Introduction
Dendritic cells (DCs) are potent bone marrow-derived
antigen-presenting cells (1). DCs
constitute a complex system of cells that is able to induce primary
immune responses (2–4). In addition, DCs are effective in
stimulating secondary immune responses (5). Thus, these cells play a central role
in antitumor immunity. However, the function of the immune system
in tumor-bearing hosts is often severely compromised, particularly
in hosts with advanced-stage disease, who present with a diminished
ability to activate immune responses against the tumor. Tumor cells
appear to have developed mechanisms to inhibit immune system
recognition and control (6). DCs
may represent a target for the inhibition of antitumor immune
responses (7).
Defective function of DCs in cancer has been
reported by several study groups (8–12);
however, the causes of DC impairment have not been fully
elucidated. One of the possible mechanisms underlying DC
dysfunction in cancer is the abnormal functional maturation of
these cells from their progenitors (13,14)
caused by tumor-derived factors (15–20).
Despite significant advances in the understanding of the mechanisms
responsible for cancer cell transformation and proliferation, the
immunological pathophysiology of cancer patients, particularly the
antitumor cytokine network, requires further investigation.
Angiogenetic processes appear to be regulated by
cytokines. The cytokine vascular endothelial growth factor (VEGF)
induced by hypoxia is produced by almost all tumors. VEGF directly
stimulates the growth of vascular endothelial cells and the
formation of tumor neovasculature (21–23).
Abnormally high blood concentrations of VEGF have been shown to be
associated with poor prognosis in solid as well as hematological
malignancies (24). Inhibition of
angiogenesis may be one of the mechanism through which the
activation of effective anticancer immunity controls neoplastic
growth (25–28).
In this study, we investigated the effect of culture
supernatants from fresh primary MCF-7 cells on DCs by evaluating
the effect of small interfering RNA (siRNA) targeting VEGF, in an
attempt to elucidate the association between VEGF and impaired DC
differentiation. We cultured mononuclear cells from human
peripheral blood mononuclear cells (PBMCs) in the presence of
culture supernatants from fresh primary MCF-7 cells to evaluate the
association of impaired DCs with tumor-derived factors.
Subsequently, we downregulated VEGF expression by siRNA in MCF-7
cells and evaluated the effects of VEGF on DCs.
Materials and methods
siRNA design
The siRNA sequences that target human VEGF were
constructed according to established guidelines (29). The primer sequences were
5′-GGAGUACCCUGAUGAGAUCUU-3′ (forward) and
5′-GAUCUCAUCAGGGUACUCCUU-3′ (reverse). The negative control
scramble siRNA (siRNASCR) primer sequences were
5′-GCGUAACGCGGGAAUUUACUU-3′ (forward) and
5′-GUAAAUUCCCGCGUUACGCUU-3′ (reverse). These sequences were
verified by DNA sequencing according to the manufacturer’s
instructions (Guangzhou RiboBio Co., Ltd., Guangzhou, Guangdong,
China).
MCF-7 cell culture and transfection
The MCF-7 human breast cancer cell line was kindly
provided by the Cell Culture Center of the Peking Union Medical
College of China and was cultured in RPMI-1640 medium containing
10% fetal calf serum in a 37°C humidified 5% CO2
incubator. The medium was changed every two days. To maintain the
cells at optimal proliferating conditions, they were passaged at
80% confluence and seeded at 20% confluence. Transfection was
performed at ~90% confluence using Lipofectamine™ 2000 (Invitrogen
Co., Carlsbad, CA, USA) following the manufacturer’s protocol. The
cells were collected at 48 h following transfection and used for
western blot analysis.
ELISA
The VEGF concentration in the conditioned medium of
MCF-7 cells was measured using a commercially available human VEGF
ELISA kit (Chemicon, Millipore Corporation, Temecula, CA, USA).
