Introduction
Non-small cell lung cancer (NSCLC) is the leading
cause of cancer-related mortality worldwide, accounting for 80–85%
of lung cancer cases (1).
Transforming growth factor-β (TGF-β) plays a critical role in
regulating the proliferation, differentiation and apoptosis of
cells, as well as the development of embryos. The TGF-β family
includes several isoforms (TGF-β 1, 2 and 3), which interact with
the specific cellular serine/threonine kinase receptors, TGF-β
receptor type I (TβRI) and type II (TβRII) (2). The heteromeric complexes of these
receptors activate Smad proteins in order to regulate the
expression of target genes. Among the members of the Smad family,
Smad4 is particularly associated with cancer (3). Hahn et al (4) identified that a TβRII and/or Smad4
gene deletion, point mutation or functional inactivation occurs in
a variety of tumors.
At present, there is increasing evidence that
impaired signal transduction is closely associated with the
occurrence of tumors. TGF-β/Smad is one of two major pathways for
adjusting cell proliferation; TGF-β and Smad may work together and
contribute to the expression of specific genes (5). Smad4 deletion or mutation can induce
precancerous diseases, promoting tumor development and affecting
the biological behavior of these tumors, such as tumor invasion and
metastasis (5). However, studies
on the expression of TβRs and DPC4/Smad4 in NSCLC are limited.
Takanami et al (6) found
that the presence of immunoreactivity for TβRI and/or TβRII is
correlated with poor prognosis in lung adenocarcinoma. In the
present study, the mRNA and protein expression levels of TβRII and
DPC4/Smad4 were compared between paired samples of NSCLC and
nonlesional lung tissues using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), western blotting and a
quantitative immunohistochemistry method. In addition, the
associations with clinical and pathological features of NSCLC were
analyzed.
Materials and methods
Patients
Lung tumor tissue specimens were obtained from 60
patients (male, 40; female, 20) that had undergone a lobectomy and
mediastinal lymph node dissection for primary lung tumors at the
Department of Thoracic Surgery at the Tangshan Gongren Hospital
(Tangshan, China) between January 2008 and December 2009. Control
nonlesional lung tissue specimens from areas distal to the tumor
were obtained from the same patients (n=24). None of the patients
had received preoperative radiotherapy or chemotherapy. The mean
age of the patients was 55.62 years (range, 33–78 years). The types
of tumors identified were squamous cell carcinoma (n=27),
adenocarcinoma (n=23), large cell carcinoma (n=3) and
adenocarcinoma-squamous cell carcinoma (n=7), which were
histologically graded as well- (n=18), moderately- (n=20) and
poorly-differentiated (n=22). In addition, lymph node metastasis
was diagnosed in 33 patients. The patients were classified into
clinical stages I (n=16), II (n=24) and III (n=20), according to
the TNM staging system (7).
Partial tumors and control nonlesional lung tissues were obtained
during surgery, frozen immediately with liquid nitrogen and stored
in a freezer at −70°C.
Furthermore, 60 paraffin-embedded specimens obtained
between 2000 and 2008, along with the five-year follow-up data,
were used in an additional investigation, which included 60
patients (male, 30; female, 30; age range, 30–74 years). All the
specimens underwent pathological analysis to determine the degree
of differentiation, histology and clinical staging. The study was
conducted in accordance with the Declaration of Helsinki and was
approved by the Ethics Committee of Tangshan Gongren Hospital.
Written informed consent was obtained from all the
participants.
