Introduction
The diagnostic approach to pleural disease is a
relatively neglected aspect of modern thoracic medicine, despite
the fact that pleural disease affects ~300 subjects per 100,000
individuals per year worldwide (1,2). If
a clinical suspicion of malignancy is high in patients with pleural
effusion, cytological examination of pleural fluid samples is
recommended; the diagnostic yield for malignancy with pleural
cytology is 50–60%, which falls to 30% in effusions associated with
malignant mesothelioma (MM) (3).
Tumor type and the availability of reliable immunocytochemistry may
influence the yield; for example, the cytological detection rate
for adenocarcinoma is higher than that for squamous cell carcinoma,
mesothelioma or lymphoma (4). When
the distinction between primary and metastatic tumors is addressed,
morphological criteria alone are not sufficient for a definite
diagnosis of MM (5). In instances
when cytology is non-diagnostic (3,4),
closed percutaneous needle biopsy has traditionally been performed
blindly using a reverse-beveled needle, such as an Abrams or Ramel
needle; however, blind closed pleural biopsy has a relatively
modest diagnostic yield of <60% for pleural malignancy (6).
Medical thoracoscopy enables the direct examination
of the pleura and biopsies taken under direct vision and has a
diagnostic yield superior to that of blind closed pleural biopsy
and thoracocentesis. The diagnostic yield is 91–95% for malignant
disease and can reach 100% for pleural tuberculosis. Furthermore,
although medical thoracoscopy is more invasive and expensive,
complications occur only infrequently (4,7).
Despite this, the most efficient and cost-effective approach to
pleural lesions remains unclear and controversial, particularly in
cases requiring the acquisition of pleural tissue. Recent studies
have proposed that image guidance may significantly increase the
diagnostic yield while simultaneously decreasing the risk of
complications. It has also been suggested that real-time computed
tomography-guided cutting needle pleural biopsy (CT-CNPB),
performed by a radiologist, is a promising technique for sampling
the pleura, as it can improve diagnostic sensitivity to ~80% for
pleural malignancy (8,9,10).
Image-assisted biopsy is more likely to be diagnostic in the
presence of pleural thickening >10 mm, pleural nodularity,
pleural-based mass lesions of >20 cm and solid pleural tumors
(7,11–16).
In the present study, the diagnostic accuracy and safety of CT-CNPB
was evaluated in patients requiring pleural tissue sampling.
Materials and methods
Study population
This study was a retrospective analysis of 92
percutaneous CT-CNPBs on 90 patients between March 2008 and May
2013. All procedures were performed by the same two radiologists
who were experienced in performing CT-guided pleural biopsies.
Percutaneous CT-CNPB was indicated in any patient with a plural
lesion requiring biopsy. Patients excluded from the study included
patients with lung-based tumors or no final diagnosis. Each patient
provided their written informed consent prior to the procedure.
This study was conducted in accordance with the Declaration of
Helsinki and with approval from the Ethics Committee of Zhongnan
Hospital of Wuhan University (Wuhan, China).
Procedure
A CT scan of the chest (Somatom Sensation16; Siemens
Healthcare, Forchheim, Germany) was initially performed to identify
the lesion. The patient was then positioned in the supine, prone or
lateral position to minimize puncture depth. An initial
localization scan with a low-dose technique (Lung CARE series:
20–50 mA; 120 kV; scanning field, 30–60 mm; Siemens Healthcare)
through the region of interest was performed at a slice thickness
of 5 mm and viewed on both lung and soft-tissue windows.
