional
practice when optimal technique is used. J Clin Oncol.
18:2560–2566. 2000.PubMed/NCBI
|
|
7
|
Clough KB, Nasr R, Nos C, Vieira M,
Inguenault C and Poulet B: New anatomical classification of the
axilla with implications for sentinel node biopsy. Br J Surg.
97:1659–1665. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwartz GF, Guiliano AE and Veronesi U;
Consensus Conference Committee. Proceeding of the consensus
conference of the role of sentinel lymph node biopsy in carcinoma
or the breast; April 19–22, 2001; Philadelphia, PA, USA. Breast J.
8. pp. 124–138. 2002
|
|
9
|
Krag DN, Anderon SJ, Julian TB, et al:
Sentinel-lymph-node resection compared with conventional
axillary-lymph-node dissection in clinically node-negative patients
with breast cancer: overall survival findings from the NSABP B-32
randomised phase 3 trial. Lancet Oncol. 11:927–933. 2011.
View Article : Google Scholar :
|
|
10
|
Goyal A, Newcombe RG, Chhabra A and Mansel
RE; ALMANAC Trialists Group. Factors affecting failed localisation
and false-negative rates of sentinel node biopsy in breast
cancer–results of the ALMANAC validation phase. Breast Cancer Res
Trea. 99:203–208. 2006. View Article : Google Scholar
|
|
11
|
Mátrai Z, Tóth L, Saeki T, et al: The
potential role of SPECT/CT in the preoperative detection of
sentinel lymph nodes in breast cancer. Orv Hetil. 152:678–688.
2011. View Article : Google Scholar
|
|
12
|
Pesek S, Ashikaga T, Krag LE and Krag D:
The false-negative rate of sentinel node biopsy in patients with
breast cancer: a meta-analysis. World J Surg. 36:2239–2251. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimazu K, Tamaki Y, Taguchi T, Takamura Y
and Noguchi S: Comparison between periareolar and peritumoral
injection of radiotracer for sentinel lymph node biopsy in patients
with breast cancer. Surgery. 131:277–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borgstein PJ, Meijer S, Pijpers RJ and van
Diest PJ: Functional lymphatic anatomy for sentinel node biopsy in
breast cancer: echoes from the past and the periareolar blue
method. Ann Surg. 232:81–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ogasawara Y, Yoshitomi S, Sato S and
Doihara H: Clinical significance of preoperative lymphoscintigraphy
for sentinel lymph node biopsy in breast cancer. J Surg Res.
148:191–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sabaté-Llobera A, Benítez-Segura A, Marí
A, et al: Lymphoscintigraphy in oral squamous cell carcinoma
sentinel node biopsy and its role in the surgical planning. Clin
Nucl Med. 39:e142–e145. 2014.
|
|
17
|
Noguchi M: Sentinel lymph node biopsy as
an alternative to routine axillary lymph node dissection in breast
cancer patients. J Surg Oncol. 76:144–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uren RF, Howman-Giles R, Renwick SB and
Gillett D: Lymphatic mapping of the breast: locating the sentinel
lymph nodes. World J Surg. 25:789–793. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kraft O and Havel M: Sentinel lymph nodes
and planar scintigraphy and SPECT/CT in various types of tumours.
Estimation of some factors influencing detection success. Nucl Med
Rev Cent East Eur. 16:17–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martínez-Rodríguez I, De Arcocha Torres M,
Banzo I, et al: Evaluation of the contribution of the dynamic phase
of lymphoscintigraphy to the detection of sentinel lymph node in
breast cancer. Q J Nucl Med Mol Imaging. 57:296–300.
2013.PubMed/NCBI
|
|
21
|
Brouwer OR, Vermeeren L, van der Ploeg IM,
et al: Lymphoscintigraphy and SPECT/CT in multicentric and
multifocal breast cancer: does each tumour have a separate drainage
pattern? Results of a Dutch multicentre study (MULTISENT). Eur J
Nucl Med Mol Imaging. 39:1137–1143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nelson KP, Choudhury KR, Coleman RE,
Shipes SW, Siler WL, Hubble WL and Wong TZ: Does the preparation
and utilization of 99mTc-sulfur colloid affect the
outcomes of breast lymphoscintigraphy? J Nucl Med Technol.
41:92–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeung HW, Cody HS III, Turlakow A, et al:
Lymphoscintigraphy and sentinel node localization in breast cancer
patients: A comparison between 1-day and 2-day protocols. J Nucl
Med. 42:420–423. 2001.PubMed/NCBI
|
|
24
|
Tanis PJ, van Sandick JW, Nieweg OE,
Valdés Olmos RA, Rutgers EJ, Hoefnagel CA and Kroon BB: The hidden
sentinel node in breast cancer. Eur J Nucl Med. 29:305–310. 2002.
View Article : Google Scholar
|