Introduction
Chronic obstructive pulmonary disease (COPD), which
is characterized by a partially irreversible and progressive
airflow limitation, is associated with an abnormal inflammatory
response of the lungs to harmful gases, such as cigarette smoke,
and harmful particles (1). COPD
primarily involves the lungs, but it can also cause a systemic
inflammatory response, seriously affecting the patients’ ability to
work and quality of life. COPD is one of the major diseases posing
a threat to public health (2);
however, despite the increasing prevalence of the disease,
treatment with conventional Western medicine is expensive and can
cause serious side effects. Furthermore, the optimal maintenance
therapy has not yet been determined due to conflicting results
regarding the efficacy and safety of the available medications
(3).
Standardized research on the traditional
medicine-based prevention and treatment of complex diseases, such
as COPD, should be considered to provide an alternative option to
Western medicine. Traditional Uyghur Medicine (TUM) has unique
theories relevant to COPD, as well as formulae that may exert
curative effects in patients with the disease.
One basic theory of TUM is that of body fluid
(Hilit); Hilit is regarded as basic matter during physiological
activity, which is produced in the liver as a result of a variety
of foods, and is thought to provide the energy for other organs.
According to the body fluid theory, Hilit includes Savda, Belghem,
Sapra and Kan, which circulate in the body and are believed to
maintain a corresponding balance. It is also believed that an
abnormal change in Hilit leads to disease, a state that is termed
abnormal Hilit; this is divided into abnormal Savda, Belghem, Sapra
and Kan. Amongst them, abnormal Savda is particularly significant
due to its links with diseases, including neoplasm, diabetes
mellitus, hypertension and asthma COPD.
According to TUM theory, abnormal changes in body
fluids, including abnormal Savda, Belghem, Sapra and Kan, are the
common underlying features of complex diseases, particularly
abnormal Savda (4,5). Abnormal Savda syndrome may be the
most common type of syndrome in patients with COPD. The concept of
the syndromes in TUM includes numerous aspects of a disease that
affects multiple organs and systems (4,6)
Using metabolomics as an approach may be beneficial in classifying
the different syndromes and their corresponding metabolic networks.
Therefore, the aim of this study was to conduct a plasma
metabolomic analysis to determine the characteristics of patients
with COPD with abnormal Savda syndrome using nuclear magnetic
resonance (NMR) spectroscopy technology.
Materials and methods
Patient classification
A total of 103 male and female patients with COPD
were enrolled in the study from the Affiliated Chinese Medicine
Hospital of Xinjiang Medical University (Urumqi, China). The
patients exhibited three disease severities, mild (n=15), moderate
(n=38) and severe (n=50), and were aged between 40 and 80 years.
According to TUM theory, the patients were classified into two
groups: Abnormal Savda syndrome (n=48, including 3 mild, 11
moderate and 34 severe) and non-abnormal Savda syndrome (n=55,
including 12 mild, 27 moderate and 16 severe; all the patients in
this group exhibited abnormal Belghem, Sapra and Kan). A total of
52 healthy volunteers were assigned to the control group. The study
protocol was approved by the Ethics Committee of Xinjiang Medical
University, and all subjects provided written informed consent.
Sample preparation
Blood samples (3 ml/individual) from the patients
and healthy volunteers were collected in the morning prior to
breakfast and centrifuged at 3,800 × g for 10 min. Following
separation, the plasma was stored at −80°C. Prior to conducting the
NMR experiments, the samples were thawed at room temperature. Each
sample (200 μl) was then mixed with 400 μl phosphate-buffered
saline and centrifuged at 4°C and 12,000 × g for 10 min. A 550-μl
sample of the supernatant was added to an NMR test tube (5 mm in
diameter) for further analysis.
Acquisition of spectral data
1H NMR spectra from the samples were
recorded on a spectrometer (Inova 600, Varian Medical Systems, Palo
Alto, CA, USA) operated at 599.95 MHz, employing a standard
one-dimensional NMR experiment with the Carr-Purcell-Meiboom-Gill
technique; the relaxation delay was set to 2 sec and the
acquisition time was 1.64 sec. For each spectrum, a total of 128
transients were collected into 32,768 data-points with a spectral
width of 20 ppm at 298 Kelvin. All free induction decays were
multiplied by an exponential function equivalent to a 0.3 Hz
line-broadening factor prior to Fourier transformation. For
assignment purposes, standard two-dimensional (2D) NMR experiments
were conducted on selected samples, including correlation
spectroscopy (COSY), total correlation spectroscopy (TOCSY) and
J-Resolved spectroscopy (J-Res) NMR spectra.