RT-qPCR
To measure the mRNA expression levels of TβRII and
DPC4/Smad4, an RT-qPCR method was employed. Total RNA was extracted
from the tumor and control nonlesional lung tissues using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. The tissue was
homogenized in 1 ml TRIzol to isolate the total RNA (2 μg), which
was reverse transcribed into cDNA. The primers used were as
follows: TβRII sense, 5′-GGG AAC AAC ATG CTA AAT GG-3′ and
antisense, 5′-CTG CAA CCA GAA CCT CAA GT-3′; β-actin sense, 5′-ACC
ACA GTC CAT GCC ATC AC-3′ and antisense, 5′-TCC ACC ACC CTG TTG CTG
TA-3′; Smad4 sense, 5′-AAAGGTGAAGGTGATGTTTGGGTC-3′ and antisense,
5′-CTGGAGCTATTCCACCTACTGATCC-3′; β-actin sense,
5′-CCACCCATGGCAAATTCCATGGCA-3′ and antisense,
5′-TCAAGACGGCAGGTCAGGTCCACC-3′. The primers were annealed at 58°C
for 28 cycles, and each sample was reverse transcribed in
duplicate. To quantify the expression of the target gene, 10-μl
samples of the PCR products were separated electrophoretically on a
1.5% agarose gel. The expression of β-actin was used as an internal
control and the products were semi-quantified by Gel-Pro Analyzer
image analysis software (Media Cybernetics, Inc., Silver Spring,
MD, USA).
Western blotting
Lung cancer and control nonlesional lung tissues
were treated with radioimmunoprecipitation assay buffer and
incubated on ice. The protein concentration was determined with the
Coomassie Brilliant Blue method. To perform polyacrylamide gel
electrophoresis, each lane of the gel was loaded with 50 g protein
mixture, and transferred to a nylon membrane. Hybridization
occurred following the addition of primary rabbit-anti-human
antibodies against TβRII and DPC4/Smad4 (1:500; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and a secondary
goat-anti-rabbit antibody (1:5,000; Santa Cruz Biotechnology,
Inc.). Color reaction and exposure in a dark room were performed to
develop the films. Band Leader software was used to analyze the
ratio of proteins to β-actin (internal control).
Immunohistochemistry
The specimens were deparaffinized in xylene for 20
min, and rehydrated with graded ethanol solutions. The endogenous
peroxidase was blocked by incubating the sections in 3% hydrogen
peroxide and methanol for 15 min. Antigen retrieval was performed
by heating the deparaffinized sections at 121°C for 10 min in l0
mmol/l citrate buffer solution (pH 6.0) in an autoclave. After
blocking nonspecific reactivity with 10% normal goat serum for 10
min at room temperature, the specimens were incubated overnight at
4°C with a primary antibody against Smad4 (1:100), followed by 30
min incubation at 37°C with a secondary antibody (goat-anti-rabbit;
1:l,000). The samples were subsequently treated with the
streptavidin biotin complex. Staining of the specimens was
performed using 3,3′-diaminobenzidine, followed by counterstaining
with hematoxylin, dehydration and cover-slipping with mounting
medium. The presence of brown yellow particles in the cells
following the immunohistochemical assay indicated
positively-stained cells. The degree of staining was determined
based on the percentage of positive cells, and the specimens were
labeled with (−) if they contained <5% positive cells, (+) for
5–20% positive cells and (++) for >20% positive cells. Positive
expression was recorded in the specimens labeled as (++) following
immunohistochemistry.