MCF-7 cells were plated on a 96-well plate at a density of
6×103 cells/ml. After 24 h of culture, the cells were
transfected with siRNA (25 nmol/l, 50 nmol/l, 100 nmol/l and 200
nmol/l), siRNASCR, or Lipofectamine reagent overnight at
37°C. The cultures were then washed twice with Hanks’ Balanced Salt
solution and incubated with fresh medium for an additional 48 h.
The supernatants were collected and VEGF concentration was
quantified (pg/ml) by ELISA according to the manufacturer’s
recommendations to determine the effect of specific downregulation
and the optimal concentration of siRNA transfection.
MTT assay
Using a 96-well plate, a total of 8×103
MCF-7 cells were seeded in each well and allowed to attach for 18
h. The cells were later treated with siRNA (25 nmol/l, 50 nmol/l,
100 nmol/l or 200 nmol/l) or siRNASCR. At 48 h following
transfection, 20 μl MTT (5 mg/ml) was added to the cells in each
well. The cells were subsequently incubated for 4 h, followed by
the addition of 100 μl dimethyl sulfoxide and the cells were
incubated for another 15 min. The optical density was determined at
492 nm with a microculture plate reader (SpectraMax M2; Molecular
Devices, Sunnyvale, CA, USA). To determine the inhibition rate, the
absorbance values were normalized to the values obtained from the
blank control group of cells.
Western blot analysis
Following treatment with 100 nmol/l siRNA or
siRNASCR for 48 h, the MCF-7 cells attached to culture
dishes were trypsinized and washed in cold phosphate-buffered
saline (PBS). Briefly, 100 μg protein samples were subjected to 10%
standard SDS-PAGE, with prestained molecular weight markers being
run in parallel to identify VEGF protein. Subsequently, the
resolved proteins were transferred to polyvinylidene difluoride
membranes. The membranes were then incubated with mouse monoclonal
anti-VEGF antibody (cat. no. sc-7269; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and mouse monoclonal anti-β-actin
antibody (cat. no. sc-47778; Santa Cruz Biotechnology, Inc.).
antibodies. Following extensive washing, the membranes were
incubated with anti-mouse IgG-horseradish peroxidase-conjugated
antibody for 1 h at room temperature and developed with a Luminol
chemiluminescence detection kit (Promega Corporation, Madison, WI,
USA). Protein expression was quantified with a Gel EDAS analysis
system and Gel-Pro Analyzer 3.1 software (Shenzhen Tianneng
Corporation, Shenzhen, Guangdong, China).
DC culture
Human PBMCs were obtained from heparinized blood of
healthy volunteers by density gradient centrifugation following
informed consent. The cells were cultured for 5–6 days in complete
RPMI-1640 medium [GlutaMAX (Invitrogen Co.), supplemented with 10%
fetal calf serum, 100 U/ml penicillin and 100 g/ml streptomycin]
with 100 ng/ml granulocyte-macrophage colony-stimulating factor
(GM-CSF) and 20 ng/ml interleukin-4 (IL-4). Maturation was induced
for 48 h in the presence of 10 ng/ml tumor necrosis factor-α
(TNF-α). The DCs were divided into the siRNA and control groups.
The DCs of the siRNA group were cultured in RPMI-1640 medium with
the addition of the culture supernatant of MCF-7 cells transfected
with siRNA (optimal concentration of 100 nmol/l) according to the
volume ratio of 1:2 (supernatant vs. RPMI-1640). The DCs of the
control group were cultured in RPMI-1640 medium with the addition
of the culture supernatant of MCF-7 cells transfected with
siRNASCR (100 nmol/l) according to the volume ratio of
1:2 (supernatant vs. RPMI-1640).
DC morphology imaging and flow
cytometry
The DCs in the two groups were observed daily under
an inverted Olympus microscope (Olympus Optical Co., Ltd., Tokyo,
Japan) with a green light filter. Following 8 days of culture, the
DCs were stained by standard direct procedure using
phycoerythrin-conjugated mouse monoclonal antibodies (mAbs) against
CD1a (cat. no. 555807), CD80 (cat. no. 557227), CD83 (cat. no.