Data processing and analysis
Fourier-transformed 1H NMR spectra were
corrected for phase and baseline distortions. The chemical shifts
of the spectra were referenced to the anomeric proton of the
α-glucose signal at 5.233 ppm, and the range of 8.5-0.5 ppm was
divided into 2,834 integrated areas. The integral values between
5.23 and 4.66 ppm were excluded from the analysis as they contained
water resonances. The spectra were normalized over the total sum of
the remaining spectral area.
Analyses of the integral results were performed with
orthogonal projection to latent structure-discriminate analysis
(OPLS-DA). OPLS-DA, including an orthogonal signal correction (OSC)
in the partial least squares DA, was used for the extraction of
COPD-related biomarkers by removing the influence of systematic
variations not associated with COPD. The OSC was capable of
eliminating the influence of dietary, age, gender and environmental
factors and of decreasing the heterogeneity of samples, a
particularly useful technique for clinical investigations (7,8).
In this study, NMR data were analyzed with OPLS-DA
in accordance with the methods outlined in previous studies
(9,10), and were processed automatically
prior to analysis (unit variance scaling). The formula utilized was
as follows: X × ij = (Xij-Mj)/Sj (Varian units using the Variance
scaling). In this formula, Sj was the variance of variable j. After
the transformation, the proportion of metabolites of the higher
contents was decreased, and the proportion of high and low levels
of metabolites could be discriminated; thus, the low signal levels
of metabolites could be improved due to the equally strong and weak
signal contribution to the model that truly contributed to the
distinction between the groups of metabolites. R2X,
R2Y and Q2 represented the established
quality evaluation indices of the model. For each model, the values
of R2X and R2Y described the variance of X
and Y, respectively, while Q2 represented the
cross-validation parameter and indicated the predictability of the
model. Thus, the authenticity of the results could be determined.
With regard to the cross-validation results, when
Q2>0.4, the prediction results were accepted