Localization was performed subsequent to the review of the CT
images using laser positioning and skin markers (Biopsy single
series: 50 mA; 120 kV; thickness, 10 mm; scanning field, 10 mm;
Siemens Healthcare) to indicate the site of needle entry and the
direction of approach for the biopsy. Subsequent to ensuring that
the direction of needle approach was perpendicular to the chest
wall, the thickness of the thoracic wall was measured from the skin
marker to the pleural surface to determine the depth of anesthesia
to be administered and the depth of needle insertion. Using an
aseptic technique, local anesthetic (lignocaine 1%) was
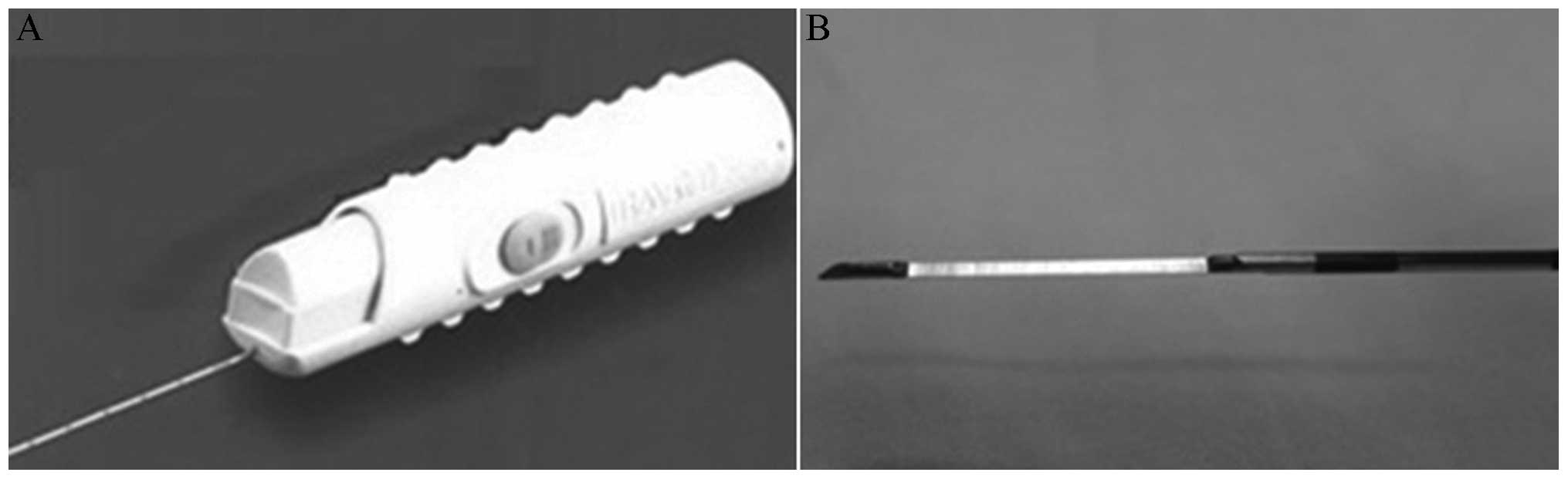
administered. An 18-gauge coaxial automated cutting needle
(Bard® Max-Core® biopsy needle; C.R. Bard,
Inc., Tempe, AZ, USA) (Fig. 1) was
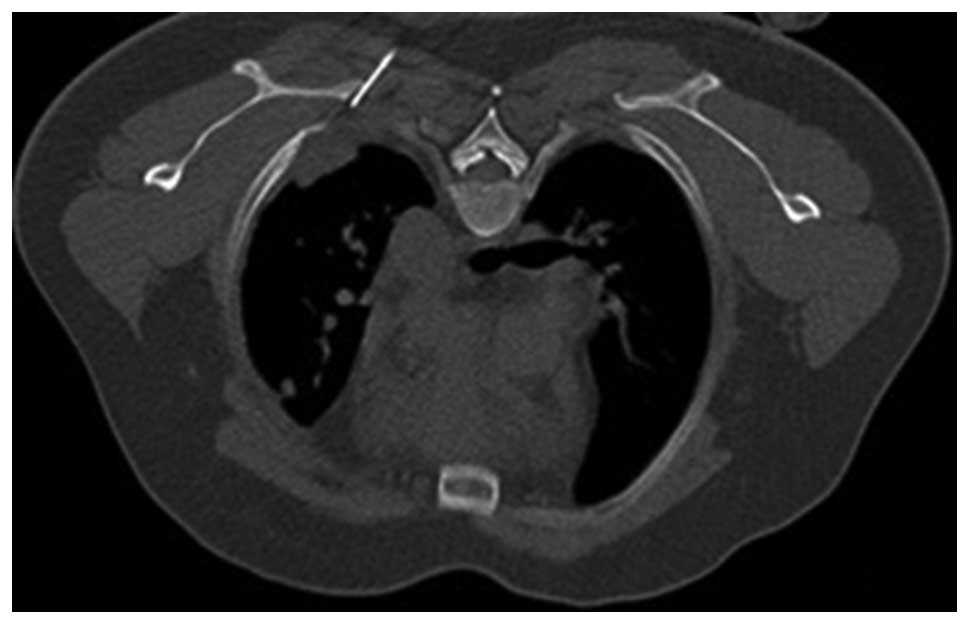
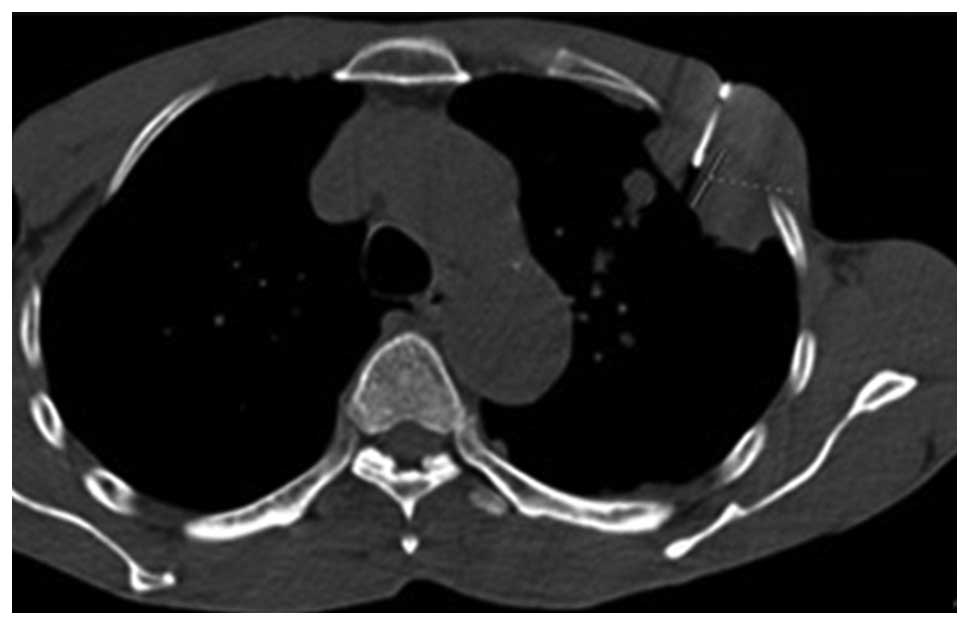
then introduced into the soft tissues without traversing the
pleural surface (Figs. 2 and
3). The position of the pleural
lesion in relation to the tip of the needle and the precise
distance to the margin of the lesion (Figs. 2 and 3) were optimized using sequential CT
scanning (Biopsy single series: 50 mA; 120 kV; thickness, 4.5 mm;
scanning field, 13.5 mm; Siemens Healthcare). According to the
precise information obtained from the repeat CT scan, the
trajectory of the needle was adjusted, and the biopsy gun was
directly advanced into the lesion and fired to obtain a core of
tissue. The procedure was stopped immediately if the patient
complained of discomfort, such as dyspnea, severe cough or
hemoptysis. The surgeon then assessed the adequacy of the sample
prior to deciding whether to proceed with additional passes. The
obtained specimens were placed in formalin solution using a
saline-filled syringe. Once the surgeon was satisfied with the
samples obtained, immediate post-biopsy CT (Biopsy single series:
50 mA; 120 kV; thickness, 4.5 mm; scanning field, 13.5 mm) was
performed over the region of the biopsy to check for pneumothorax
or hemorrhage.