556855), CD86 (cat. no. 555658) or HLA-DR (cat. no. 555812; all
from BD Biosciences, Franklin Lakes, NJ, USA). The cells were
incubated with mAbs for 20–30 min at ambient temperature, washed
twice with PBS and resuspended in 100 μl PBS. Cell fluorescence was
analyzed by the Epics XL flow cytometer (Beckman Coulter Inc.,
Brea, CA, USA).
Mixed lymphocyte reaction
Responder T cells were purified from the PBMCs of
healthy volunteers. T cells (1×106/ml) were co-cultured
with DCs loaded with MCF-7 antigen for 72 h to induce cytotoxic T
lymphocytes (CTLs). The CTLs were then collected and used as the
effector cells in CTL assays. As the target cells, MCF-7 cells were
placed in 96-well culture plates at lx104 cells per well
and co-cultured with the effector cells at varied effector/target
cell ratios (E/T) of l:10, 1:25 and l:50 for 48 h. The cytotoxic
activity was detected with the MTT assay. The tests were performed
in triplicate and the results are expressed as mean counts per
minute with standard deviation.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 11.0 (SPSS Inc., Chicago, IL, USA). Data are
expressed as the means ± SD. The results were considered
statistically significant if P<0.05 was obtained by the
appropriate ANOVA procedure and the Student’s t-test.
Results
VEGF-targeted siRNA inhibits the
expression of VEGF in MCF-7 cells
In order to test whether siRNAs affect the
expression of VEGF, we measured the VEGF concentration in the
culture supernatants of MCF-7 cells transfected with siRNA (25
nmol/l, 50 nmol/l, 100 nmol/l or 200 nmol/l), siRNASCR
or Lipofectamine reagent using ELISA and analyzed the proteins by
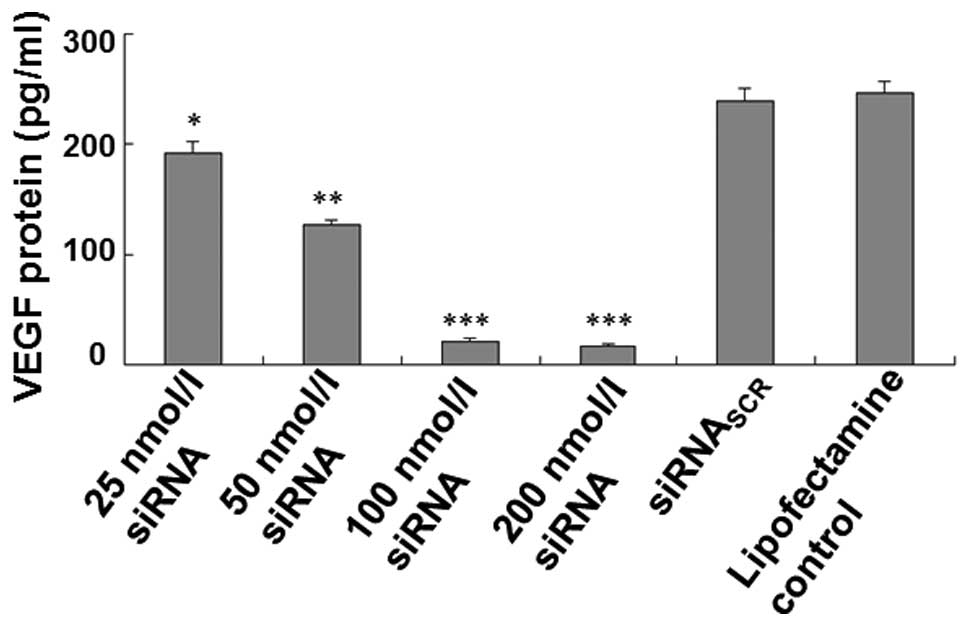
western blotting. The ELISA data demonstrated that the VEGF
expression and protein production in the supernatants of MCF-7
cells transfected with siRNA (100 nmol/l and 200 nmol/l) were