Statistical analysis
Statistical analysis was done using the SPSS
statistical software (SPSS, Chicago, IL, USA). The difference
between TβRII and DPC4/Smad4 expression in tumor tissues and normal
tissues was performed by unpaired Student’s t test. The correlation
between TβRII and Smad4 expression and the clinicopathological
characteristics were analyzed using the Chi-squared test and
Spearman’s correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
mRNA and protein expression levels of
TβRII
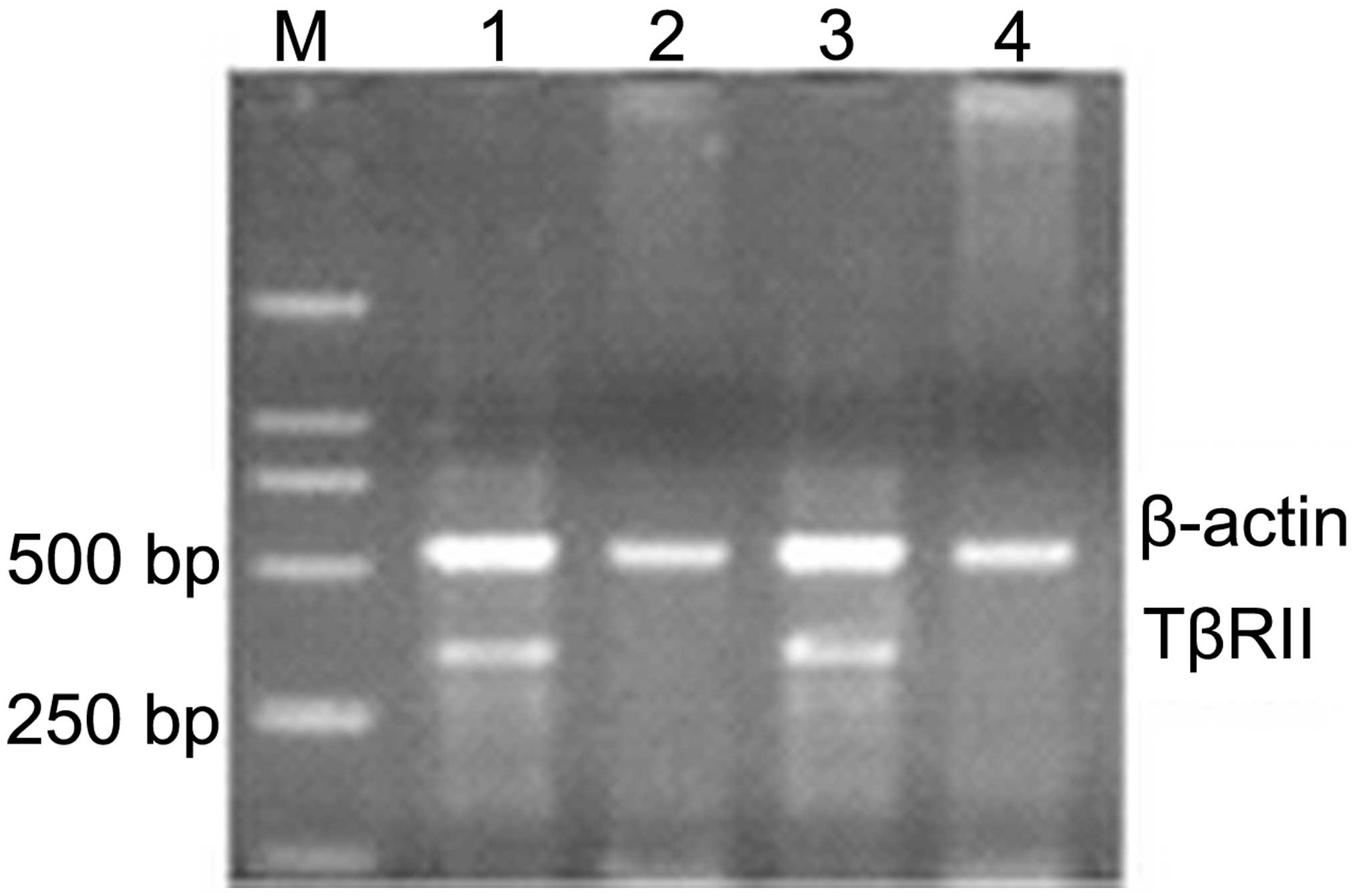
RT-qPCR analysis demonstrated that the relative
expression of TβRII in NSCLC tissues was 0.498±0.198, which was
markedly lower compared with the control nonlesional lung tissues
(1.820±0.672; P<0.05; Fig. 1).