Classification of diagnoses and
complications
The biopsy specimens were evaluated by the same
experienced pathologist. The cases were categorized primarily as
benign and malignant, and those that were malignant were also
categorized according to the cell properties. Immunohistochemical
stains were used to differentiate tumors when required. The
immunohistochemical markers used were: cytokeratin 7, thyroid
transcription factor-1, vimentin, mesothelial cell and calretinin.
A positive expression of calretinin, Vim and mesothelial cell would
indicate that pleural mesothelioma occured, whereas, positive
expression of cytokeratin 7 and thyroid transcription factor-1
would support that lung metastasis has occured. The pathologist
also stained the specimens with Ziehl-Neelsen (Baso Diagnostics
Inc., Zhuhai, China) to investigate for acid-resistant bacilli. A
specific histological diagnosis was defined as a definite
histological type; the results were considered non-specific when no
particular diagnosis could be established from the specimen
obtained and in false-negative cases of malignancy. The final
diagnosis was confirmed at surgery. Histological findings obtained
by biopsy were compatible with the patient’s clinical
manifestations of disease.
The presence of pneumothorax was assessed by a
low-dose CT technique. If the patient was clinically stable, he or
she was kept under medical observation for 12 h. All patients had a
chest radiograph performed 4 h after the procedure or sooner if
they became symptomatic. Pneumothorax (17) and hemorrhage (18) were graded as mild, moderate or
severe.
Statistical analysis
Details regarding the nature of the lesion (lesion
location and thickness), the procedure itself (false-negative rate,
requirement for CT contrast enhancement to distinguish a lesion
from the thorax wall and vessels and number of attempts) and
complications were calculated. Categorical variables are presented
as counts and percentages, and χ2 tests were conducted
for group comparisons. Logistic regression models were performed to
detect the risk factors for diagnostic accuracy (false-negative
rate). Factors with a significance level of P<0.05 in the
univariate analyses were included in the multivariate model. A
two-sided P-value of <0.05 was considered statistically
significant. Statistical Analysis System (SAS) version 9.2
statistical software (SAS Inc., Cary, NC, USA) was used for all
analyses.
Results
Patient characteristics
In the present study, 90 patients, including 53
males and 37 females (mean age, 55.2±14.5 years) underwent 92
CT-CNPBs. There were 64 (71.1%) inpatients and 26 (28.9%)
outpatients. Pleural thickening in the 92 cases varied between 6
and 99 mm, and 23 cases (25%) had pleural effusion. A total of 21
cases underwent CT contrast enhancement. The number of attempts
ranged between two and four, and the duration of the procedure was
16±3 min.
Biopsy yield
The distribution of diagnoses for cases included in
the study is shown in Table I.
Immunohistochemical stains were used in 19 cases. The sensitivity
of diagnostic malignant lesion was 90.9% (50/55), the specificity
was 100% (37/37), the positive predictive value was 100% (50/50)
and the negative predictive value was 88.1% (37/42). The overall
diagnostic accuracy was 94.6% (87/92). A specific histological
diagnosis was achieved in 89.1% (49/55) of malignant lesions and
86.5% (32/37) of benign lesions. The non-specific results and
confirmation methods are shown in Table II.
 | Table IDistribution of the diagnoses of the
92 cases included in the study. |
Table I
Distribution of the diagnoses of the
92 cases included in the study.