(11) and when
Q2>0.9, the prediction results of the model were
considered to be the most reliable (12).
Results
1H NMR spectra of plasma
samples
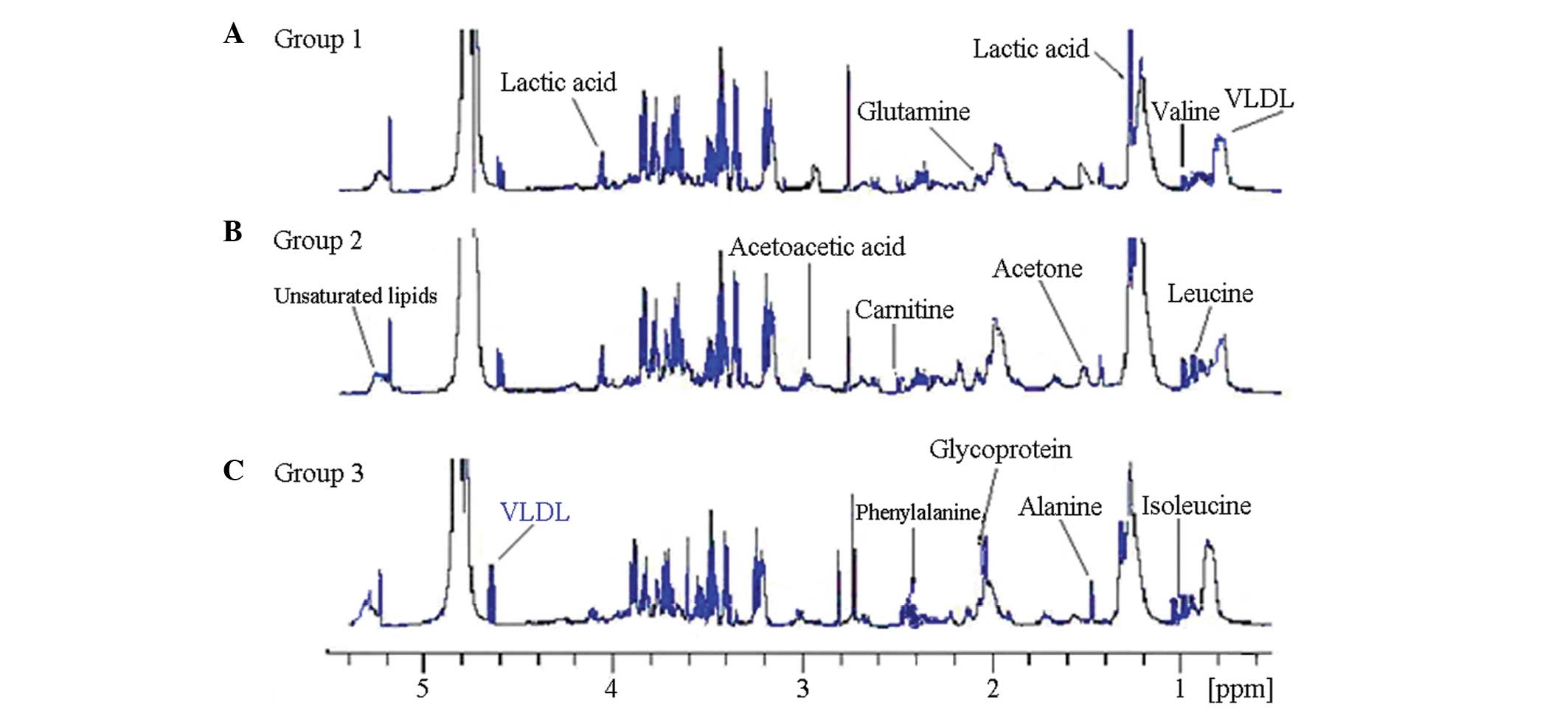
Fig. 1 shows the
original record of the 1H NMR spectra of the plasma from
the patients with COPD and healthy controls. The
metabolomics-related 1H NMR chemical shift spectrum can
be found in the Human Metabolome Project library (http://www.hmdb.ca) and has been presented in previous
studies (13,14). The 1H NMR spectrum can
be identified in numerous endogenous products of the metabolism,
including amino acids and fatty acids. These substances are
involved in various biological processes, such as sugar, lipid and
amino acid metabolism; therefore, the hydrogen spectrum diagram can
be used as a chemical fingerprint spectrum to describe the extent
of the changes in levels of endogenous products of metabolism in
patients with COPD. According to the number of samples, |r|=0.273
was used as the cutoff value of discriminative significance at the
level of P=0.05.
Typical 1H NMR spectra of the plasma from