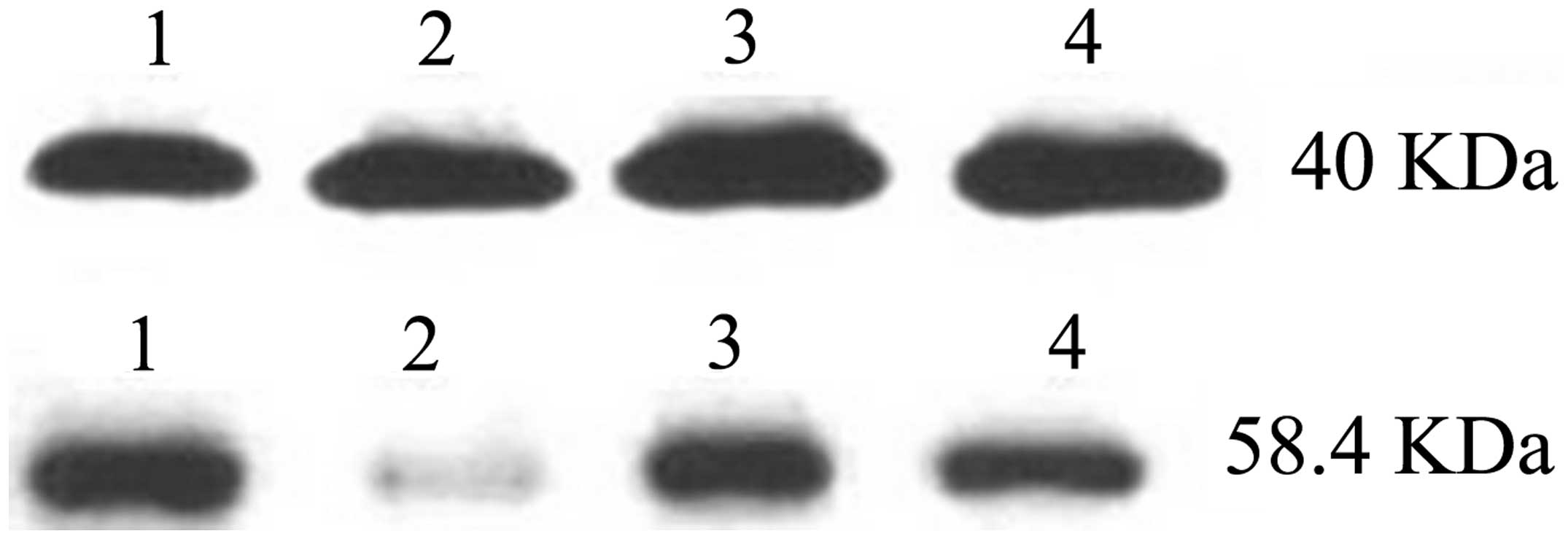
Similarly, the western blotting results demonstrated that the
relative expression of TβRII was 0.203±0.142 in the NSCLC tissues
and 0.882±0.334 in the control nonlesional lung tissues, revealing
a statistically significant difference (P<0.05; Fig. 2). β-actin (40 kDa) was used as an
internal control.
Correlation between TβRII and Smad4
protein expression
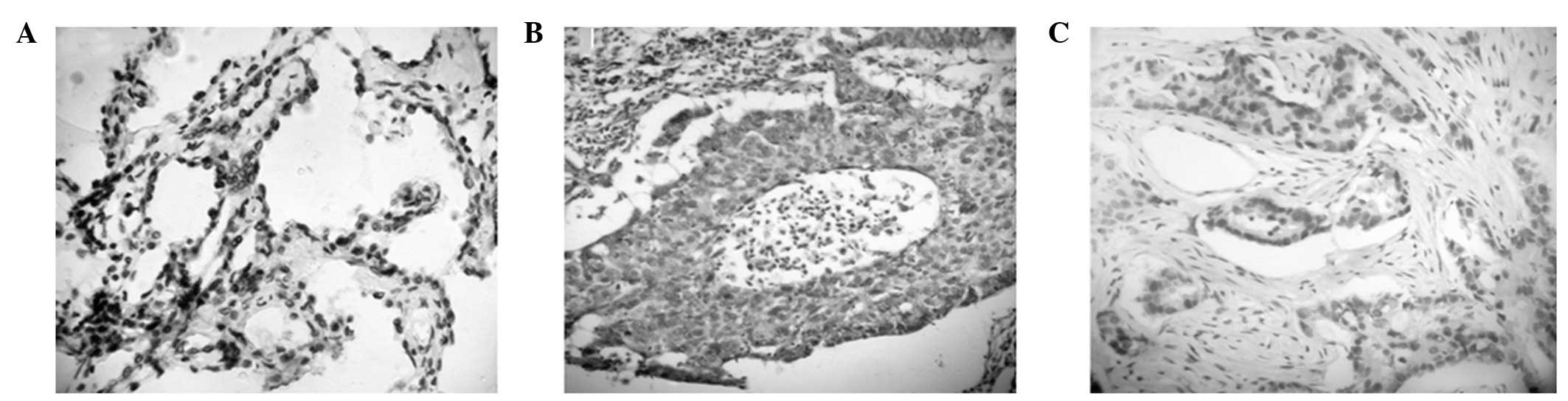
Protein expression levels of TβRII and Smad4 were
investigated. An immunohistochemical assay revealed that Smad4 was
mainly expressed in the cell nucleus of NSCLC and control
nonlesional lung tissues. Positive expression of both TβRII and
Smad4 was identified in eight NSCLC tissue samples, while negative
expression of the two proteins was identified in 36 cases (Fig. 3). Correlation analysis indicated
that the expression of Smad4 was positively associated with the
expression of TβRII in NSCLC tissues (r=0.2326, P<0.01).
Correlation between TβRII and Smad4
expression with clinical pathology parameters
The positive expression rate of TβRII and Smad4 in
the poorly-differentiated lung carcinoma group was significantly
lower when compared with the well- and moderately-differentiated
lung carcinoma groups, exhibiting a statistically significant
difference (P<0.05). In addition, the expression levels in the
patients with lymph node metastasis were significantly lower when
compared with the patients without lymph node metastasis, and a
statistically significant difference was observed between the two
groups (P<0.05). The positive expression rate of TβRII and Smad4
was also reduced in the patients with higher tumor stages.
Moreover, a statistically significant difference was observed