| A, Malignant, n=55
(59.8%) |
|---|
|
|---|
| Diagnosis | N (%) |
|---|
| Mesothelioma | 12 (13.0) |
| Metastatic pleural
disease | 36 (39.1) |
| Adenocarcinoma | 24 (26.1) |
| Squamous
carcinoma | 5 (5.4) |
| Clear cell
carcinoma | 2 (2.2) |
| Adenosquamous
carcinoma | 1 (1.1) |
| Small cell
carcinoma | 1 (1.1) |
| Carcinocarcoma | 1 (1.1) |
| Plasmocytoma | 1 (1.1) |
| Osteosarcoma | 1 (1.1) |
| Synovial sarcoma | 1 (1.1) |
| Malignant cell | 1 (1.1) |
| False-negative
lesion | 5 (5.4) |
|
| B, Benign, n=37
(40.2%) |
|
| Diagnosis | N (%) |
|
| Inflammation | 15 (16.3) |
| Tuberculosis | 10 (10.9) |
| Granuloma | 3 (3.3) |
| Solitary fibrous
tumor | 2 (2.2) |
| Hematoma | 1 (1.1) |
| Fungus | 1 (1.1) |
| Indeterminate-origin
disease | 5 (5.4) |
 | Table IINon-specific specimen results and the
final diagnoses. |
Table II
Non-specific specimen results and the
final diagnoses.
| Case | Specimen results | Confirmation
methods | Final diagnoses |
|---|
| 1 | Malignant cell | Secondary biopsy | Adenocarcinoma |
| 2 | Atypical mesothelial
proliferation | Surgery | Adenosquamous
carcinoma |
| 3 | Atypical mesothelial
proliferation | Secondary biopsy | Adenocarcinoma |
| 4 | Fibrous tissue | Surgery | Adenosquamous
carcinoma |
| 5 | Fibrous tissue | Clinical
manifestation: Lesion enlarged | Metastatic pleural
disease of lung adenocarcinoma |
| 6 | Fibrous tissue | Adenocarcinoma cell
in hydrothorax | Metastatic pleural
disease of ovarian adenocarcinoma |
| 7/8 | Glassy degeneration
tissue and fibrous connective tissue | Clinical follow-up in
two years | Benign lesion |
| 9 | Necrotic tissue | Absorbed after
antituberculosis therapy | Tuberculosis |
| 10 | Fibrous connective
tissue | Surgery | Inflammation |
| 11 | Fibrous tissue
hyperplasia | Surgery | Solitary fibrous
tumor |
There were five false-negative cases in 55 malignant
cases. Univariate analysis of the risk factors affecting accuracy
(false-negative rate) did not reveal any significant differences
(all P>0.05) (Table III).
 | Table IIIUnivariate analysis of the risk
factors affecting accuracy (false-negative rate). |
Table III
Univariate analysis of the risk
factors affecting accuracy (false-negative rate).
| False-negative
cases/total (%) | P-value |
|---|
| Gender | | 1.000 |
| Male | 3/31 (9.67) | |
| Female | 2/24 (8.33) | |
| Age, years | | 0.878 |
| ≤50 | 1/14 (7.14) | |
| 51–60 | 1/16 (6.25) | |
| 61–70 | 2/14 (8.33) | |
| >70 | 1/11 (9.09) | |
| Lesion location | | 0.061 |
| Right upper | 0/10 (0.00) | |
| Right middle | 0/2 (0.00) | |
| Right lower | 1/18 (5.56) | |
| Left upper | 0/11 (0.00) | |
| Left lower | 4/14 (28.57) | |
| Lesion thickness,
mm | | 0.144 |
| ≤10 | 2/6 (33.33) | |
| 11–20 | 0/21 (0.00) | |
| 21–30 | 1/6 (16.67) | |
| 31–40 | 1/9 (11.11) | |
| >40 | 1/13 (7.69) | |
| Number of
attempts | | 0.747 |
| ≤2 | 1/9 (11.11) | |
| 3 | 3/40 (7.5) | |
| ≥4 | 1/6 (16.67) | |
| Pleural
effusion | | 0.649 |
| Yes | 1/19 (5.26) | |
| No | 4/36 (11.11) | |
| Contrast
enhancement | | 0.592 |
| Yes | 2/14 (14.29) | |
| No | 3/41 (7.31) | |
Complications
Pneumothorax occurred in six cases (6.5%) in the
group with a pleural thickness of >30 mm; five (5.4%) of the
cases were graded as mild and one case (1.1%) was graded as severe
and required a chest drain. No further treatment was required for
these cases. Mild lung hemorrhage around the entry site occurred in
eight cases (8.7%) subsequent to the CT-CNPB. One patient (1.1%),
who had a history of long-term oral aspirin administration,
suffered from a hemothorax, which required tube thoracostomy. No
cases required blood transfusion and no patients succumbed in the
three days following the procedure. No patients required additional
analgesics due to the pain.