significantly decreased compared to that in the controls; however,
the level of VEGF protein was not significantly different between
the 100 and the 200 nmol/l siRNA groups, or among the
siRNASCR, Lipofectamine control and the blank control
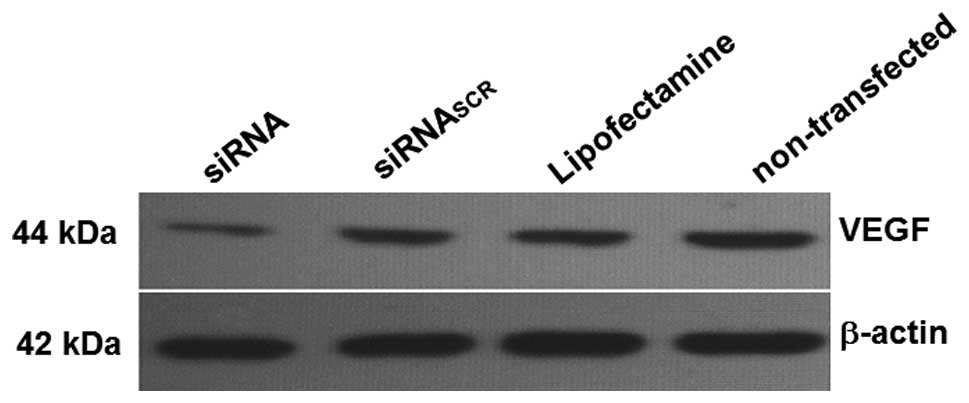
groups (Fig. 1). The western blot
analysis demonstrated that VEGF expression was significantly
reduced in cells transfected with siRNA compared to those
transfected with siRNASCR or Lipofectamine (P<0.05)
(Fig. 1). There was no significant
difference among the siRNASCR, Lipofectamine and
non-transfected groups (Fig. 2).
These data suggested that the constructed VEGF-targeted siRNA
inhibited the expression of VEGF.
Cultivation with the culture supernatants
from MCF-7 cells treated with siRNA affects DC morphology
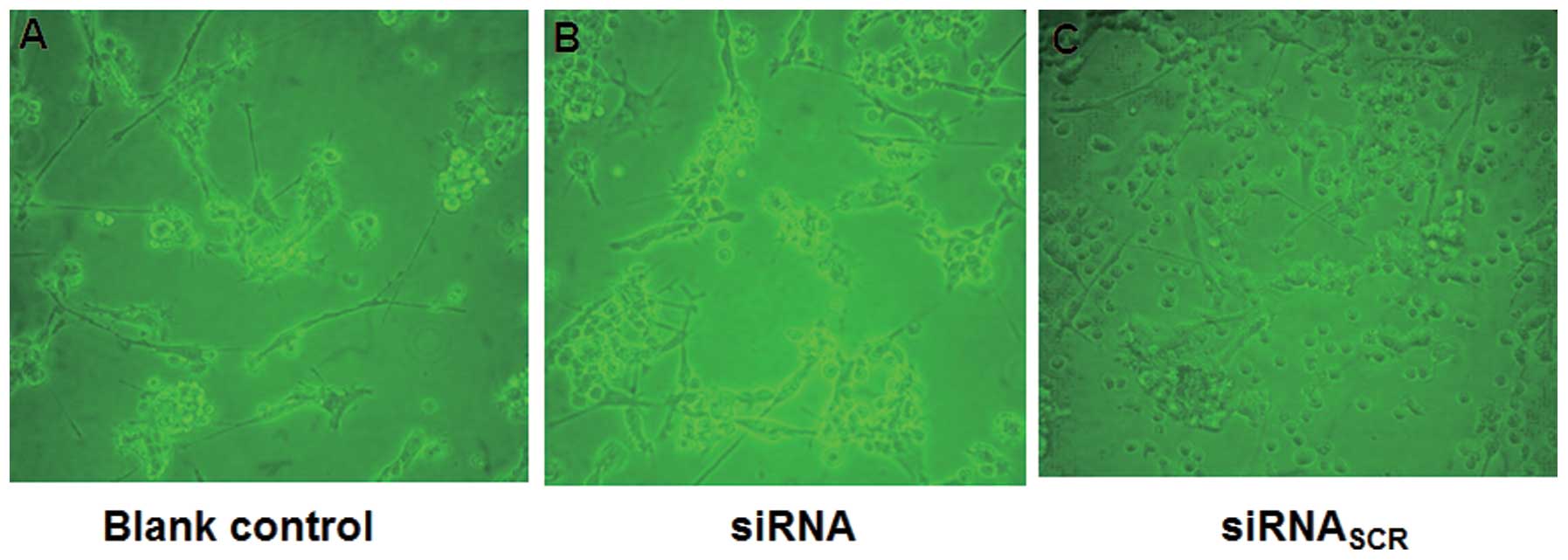
Optical microscopy was employed to investigate the
effect of siRNA on DC morphology. Representative photographs taken
on day 8 demonstrated that DCs generated from adherent human PBMCs
of healthy donors in the presence of GM-CSF, IL-4 and TNF-α
exhibited significant differences in morphology between the siRNA
and siRNASCR groups (Fig.
3). The DCs in the blank control group exhibited typical
arborizations (Fig. 3A). The PBMCs
cultured with the culture supernatants from MCF-7 cells treated
with siRNA started to elongate into irregular shapes on the 2nd or
3rd day and exhibited the morphological characteristics of DCs on
the 8th day (Fig. 3B). The DCs in
the siRNASCR group, cultured with the culture
supernatants from MCF-7 cells transfected with siRNASCR,
started to elongate marginally on the 3rd or 4th day, but exhibited
no distinct DC characteristics until the 8th day (Fig. 3C). These data demonstrated that DC
morphology was affected by cultivation with the culture