the patients with COPD and healthy controls are shown in Fig. 1. Resonance assignments were
performed by The Metabolomics Innovation Center (http:/metabolomics.ca) and confirmed with the use of
2D NMR methods, such as COSY, TOCSY and J-Res spectra. The
metabolites confirmed in the spectra of plasma included a number of
amino acids, such as leucine, isoleucine, valine, alanine,
citrulline, tyrosine, histidine and glutamine; a range of sugars,
including α- and β-glucose; lipid metabolites, such as very
low-density lipoprotein (VLDL), low-density lipoprotein (LDL) and
unsaturated lipids; acidic metabolites, such as lactate, formate,
acetate, acetoacetate, pyruvate, malonic acid and
β-hydroxybutyrate; and other metabolites, such as glycoprotein,
myo-inositol, scyllo-inositol, creatine, acetone and carnitine.
OPLS-DA results
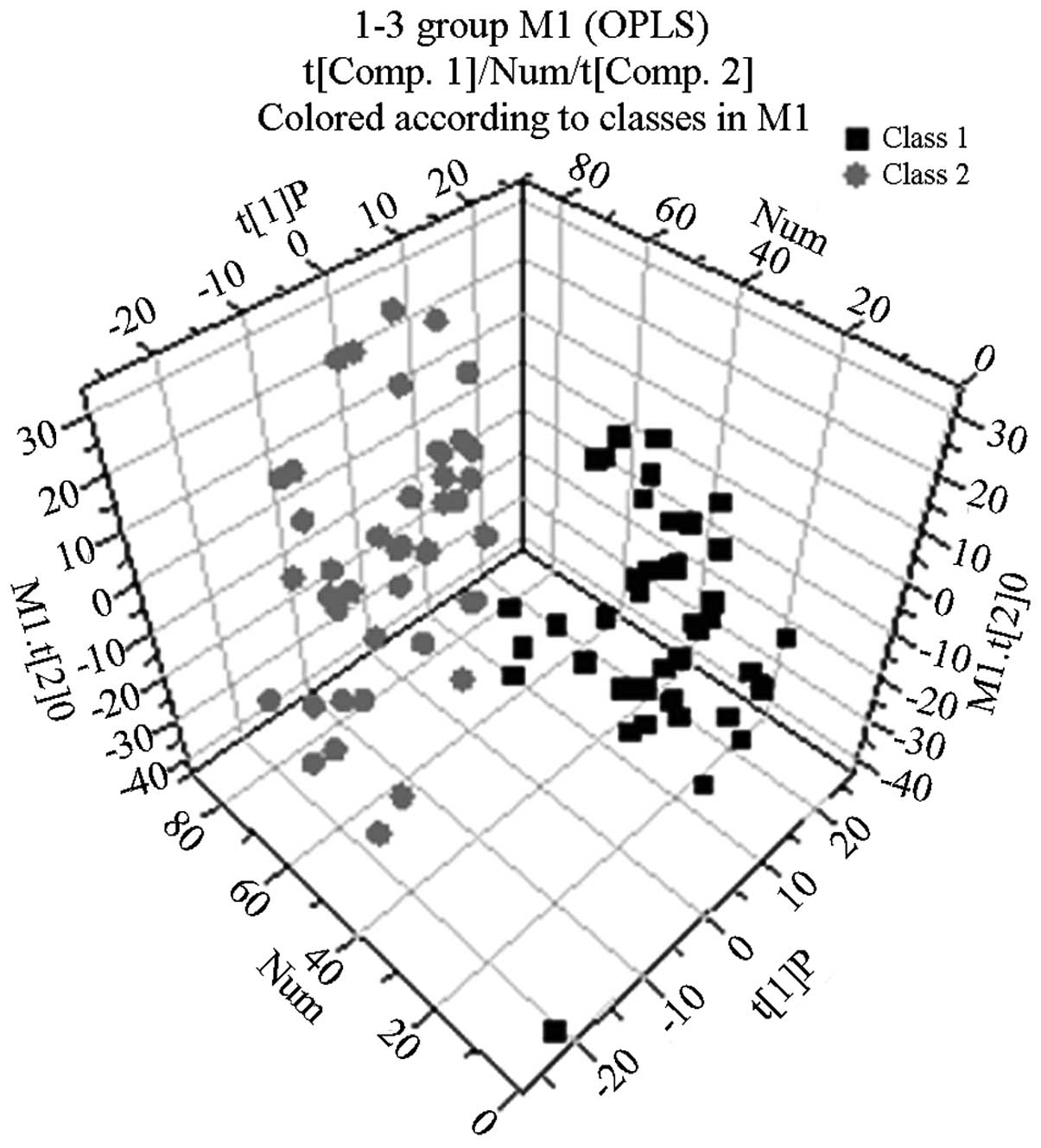
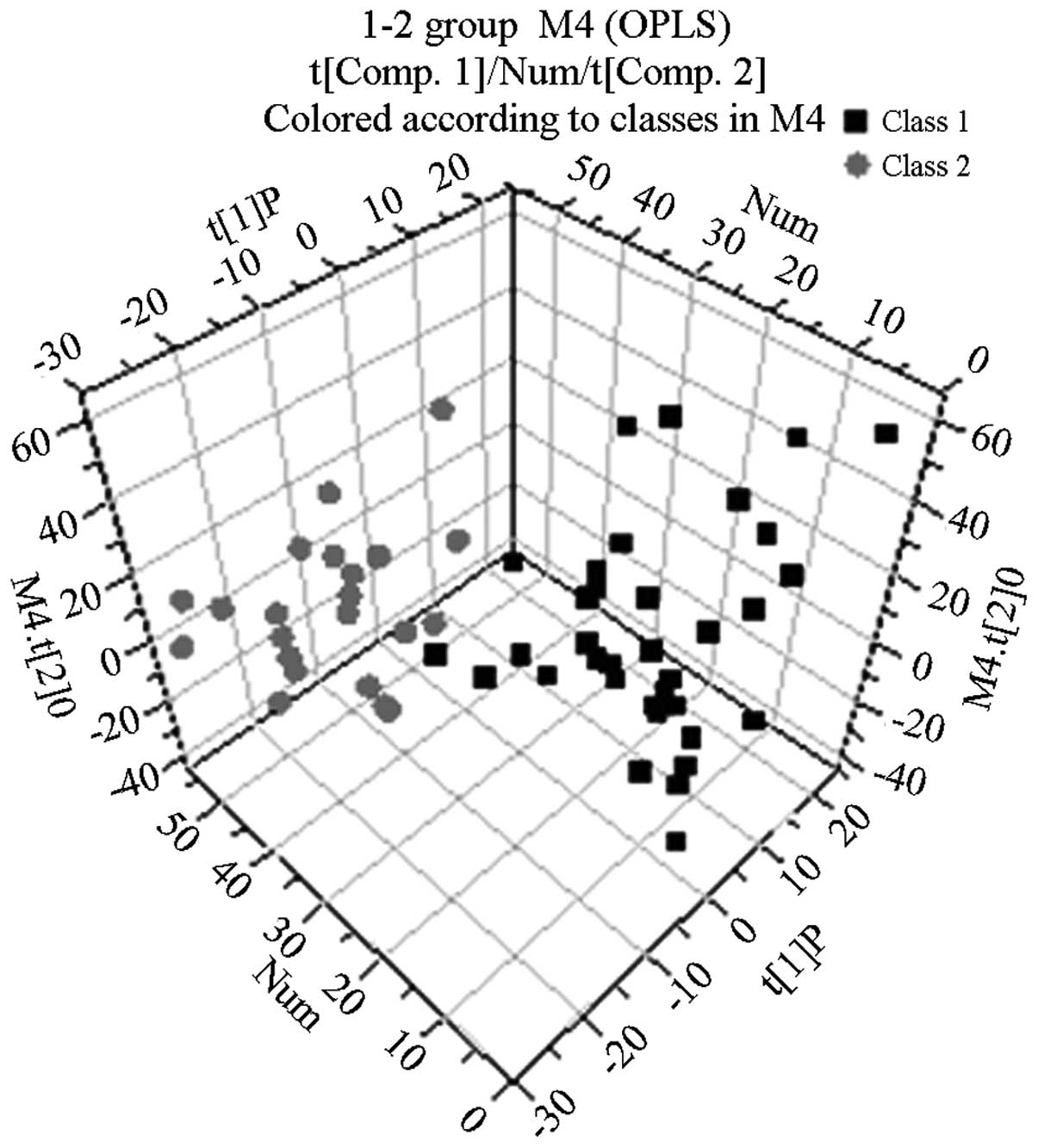
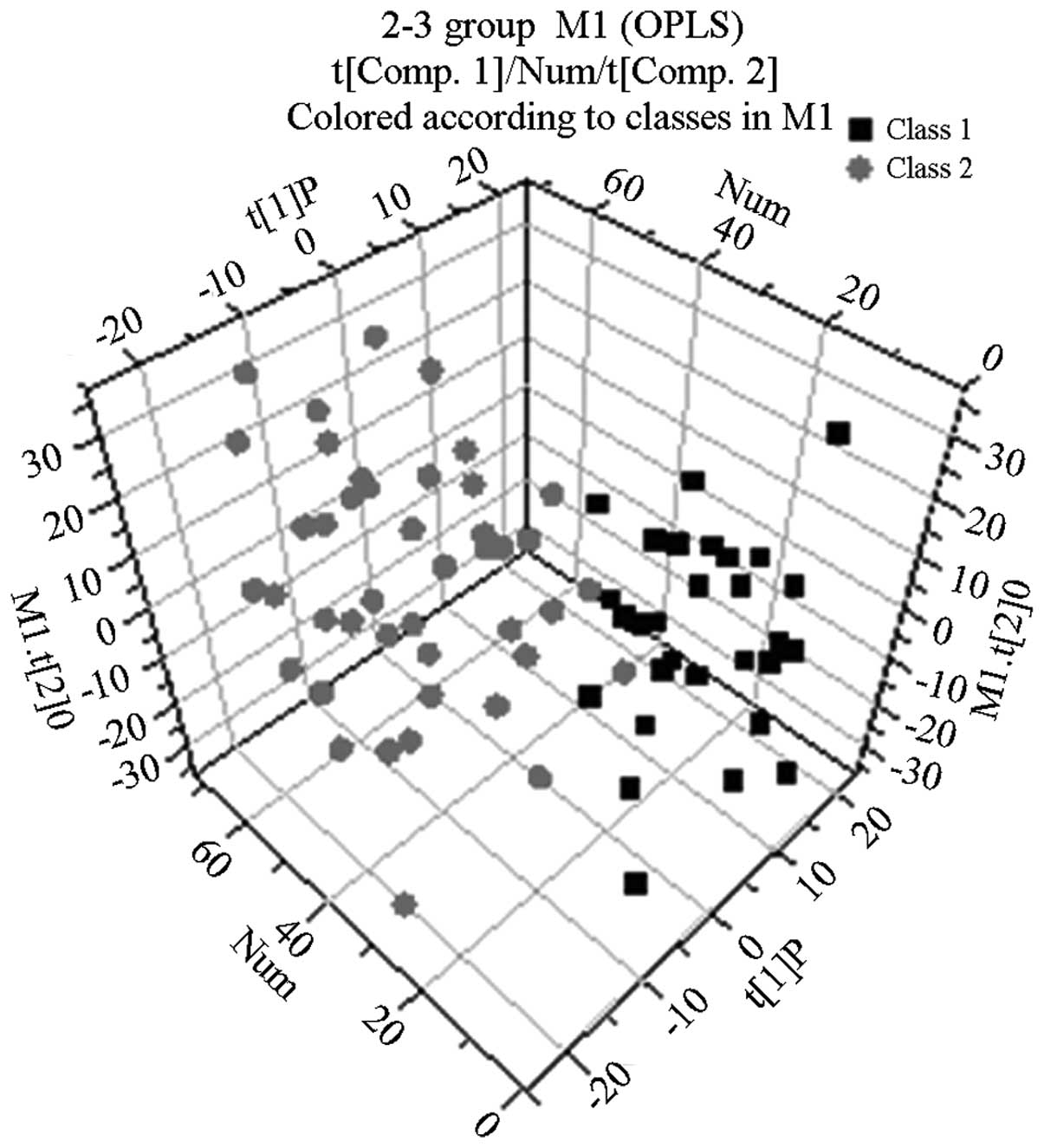
Following the use of OPLS-DA to analyze the
1H NMR spectra integral values, the 3D spatial
distribution was determined (Fig.