Discussion
Pleural diseases are a frequently occurring medical
problem and the differential diagnosis is wide. Pleural aspiration
is recommended as the first diagnostic procedure in patients with
pleural effusion. This procedure is simple and safe and can often
be performed at the bedside or in the clinic. The success rate of
ultrasound-guided pleural aspirations can be ≤97% (16). For cases in which malignancy is
suspected, fluid should be sent for cytological examination. The
diagnosis of MM, metastasis or benign mesothelial proliferation in
effusion samples is often challenging for medical professionals due
to sampling problems (few malignant cells shedding and hemorrhagic
or inflammatory effusion) and/or errors in interpretation. The
reported sensitivity for the cytological diagnosis of MM ranges
between 31.9 and 86.3% for malignancy without further specification
and between 11.7 and 75.3% for a correct diagnosis of primary
neoplasm (20). A significant
proportion of biopsies may be technically inadequate and there is a
significant false-negative rate of 35–50% (18). Furthermore, a percentage of
false-positive cases still occur, with reactive mesothelial cells
mimicking malignancy (5). In the
present series, 25% of cases had pleural effusion.
The diagnostic yield of unaided (blind) closed
pleural biopsy for pleural malignancy is a relatively modest
<60%. Of note is the fact that the overall diagnostic yield for
malignancy is only increased by 7–27% when compared with pleural
fluid cytology (22). CNPB is a
relative recent technique to be adopted. Image-guided cutting
needle biopsy of mass lesions associated with pleural effusion is a
well-validated modality, producing diagnostic yields higher than
those for closed pleural biopsy. A contrast-enhanced thoracic CT
scan of a patient with a pleural effusion may show focal areas of
abnormal thickening (7). A study
by Maskell et al (12)
found that CT guidance significantly increased the diagnostic yield
with regard to pleural thickening. In their study, CT-CNPB had a
sensitivity of 87%, whereas unaided Abrams needle biopsy had a
sensitivity of 44% (P=0.02) (12).
Furthermore, Adams and Gleeson (24) previously found that CT-guided
biopsies had a sensitivity of 93% for MM. The sensitivity of
diagnostic malignant lesion calculated in the present study is
among the highest of those previously published, and there were no
false-positive cases in the group.
For pleural thickening of ≤5 mm, the sensitivity of
CT-guided needle biopsy is 75%. The frequency of non-diagnostic
biopsies ranges between 0 and 9% (11,25); therefore, if a CT-guided
biopsy is performed in cases with minor pleural thickness, there
may be a lower probability that a sufficient amount of tissue will
be obtained. The rate of non-diagnostic pleural biopsies in the
present series was 0%, and the rate of non-specific histological
diagnosis was 12.0% (11/92). The non-specific cases may have been a
result of inadequate biopsy samples and lesion complexity. In
certain cases, biopsy would be insufficient for the final
diagnosis; these cases would require further clinical and
radiological investigations, as well as follow-up.
CT-guided biopsy may significantly increase the
diagnostic yield while decreasing the risk of complications; the
procedure can be performed in outpatient conditions and can be used
for patients without pleural effusion. Furthermore, CT-CNPB can be
performed in patients with pleural thickening in the absence of
pleural fluid, which is not convenient when using an Abrams needle.