supernatants from MCF-7 cells treated with siRNA.
siRNA alters the effect of VEGF on DC
surface phenotypes
In order to investigate the effects of VEGF on DC
differentiation in the culture supernatants from MCF-7 cells and
determine how this effect is affected by VEGF-targeted siRNA, DCs
were labeled with a mixture of phycoerythrin-conjugated
lineage-specific antibodies (anti-HLA-DR, CD80, CD83, CD1a, CD86)
and analyzed directly using flow cytometry. The expression of
HLA-DR on DCs in the siRNA group was higher compared to that in the
control group that was transfected with siRNASCR
(Table I). Folllowing siRNA
transfection, the percentage of cells expressing CD80, CD86 and
CD83 was increased (Table I).
However, the expression of CD1a in the siRNA group was lower
compared to that in the control group (Table I). These data demonstrated that
siRNA altered the effect of VEGF on DC surface phenotypes.
 | Table IExpression of dendritic cell
phenotypes cultured during 8 days. |
Table I
Expression of dendritic cell
phenotypes cultured during 8 days.
| Group | CD1a | CD80 | CD83 | CD86 | HLA-DR |
|---|
| siRNA | 14.56±1.26a | 81.45±2.27a | 82.24±3.24a | 91.15±2.71a | 98.64±2.35a |
| Control | 48.26±2.26 | 34.21±2.11 | 48.34±2.74 | 36.82±1.67 | 58.12±2.12 |
siRNA enhances the toxicity of CTLs
induced by DCs
In order to detect the inhibition rates of
tumor-specific CTLs against MCF-7 cells mediated by DCs cultured in
different media, the MTT assay was used. The results of the MTT
assay demonstrated that the inducing activity of DCs in the siRNA
group was enhanced in comparison to DCs in the control group
(siRNASCR). The cytotoxic activity of CTLs was most
significant at E/T 1:50 (P<0.01) (Table II). These data indicated that the
cytotoxic activity of CTLs mediated by DCs was significantly
increased by transfection with siRNA.