between the poorly-differentiated and the well- and
moderately-differentiated groups (P<0.05; Table I).
 | Table IAssociations between the expression of
TβRII and DPC4/Smad4 and clinical pathology in patients with
NSCLC. |
Table I
Associations between the expression of
TβRII and DPC4/Smad4 and clinical pathology in patients with
NSCLC.
| Clinical pathology
parameter | Patients (n) | TβRII (n) | χ2 | P-value | DPC4/Smad4 (n) | χ2 | P-value |
|---|
|
|
|---|
| + | − | + | − |
|---|
| Histological
grade |
| Well- and
moderately-differentiated | 38 | 24 | 14 | | | 20 | 13 | | |
|
Poorly-differentiated | 22 | 6 | 16 | 7.17 | <0.05 | 4 | 18 | 9.15 | <0.05 |
| Lymph node
metastasis |
| No metastasis | 27 | 17 | 10 | | | 16 | 11 | | |
| Metastasis | 33 | 7 | 26 | 10.79 | <0.05 | 10 | 23 | 5.07 | <0.05 |
| Clinical stage |
| I+II | 40 | 24 | 16 | | | 22 | 18 | | |
| III | 20 | 4 | 16 | 8.57 | <0.05 | 3 | 17 | 8.78 | <0.05 |
Discussion
Smad4 was identified as a candidate tumor suppressor
gene in pancreatic carcinomas and was initially known as ‘deleted
in pancreatic carcinoma locus 4 (DPC4)’, since almost 40% of
patients had a deleted or inactivated version of Smad4 (4). The Smad4 protein is a critical
transcription factor in the TGF-β signaling pathways. Ke et
al (8) indicated that DPC4 may
be involved in preventing tumor metastasis by inhibiting tumor
angiogenesis.
Decreased expression of TβR was considered to be one
of the mechanisms underlying the loss of TGF-β sensitivity and the
enhanced tumor progression in numerous types of cancer (9–11).
Decreased mRNA and corresponding protein expression levels of TβRII
have been reported in gastric cancer cell lines (12). However, studies on the expression
of TβR in NSCLC have been rarely reported (6,13,14).