2–4). The regional
distributions of the three groups were completely separate, as
demonstrated by comparisons among the groups. In addition, notable
differences were observed among the metabolic components present in
the plasma from the three groups.
By analyzing the 1H NMR spectra and the
2D spectra obtained using the COSY, TOCSY and J-RES spectrum
techniques, the signals belonging to the different plasma metabolic
components in the normal control, abnormal Savda syndrome and
non-abnormal Savda syndrome groups were determined.
Western medicine diagnosis on COPD
cases
General material distribution in COPD
patients
A total of 103 cases of patients were examined.
Among them there were 50 patients with severe COPD (level III),
including 31 males and 19 females with an average age of 68.56 ±
12.451 years; there were also cases with moderate COPD (level II),
including 25 males and 13 females at an average age of 58.727 ±
13.253 years. Finally, there were 15 patients with mild COPD (I
level), including 9 males and 6 females, at an average age of
49.820 ± 14.278 years.
Distribution of patients with TUM
sydrome
A total of 48 patients with abnormal Savda syndrome
were examined. In total, 34 cases had severe COPD (level III), 11
cases had moderate COPD (level III) and 3 cases had mild COPD
(level I). A total of 55 cases with normal Savda syndrome were
examined. Amongst them, there were 16 cases with severe COPD (level
II), 27 cases with moderate COPD (level II) and 12 cases with mild
COPD (level I).
Discussion
According to the TUM theory, patients with COPD can
be classified according to whether they exhibit abnormal Savda
syndrome or non-abnormal Savda syndrome. The main purpose of this
study was to find biomarkers to analyze the characteristic
mechanism of patients with COPD with abnormal Savda syndrome. The
results showed that abnormal Savda syndrome was the predominant
type of syndrome among patients with COPD; increased age, a longer
duration of illness and a higher disease severity were
characteristic of this type of syndrome compared with the
non-abnormal Savda syndrome. This suggests that the appearance of
the abnormal Savda syndrome in patients with COPD may take a longer
time to develop in the course of disease. In addition, the OPLS-DA
showed that the regional distributions of the COPD with abnormal
Savda syndrome, COPD with non-abnormal Savda syndrome and healthy
control groups were completely separate. Furthermore, compared with
the healthy controls, the blood plasma of the patients with COPD
with abnormal Savda syndrome exhibited a lower concentration of
amino acids (including isoleucine, leucine, valine, phenylalanine,
alanine, glutamine and tyrosine), glycoprotein and LDL, but a
higher concentration of unsaturated lipids, lactic acid, carnitine,
acetone and acetoacetic acid (Table
I).
 | Table IChanges in metabolites observed in
plasma obtained from the three groups and the correlation
coefficients. |
Table I
Changes in metabolites observed in
plasma obtained from the three groups and the correlation
coefficients.
| Metabolite | Chemical shift in ppm
(multiplicity) | Assignment | Abnormal Savda
syndrome/Healthy (r) | Non-abnormal Savda
syndrome/Healthy (r) | Abnormal Savda
syndrome/Non-abnormal Savda syndrome (r) |
|---|
| Lipid (VLDL) | 0.85 (m) |
CH3(CH2)n | 0.81 | 0.72 | 0.66 |
| 0.88 (m) |
CH3CH2CH2C | | | |
| Isoleucine | 0.93 (t) | δ-CH3 | 0.80 | 0.68 | |
| 1.00 (d) | β-CH3 | | | |
| 1.96 (m) | β-CH | | | |
| Leucine | 0.95 (d) | δ-CH3 | 0.73 | 0.70 | 0.56 |
| 0.97 (d) | δ-CH3 | | | |
| 1.72 (m) |
β-CH2/γ-CH | | | |
| 3.65 (dd) | α-CH | | | |
| Valine | 0.98 (d) | CH3 | 0.77 | 0.71 | 0.63 |
| 1.03 (d) | CH3 | | | |
| 2.26 (d) | β-CH2 | | | |
| 3.60 (d) | α-CH2 | | | |
| Lactate | 1.33 (d) | CH3 | 0.68 | | −0.48 |
| 4.11 (q) | CH | | | |
| Alanine | 1.47 (d) | CH3 | 0.71 | 0.69 | |
| 3.76 (q) | α-CH | | | |
| Glycoprotein | 2.03 (s) |
NHCO-CH3 | 0.74 | 0.69 | −0.49 |
| Glutamate | 2.13 (m) | half
β-CH2 | 0.84 | 0.85 | 0.65 |
| 2.36 (m) | half
γ-CH2 | | | |
| 3.75 (t) | α-CH | | | |
| Carnitine | 2.22 (s) | CH3 | −0.65 | −0.57 | −0.54 |
| Tyrosine | 2.52 (d) | half
CH2 | 0.79 | 0.69 | 0.60 |
| 2.67 (d) | half
CH2 | | | |
| Phenylalanine | 3.03 (s) | CH3 | 0.78 | | 0.61 |
| 3.93 (s) | CH2 | | | |
| Glutamine | 2.10 (m) | half
β-CH2 | 0.75 | 0.56 | 0.51 |
| 2.14 (m) | half
γ-CH2 | | | |
| Unsaturated
lipid | 5.28 (m) |
CHCH2CH2 | 0.78 | 0.76 | 0.36 |
| 5.30 (m) |
CH=CHCH2CH=CH | | | |
| Acetone | 2.22 (s) |
-CH3 | −0.46 | −0.30 | −0.36 |
| Acetoacetate | 2.27 (s) |
-CH3 | −0.39 | −0.31 | −0.33 |
With the exception of lactic acid and phenylalanine,
the metabolic components in the blood plasma of the patients with
COPD with non-abnormal Savda syndrome differed from those in the
plasma of the healthy controls (Fig.