 | Table IIKilling abilities of CTLs in
different groups. |
Table II
Killing abilities of CTLs in
different groups.
| Group | E/T=1:10 | E/T=1:25 | E/T=1:50 |
|---|
| siRNA | 26.1±4.6a | 36.2±3.6a | 66.3±6.8a |
| Control | 4.9±2.0 | 10.4±2.1 | 17.3±1.8 |
| T cells | 3.1±1.3 | 3.5±0.8 | 3.04±1.7 |
Discussion
DCs play a central role in the induction of
antitumor immune responses (30,31).
Adequate DC function is crucial for effective antitumor control and
successful cancer immunotherapy. The inadequate function of DCs in
cancer may be one of the important mechanisms through which tumors
escape immune system control. Cancer patients, particularly those
with advanced-stage disease, have diminished ability to activate
immune responses against the tumor. Although the mechanisms
underlying this immune ‘defect’ are multifactorial, DC dysfunction
plays a key role (32–36). DCs not only initiate T-cell
responses, but are also involved in silencing T-cell immune
responses. The functional activities of DCs mainly depend on their
state of activation and differentiation. Terminally differentiated
mature DCs may efficiently induce the development of effector T
cells, whereas DCs are also involved in the maintenance of
peripheral tolerance. However, accumulated DCs that are educated at
the tumor site, act as functional inhibitors of tumor-specific
immune responses in cancer. Our study demonstrated that the culture
supernatants from primary MCF-7 cells inhibited the
differentiation, maturation and function of DCs induced from the
PBMCs of healthy donors.
We demonstrated that DCs cultured in the culture
supernatants from MCF-7 cells transfected with VEGF-targeted siRNA
exhibited upregulated expression of CD80, CD83, CD86 and HLA-DR,
but a lower level of CD1a expression. T-lymphocyte stimulating
activities and the capacity to induce CTL cytotoxicity were all
enhanced. Our results strongly suggest that the soluble factor VEGF
secreted by MCF-7 cells played an important role in tumor evasion
from immune surveillance, which is one of the factors responsible
for defective DC maturation (37).
However, the detailed mechanisms underlying the effect of VEGF on
DC maturation and function have not been fully elucidated. It was
previously indicated that DCs play a dual role in immune regulation
through cross-priming of T cells and immunosuppression (38). Dikov et al (39) reported that the direct impairment
of DC function by VEGF is mediated primarily by VEGFR1/Flt-1.
Therefore, further studies are required to determine the detailed
mechanisms of the effect of VEGF on human DCs.
VEGF is produced by almost all tumor cells and is
responsible for the formation of tumor neovasculature (40). Our data indicated that the designed
siRNAs specifically inhibited VEGF expression in MCF-7 cells and
the inhibition of DC maturation and function in the culture
supernatants from siRNA-transfected cells was significantly
decreased. These data suggested that VEGF may play a significant
role in tumor development, progression and immunosuppression.
VEGF-targeted siRNA may be effective in the induction of antitumor
immune responses and the treatment of breast cancer.
Acknowledgements
This study was supported by grants from the Qingdao
Science and Technology Project (no. 07-2-1-7-nsh) and the Young
Investigator Award of Qingdao University (no. 2007-1-11).
References
|
1
|
Steinman RM and Cohn ZA: Identification of
a novel cell type in peripheral lymphoid organs of mice. I
Morphology, quantitation, tissue distribution. J Exp Med.
137:1142–1162. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cella M, Sallusto F and Lanzavecchia A:
Origin, maturation and antigen presenting function of dendritic
cells. Curr Opin Immunol. 9:10–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hart DN: Dendritic cells: unique leukocyte
populations which control the primary immune response. Blood.