In the present study, RT-qPCR, western blotting and
immunohistochemistry were performed to analyze the mRNA and
immunoreactive protein expression levels of TβRII and Smad4. The
aim of the study was to compare the mRNA and protein expression
levels of TβRII and Smad4 in NSCLC and control nonlesional lung
tissues.
The present study demonstrated that immunoreactive
Smad4 protein is expressed significantly less in NSCLC tissues
compared with control nonlesional lung tissues, and a similar trend
is present for the mRNA expression levels. The mRNA expression of
Smad4 in poorly-differentiated NSCLC tissues was significantly
lower compared with moderately- or well-differentiated NSCLC
tissues (P<0.05). In addition, the mRNA expression levels of
Smad4 were significantly lower in NSCLC tissues with metastatic
lymph nodes compared with tissues without metastatic lymph nodes
(P<0.05). Protein expression was found to be significantly
decreased in cancer tissues, and the expression was demonstrated to
be closely associated with higher clinical staging, the presence of
metastatic lymph nodes and poor differentiation. In addition, a
decrease in the mRNA expression of Smad4 was found to be associated
with a decrease in the protein expression of Smad4. The results
indicated that the expression of Smad4 was associated with the
tumorigenesis, differentiation and progression of NSCLC. Previous
studies have indicated that patients with Smad4-positive tumors
have a longer survival rate compared with patients without
Smad4-labeled tumors (15,16).
TGF-β is a multifunctional cytokine that inhibits
epithelial cell proliferation, and a strong correlation has been
demonstrated between malignant progression and loss of sensitivity
to the antiproliferative effects of TGF-β. Tumor cells often escape
the antiproliferative effects of TGF-β by the mutational
inactivation or dysregulated expression of components in the TGF-β
signaling pathway (17). A
decreased expression of TβRs is considered to be a possible
mechanism underlying the loss of TGF-β sensitivity and the enhanced
tumor progression in numerous types of cancer (9–11).
Accordingly, the loss of growth regulation by TGF-β is considered
to be an important step in tumor progression in several types of
cancer (18–20).
Decreased mRNA and corresponding protein expression
of TβRII has been reported in a number of tumor cell lines
(14,16,18,19).
In the present study, the mRNA and protein expression levels of
TβRII were further investigated in NSCLC and control nonlesional
lung tissues, revealing that the expression levels in NSCLC tissues
were lower compared with the control nonlesional lung tissues.
Positive expression rates of TβRII in the poorly-differentiated and
lymph node metastasis groups were significantly lower compared with
the well-differentiated and no lymph node metastasis groups,
respectively, and the differences were found to be statistically
significant (P<0.05). The positive expression of TβRII decreased
with increasing pathological stage. In addition, the present study
further demonstrated that the protein expression levels of TβRII
and Smad4 in NSCLC were positively correlated, indicating that
TβRII and Smad4 proteins may have a synergistic effect in the
development of NSCLC.
In conclusion, the present study demonstrated that
downregulation of Smad4 gene expression may be involved in lung
carcinogenesis. The results indicated that loss of the growth
inhibitory response to TGF-β signaling may be crucial in promoting
tumor development in NSCLC.
References
|
1
|
Ginsberg RJ, Vokes EE and Raben A:
Non-small cell lung cancer. Cancer Principles and Practice of
Oncology. DeVita VT Jr, Hellman S and Rosenberg SA: 5th edition.
Lippincott-Raven Publishers; Philadelphia, PA: pp. 858–911.
1997
|
|
2
|
Wrana JL, Attisano L, Weiser R, Ventura F
and Massagué J: Mechanism of activation of the TGF-beta receptor.
Nature. 370:341–347. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moustakas A, Souchelnytskyi S and Heldin
CH: Smad regulation in TGF-beta signal transduction. J Cell Sci.
114:4359–4369. 2001.
|
|
4
|
Hahn SA, Schutte M, Hoque AT, et al: DPC4,
a candidate tumor suppressor gene at human chromosome 18q21.1.