The main complication associated with the procedure is
pneumothorax. Although pneumothorax occurs in ≤15% of patients
undergoing biopsies, very few require intervention. Other
complications may include site pain (1–15%), vasovagal reaction
with potential syncope (1–5%), hemothorax (<2%) and site
hemorrhage with hematoma formation (<1%) (4). In the present series, the
pneumothorax rate was 6.5%, and one patient required a chest drain.
Bleeding occurred in 8.7% of the cases at the time of biopsy,
although no subsequent blood transfusions were necessary. When an
17/18 or 20/21 G needle with a 2-cm throw is utilized to sample
minimal pleural thickening in the absence of a pleural effusion,
the visceral pleura and adjacent lung are likely to be traversed. A
number of the observed pneumothoraces may have been a result of the
introduction of air by the biopsy or drain rather than due to a
direct communication with the airway.
Thoracoscopy has the advantage that it enables
direct visualization of the pleura, although the visceral and
parietal pleura must not be adherent for the technique to be
performed. In a previous study, the CT-guided pleural Abrams needle
biopsy group had a diagnostic sensitivity of 87.5%, whereas the
medical thoracoscopy group had a sensitivity of 94.1% (P=0.252).
Furthermore, CT-guided Abrams needle biopsy had a sensitivity of
95% in cases with pleural thickening ≥1 cm, which was similar to
the sensitivity obtained with thoracoscopy (96%). Thoracoscopy
achieved higher sensitivity in cases with <1 cm thickening (93
vs. 82%, P=0.42). The authors concluded that CT-guided Abrams
needle biopsy should be used as the primary method of diagnosis in
patients with pleural thickening or lesions observed by CT scan,
but suggested that patients with the appearance of only pleural
fluid on the CT scan may still benefit from primary medical
thoracoscopy (14). Medical
thoracoscopy has the advantage that it may additionally be used for
therapeutic purposes, for example for the direct insufflation of
talc in order to achieve pleurodesis and the breakdown of
loculations. Surgical thoracoscopy, requiring complete deflation of
a lung and superior access for therapeutic interventions, is
significantly more invasive and expensive (4).
In conclusion, CT-CNPB is a safe and accurate
diagnostic technique. It is suggested that the present method of
CT-CNPB be used as a first diagnostic evaluation in those cases
with pleural thickness or pleural lesion observed in the thoracic
CT scan.
Acknowledgements
This study was supported by the key foundation of
Hubei Nature Scientific Funds (grant no. 2012FFB04414).
References
|
1
|
Du Rand I and Maskell N: Introduction and
methods: British Thoracic Society Pleural Disease Guideline 2010.
Thorax. 65(Suppl 2): ii1–ii3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koegelenberg CF and Diacon AH: Pleural
controversy: closed needle pleural biopsy or thoracoscopy-which
first? Respirology. 16:738–746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Renshaw AA, Dean BR, Antman KH, Sugarbaker
DJ and Cibas ES: The role of cytologic evaluation of pleural fluid
in the diagnosis of malignant mesothelioma. Chest. 111:106–109.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hooper C, Lee YC and Maskell N: BTS
Pleural Guideline Group: Investigation of a unilateral pleural
effusion in adults: British Thoracic Society pleural disease
guideline 2010. Thorax. 65(Suppl 2): ii4–ii17. 2010. View Article : Google Scholar
|
|
5
|
Fassina A, Fedeli U, Corradin M, Da Frè M
and Fabbris L: Accuracy and reproducibility of pleural effusion
cytology. Leg Med (Tokyo). 10:20–25. 2008. View Article : Google Scholar
|
|
6
|
Tomlinson JR: Invasive procedures in the
diagnosis of pleural disease. Semin Respir Med. 9:30–60. 1987.
View Article : Google Scholar
|
|
7
|
Lee P, Hsu A, Lo C and Colt HG:
Prospective evaluation of flex-rigid pleuroscopy for indeterminate
pleural effusion: Accuracy, safety and outcome. Respirology.