90:3245–3287. 1997.PubMed/NCBI
|
|
4
|
Steinman RM: The dendritic cell system and
its role in immunogenicity. Annu Rev Immunol. 9:271–296. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baird JR, Fox BA, Sanders KL, et al:
Avirulent Toxoplasma gondii generates therapeutic antitumor
immunity by reversing immunosuppression in the ovarian cancer
microenvironment. Cancer Res. 73:3842–3851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schuler G and Steinman RM: Dendritic cells
as adjuvants for immune-mediated resistance to tumors. J Exp Med.
186:1183–1187. 1997. View Article : Google Scholar
|
|
8
|
Gabrilovich D, Ciernik F and Carbone DP:
Dendritic cells in anti-tumor immune responses. I Defective antigen
presentation in tumor-bearinghosts. Cell Immunol. 170:101–110.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaux P, Moutet M, Faivre J, Martin F and
Martin M: Inflammatory cells infiltrating human colorectal
carcinomas express HLA class II but not B7-1 and B7-2 costimulatory
molecules of the T-cell activation. Lab Invest. 74:975–983.
1996.PubMed/NCBI
|
|
10
|
Chaux P, Favre N, Martin M and Martin F:
Tumor-infiltrating dendritic cells are defective in their
antigen-presenting function and inducible B7 expression in rats.
Int J Cancer. 72:619–624. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gabrilovich DI, Corak J, Ciernik IF,
Kavanaugh D and Carbone DP: Decreased antigen presentation by
dendritic cells in patients with breast cancer. Clin Cancer Res.
3:483–490. 1997.PubMed/NCBI
|
|
12
|
Nestle FO, Burg G, Fah J, Wrone-Smith T
and Nickoloff BJ: Human sunlight-induced
basal-cell-carcinoma-associated dendritic cells are deficient in T
cell co-stimulatory molecules and are impaired as
antigen-presenting cells. Am J Pathol. 150:641–651. 1997.PubMed/NCBI
|
|
13
|
Gabrilovich DI, Nadaf S, Corak J,
Berzofsky JA and Carbone DP: Dendritic cells in antitumor immune
responses. II Dendritic cells grown from bone marrow precursors,
but not mature DC from tumor-bearing mice are effective antigen
carriers in the therapy of established tumors. Cell Immunol.
170:111–119. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gabrilovich DI, Chen HL, Girgis KR, et al:
Production of vascular endothelial growth factor by human tumors
inhibits the functional maturation of dendritic cells. Nat Med.
2:1096–1103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ratta M, Fagnoni F, Curti A, et al:
Dendritic cells are functionally defective in multiple myeloma: the
role of interleukin-6. Blood. 100:230–237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Um SH, Mulhall C, Alisa A, et al:
Alpha-fetoprotein impairs APC function and induces their apoptosis.
J Immunol. 173:1772–1778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Péguet-Navarro J, Sportouch M, Popa I, et
al: Gangliosides from human melanoma tumors impair dendritic cell
differentiation from monocytes and induce their apoptosis. J
Immunol. 170:3488–3494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sombroek CC, Stam AG, Masterson AJ, et al:
Prostanoids play a major role in the primary tumor-induced
inhibition of dendritic cell differentiation. J Immunol.
168:4333–4343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bockorny B and Dasanu CA: Intrinsic immune
alterations in renal cell carcinoma and emerging immunotherapeutic
approaches. Expert Opin Biol Ther. 13:911–925. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harimoto H, Shimizu M, Nakagawa Y,
Nakatsuka K, Wakabayashi A, Sakamoto C and Takahashi H:
Inactivation of tumor-specific CD8+ CTLs by
tumor-infiltrating tolerogenic dendritic cells. Immunol Cell Biol.
91:545–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toi M, Taniguchi T, Yamamoto Y, Kurisaki
T, Suzuki H and Tominaga T: Clinical significance of the
determination of angiogenic actors. Eur J Cancer. 32A:2513–2519.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ellis LM and Fidler IJ: Angiogenesis and
metastasis. Eur J Cancer. 32A:2451–2460. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salven P, Mänpää H, Orpana A, Alitalo K
and Joensuu H: Serum vascular endothelial growth factor is often
elevated in disseminated cancer. Clin Cancer Res. 3:647–651.