Science. 271:350–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dumont N, Bakin AV and Arteaga CL:
Autocrine transforming growth factor-beta signaling mediates
Smad-independent motility in human cancer cells. J Biol Chem.
278:3275–3285. 2003. View Article : Google Scholar
|
|
6
|
Takanami I, Tanaka F, Hashizume T and
Kodaira S: Roles of the transforming growth factor beta 1 and its
type I and II receptors in the development of a pulmonary
adenocarcinoma: results of an immunohistochemical study. J Surg
Oncol. 64:262–267. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
UICC. International Union Against Cancer.
TNM Classification of Malignant Tumours. Sixth edition. Wiley-Liss;
New York, NY: 2002
|
|
8
|
Ke Z, Zhang X, Ma L and Wang L: Expression
of DPC4/Smad4 in non-small-cell lung carcinoma and its relationship
with angiogenesis. Neoplasma. 55:323–329. 2008.PubMed/NCBI
|
|
9
|
Knaus PI, Lindemann D, DeCoteau JF, et al:
A dominant inhibitory mutant of the type II transforming growth
factor beta receptor in the malignant progression of a cutaneous
T-cell lymphoma. Mol Cell Biol. 16:3480–3489. 1996.PubMed/NCBI
|
|
10
|
Kim IY, Ahn HJ, Lang S, et al: Loss of
expression of transforming growth factor-beta receptors is
associated with poor prognosis in prostate cancer patients. Clin
Cancer Res. 4:1625–1630. 1998.PubMed/NCBI
|
|
11
|
Tokunaga H, Lee DH, Kim IY, Wheeler TM and
Lerner SP: Decreased expression of transforming growth factor beta
receptor type I is associated with poor prognosis in bladder
transitional cell carcinoma patients. Clin Cancer Res. 5:2520–2525.
1999.PubMed/NCBI
|
|
12
|
Park K, Kim SJ, Bang YJ, Park JG, Kim NK,
Roberts AB and Sporn MB: Genetic changes in the transforming growth
factor beta (TGF-beta) type II receptor gene in human gastric
cancer cells: correlation with sensitivity to growth inhibition by
TGF-beta. Proc Natl Acad Sci USA. 91:8772–8776. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim WS, Park C, Jung YS, et al: Reduced
transforming growth factor-beta type II receptor (TGF-beta RII)
expression in adenocarcinoma of the lung. Anticancer Res.
19:301–306. 1999.PubMed/NCBI
|
|
14
|
Kang Y, Prentice MA, Mariano JM, et al:
Transforming growth factor-beta 1 and its receptors in human lung
cancer and mouse lung carcinogenesis. Exp Lung Res. 26:685–707.
2000. View Article : Google Scholar
|
|
15
|
Wilentz RE, Iacobuzio-Donahue CA, Argani
P, et al: Loss of expression of Dpc4 in pancreatic intraepithelial
neoplasia: evidence that DPC4 inactivation occurs late in
neoplastic progression. Cancer Res. 60:2002–2006. 2000.PubMed/NCBI
|
|
16
|
Wilentz RE, Su GH, Dai JL, et al:
Immunohistochemical labeling for dpc4 mirrors genetic status in
pancreatic adenocarcinomas: a new marker of DPC4 inactivation. Am J
Pathol. 156:37–43. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Caestecker MP, Piek E and Roberts AB:
Role of transforming growth factor-beta signaling in cancer. J Natl
Cancer Inst. 92:1388–1402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garrigue-Antar L, Muñoz-Antonia T, Antonia
SJ, Gesmonde J, Vellucci VF and Reiss M: Missense mutations of the
transforming growth factor beta type II receptor in human head and
neck squamous carcinoma cells. Cancer Res. 55:3982–3987.
1995.PubMed/NCBI
|
|
19
|
Markowitz S, Wang J, Myeroff L, et al:
Inactivation of the type II TGF-beta receptor in colon cancer cells
with microsatellite instability. Science. 268:1336–1338. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signalling in tumor suppression and cancer progression.
Nat Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|