12:881–886. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sconfienza LM, Mauri G, Grossi F, et al:
Pleural and peripheral lung lesions: comparison of US- and
CT-guided biopsy. Radiology. 266:930–935. 2013. View Article : Google Scholar
|
|
9
|
Chira R, Chira A and Mircea PA:
Intrathoracic tumors in contact with the chest wall -
ultrasonographic and computed tomography comparative evaluation.
Med Ultrason. 14:115–119. 2012.PubMed/NCBI
|
|
10
|
Ferretti GR, Busser B, de Fraipont F,
Reymond E, et al: Adequacy of CT-guided biopsies with
histomolecular subtyping of pulmonary adenocarcinomas: influence of
ATS/ERS/IASLC guidelines. Lung Cancer. 82:69–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta S and Madoff DC: Image-guided
percutaneous needle biopsy in cancer diagnosis and staging. Tech
Vasc Interv Radiol. 10:88–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maskell NA, Gleeson FV and Davies RJ:
Standard pleural biopsy versus CT-guided cutting-needle biopsy for
diagnosis of malignant disease in pleural effusions: a randomised
controlled trial. Lancet. 361:1326–1330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benamore RE, Scott K, Richards CJ, et al:
Image-guided pleural biopsy: diagnostic yield and complications.
Clin Radiol. 61:700–705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Metintas M, Ak G, Dundar E, et al: Medical
thoracoscopy vs. CT scan-guided Abrams pleural needle biopsy for
diagnosis of patients with pleural effusions: a randomized,
controlled trial. Chest. 137:1362–1368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheung YC, Chang JW, Hsieh JJ, et al:
Adequacy and complications of computed tomography-guided core
needle biopsy on non-small cell lung cancers for epidermal growth
factors receptor mutations demonstration: 18-gauge or 20-gauge
biopsy needle. Lung Cancer. 67:166–169. 2010. View Article : Google Scholar
|
|
16
|
Solomon SB, Zakowski MF, Pao W, et al:
Core needle lung biopsy specimens: adequacy for EGFR and KRAS
mutational analysis. AJR Am J Roentgenol. 194:266–269. 2010.
View Article : Google Scholar
|
|
17
|
Yeow KM, See LC, Lui KW, et al: Risk
factors for pneumothorax and bleeding after CT-guided percutaneous
coaxial cutting needle biopsy of lung lesions. J Vasc Interv
Radiol. 12:1305–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yeow KM, Su IH, Pan KT, et al: Risk
factors of pneumothorax and bleeding: multivariate analysis of 660
CT-guided coaxial cutting needle lung biopsies. Chest. 126:748–754.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang PC, Kuo SH and Luh KT:
Ultrasonography and ultrasoundguided needle biopsy of chest
diseases: indications, techniques, diagnostic yields and
complications. J Med Ultrasound. 1:53–63. 1993.
|
|
20
|
Di Bonito L, Falconieri G, Colautti I, et
al: Cytopathology of malignant mesothelioma: a study of its
patterns and histological bases. Diagn Cytopathol. 9:25–31. 1993.
View Article : Google Scholar
|
|
21
|
Poe RH, Israel RH, et al: Sensitivity,
specificity and predictive values of closed pleural biopsy. Arch
Int Med. 144:325–328. 1984. View Article : Google Scholar
|
|
22
|
Nance KV, Shermer RW and Askin FB:
Diagnostic efficacy of pleural biopsy as compared with that of
pleural fluid examination. Mod Pathol. 4:320–324. 1991.PubMed/NCBI
|
|
23
|
Adams RF and Gleeson FV: Percutaneous
image-guided cutting needle biopsy of the pleura in the diagnosis
of malignant mesothelioma. Chest. 120:1798–1802. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams RF and Gleeson FV: Percutaneous
image-guided cutting needle biopsy of the pleura in the presence of
a suspected malignant effusion. Radiology. 219:510–514. 2001.
View Article : Google Scholar : PubMed/NCBI
|