1997.PubMed/NCBI
|
|
25
|
Ni YH, Wang ZY, Huang XF, et al: Effect of
siRNA-mediated downregulation of VEGF in Tca8113 cells on the
activity of monocyte-derived dendritic cells. Oncol Lett.
3:885–892. 2012.PubMed/NCBI
|
|
26
|
Hajrasouliha AR, Funaki T, Sadrai Z,
Hattori T, Chauhan SK and Dana R: Vascular endothelial growth
factor-C promotes alloimmunity by amplifying antigen-presenting
cell maturation and lymphangiogenesis. Invest Ophthalmol Vis Sci.
53:1244–1250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marti LC, Pavon L, Severino P, Sibov T,
Guilhen D and Moreira-Filho CA: Vascular endothelial growth
factor-A enhances indoleamine 2,3-dioxygenase expression by
dendritic cells and subsequently impacts lymphocyte proliferation.
Mem Inst Oswaldo Cruz. 109:70–79. 2014. View Article : Google Scholar :
|
|
28
|
Liu CZ, Zhang L, Chang XH, et al:
Overexpression and immunosuppressive functions of transforming
growth factor 1, vascular endothelial growth factor and
interleukin-10 in epithelial ovarian cancer. Chin J Cancer Res.
24:130–137. 2012. View Article : Google Scholar
|
|
29
|
Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S
and Muramatsu T: A small interfering RNA targeting vascular
endothelial growth factor as cancer therapeutics. Cancer Res.
64:3365–3370. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Walter S, Weinschenk T, Stenzl A, et al:
Multipeptide immune response to cancer vaccine IMA901 after
single-dose cyclophosphamide associates with longer patient
survival. Nat Med. 18:1254–1261. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cubillos-Ruiz JR, Baird JR, Tesone AJ, et
al: Reprogramming tumor-associated dendritic cells in vivo using
miRNA mimetics triggers protective immunity against ovarian cancer.
Cancer Res. 72:1683–1693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gabrilovich DI, Ciernik IF and Carbone DP:
Dendritic cells in antitumor immune responses. I Defective antigen
presentation in tumor-bearing hosts. Cell Immunol. 170:101–110.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gabrilovich DI, Corak J, Ciernik IF,
Kavanaugh D and Carbone DP: Decreased antigen presentation by
dendritic cells in patients with breast cancer. Clin Cancer Res.
3:483–490. 1997.PubMed/NCBI
|
|
34
|
Gabrilovich DI, Chen HL, Girgis KR, et al:
Production of vascular endothelial growth factor by human tumors
inhibits the functional maturation of dendritic cells. Nat Med.
2:1096–1103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mosca PJ, Hobeika AC, Colling K, et al:
Multiple signals are required for maturation of human dendritic
cells mobilized in vivo with Flt3 ligand. J Leukoc Biol.
72:546–553. 2002.PubMed/NCBI
|
|
36
|
Scarlett UK, Rutkowski MR, Rauwerdink AM,
et al: Ovarian cancer progression is controlled by phenotypic
changes in dendritic cells. J Exp Med. 209:495–506. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gabrilovich DI, Chen HL, Girgis KR, et al:
Production of vascular endothelial growth factor by human tumors
inhibits the functional maturation of dendritic cells. Nat Med.
2:1096–1103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Woltman AM and van Kooten C: Functional
modulation of dendritic cells to suppress adaptive immune
responses. J Leukoc Biol. 73:428–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dikov MM, Ohm JE, Ray N, et al:
Differential roles of vascular endothelial growth factor receptors
1 and 2 in dendritic cell differentiation. J Immunol. 174:215–222.
2005. View Article : Google Scholar
|
|
40
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|