3 and Table I). The OPLS-DA
showed that the distribution areas of the metabolic components in
the blood plasma of the patients with COPD and those with abnormal
Savda syndrome and non-abnormal Savda syndrome could be
distinguished (Fig 4). Two types
of amino acids, namely alanine and isoleucine, were absent;
however, there were another 13 types of metabolites of which the
content was lower in the group of patients with COPD. Therefore,
the differences of these 13 types of metabolites between the two
groups of patients could be used as specific plasma biomarkers to
identify abnormal Savda syndrome.
The present study analyzed the plasma metabolomic
characteristics of patients with COPD with Uyghur syndrome
(patients with abnormal and non-abnormal Savda syndrome). The blood
plasma of patients with COPD exhibited a low concentration of amino
acids, glycometabolism abnormalities, decreases in metabolites
associated with immune function, including glutamine, and enhanced
fat metabolism; signals for acetoacetic acid and acetone, which are
the products of fatty acid catabolism, known as ketone bodies, were
increased significantly in the plasma of patients with COPD
compared with those in the plasma of healthy controls.
COPD patients with abnormal Savda syndrome rely
mainly on energy from fatty acids. In the present study, such
patients showed the most significant increases in ketone body
acetoacetic acid (acetoacetate) and acetone content in the plasma
(P<0.05). Ketone bodies are produced by the mitochondria in
liver cells from fatty acids; a high content of blood ketone in the
normal body is rare, but levels increase with an increased usage of
fat. Furthermore, the content of carnitine from the fat metabolism
increases accordingly (15).
Amino acids are the basic unit of protein
composition, and the three branched chain amino acids (BCAAs),
valine, leucine and isoleucine, are important and effective
nutrition supplements. Numerous types of amino acid, including
BCAAs, affect protein synthesis and decomposition (14), increase the body’s immune
protection (16) and improve
endurance in sports. Reduced production of these amino acids can
cause fatigue; however, brain serotonin generation can reduce
mental fatigue to help the organism naturally, without any side
effects, thereby enhancing muscle activity or increasing energy,
particularly at the cellular level, and reducing the
characteristics of diseases, such as heart failure or chronic lung
disease. COPD primarily affects the lungs, but the systemic
inflammatory response, muscle malnutrition, languid performance and
emaciation are associated with the various types of amino acid
deficiency, including BCAA deficiency. In addition, the energy
consumption associated with patients with COPD is enhanced,
requiring an increase in the energy produced from fat
metabolism.
In the present study, the blood plasma of patients
with COPD exhibited significantly reduced lipoprotein and
unsaturated lipid concentrations (P<0.01). We speculate that in
patients with COPD with reduced pulmonary ventilation function
there is an increased demand for energy metabolism; therefore, the
catabolism of the energy-giving nutrients, carbohydrate, protein
and fat, is increased as a compensatory mechanism. As a result, the
carbohydrate, protein and fat resources are consumed at a higher
rate, reducing the amino acid and fat content in the plasma. In
order for the consumption of carbohydrate, protein and fat in the
body to be increased, the metabolite content of plasma amino acids
and lipids was significantly decreased in patients with COPD. A
lack of protein may lead to a reduced synthesis of apolipoprotein
and a reduction in the secretion of triglycerides into the blood
following synthesis in liver cells. This may ultimately lead to a
reduced fat metabolism.
Persistent COPD airway inflammation leads to smooth
muscle cell proliferation, airway wall thickening and remodeling,
airway mucosa congestion and edema, airway obstruction and
respiratory muscle load increase. In contrast to skeletal muscle,
respiratory muscle gains approximately half of its energy from
sugar metabolism, while the other half is predominantly from lipid
metabolism with fatty acids (17,18).
In cases of severe COPD with abnormal Savda syndrome, the patients’
ability to breathe is poor, oxygen is limited, the aerobic
oxidation pathway is restrained and the demand of the respiratory
muscles for energy is increased. The supply of energy to the
respiratory muscle either relies on glycolysis, which is low in
efficiency and produces a large amount of lactic acid, which leads
to a significant increase in the lactic acid (lactate) levels, or
on energy from fatty acids. This study showed that the
concentration of ketone bodies, such as acetoacetic acid
(acetoacetate) and acetone, was increased significantly in the
plasma of patients with COPD with abnormal Savda syndrome compared
with that in the plasma from healthy controls (P<0.05). Ketone
bodies are a type of metabolite that are catabolized from fatty
acids in the mitochondria in liver cells; the concentration of
blood ketone bodies in healthy individuals is low, and only
increases naturally if the amount of fat utilized increases
(18). The concentration of
carnitine, which is associated with fat metabolism, was also
increased.
It was also observed in this study that, in the
plasma of patients with COPD with abnormal Savda syndrome, the
concentration of other metabolites, such as glycoprotein, was lower
than that in the plasma of patients with COPD with non-abnormal
Savda syndrome and that in the plasma of healthy subjects. Certain
types of plasma immune globulins belonging to the glycoprotein
family exhibit efficacy in blood coagulation and antibody activity.
Another important function of glycoprotein is a direct or indirect
involvement in cell surface recognition, in order to protect the
integrity of epithelial cells and increase the total number of
lymphocytes to boost the function of the immune system (19). While the injury to the lung and
airway epithelial cell integrity of patients with COPD with
abnormal Savda syndrome is particularly serious, decreased immune
function leads to the repeated aggravation of the illness.
COPD with abnormal Savda syndrome is the most
serious syndrome of TUM, with persisting airway inflammation,
irreversible airflow obstruction and an existing systemic
inflammatory response, which results in the consumption of body
energy and malnutrition. This leads to disordered energy
metabolism, including the metabolism of amino acids, protein and
fat. The pathological mechanism affects numerous parts of the body
and multiple organs or organ systems and causes a loss of balance;
thus, a vicious circle exists between diseases and disorders of
overall function.
In conclusion, through metabolomic study, the
specific metabolic markers reflecting the pathophysiological
changes in patients with COPD with abnormal Savda syndrome were
determined. The results of this study may enrich the theory of TUM
and provide an objective view of the syndromes associated with TUM,
thus enhancing their significance. When lesions are of the TUM
abnormal Savda Syndrome type, targeted metabolomics can be
performed with effective drug intervention targets, and an
intervention with traditional medicine in advance may ultimately
provide a good platform for improving the treatment of COPD and
other complex diseases.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81060281).
References
|
1
|
The Chinese medical association
respiratory neurology, chronic obstructive pulmonary disease
committee. Chronic obstructive pulmonary disease diagnosis and
treatment guideline (2007 revision). The tuberculosis and
respiratory journal. 30:8–17. 2007.
|
|
2
|
Barnes N, Calverley PM, Kaplan A and Rabe
KF: Chronic obstructive pulmonary disease and exacerbations:
patient insights from the global Hidden Depths of COPD survey. BMC
Pulm Med. 13:542013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fabbri LM, Calverley PM, Izquierdo-Alonso
JL, et al; M2-127 and M2-128 study groups. Roflumilast in
moderate-to-severe chronic obstructive pulmonary disease treated
with longacting bronchodilators: two randomised clinical trials.
Lancet. 374:695–703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tohti K, Yusup A and Upur H: Comparative
study of normal Hilit and abnormal Hilit described in traditional
Uyghur medicine. J Med Pharm Chin Minorit. 3:3–4. 2004.(In
Chinese).
|
|
5
|
Yusup A, Li L, Upur H, Upur T, Hasan H and
Yusup B: Classify abnormal body fluid in Uyghur medicine and
relationship with oxidation and antioxidation system. Chin J Basic
Med Tradit Chin Med. 10:61–62. 2004.(In Chinese).
|
|
6
|
Lindon JC, Holmes E and Nicholson JK:
Pattern recognition methods and applications in biomedical magnetic
resonance. Prog Nucl Magn Reson Spectrosc. 39:1–40. 2001.
View Article : Google Scholar
|
|
7
|
Yin P, Mohemaiti P, Chen J, et al: Serum
metabolic profiling of abnormal savda by liquid chromatography/mass
spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci.
871:322–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gavaghan CL, Wilson ID and Nicholson JK:
Physiological variation in metabolic phenotyping and functional
genomic studies: use of orthogonal signal correction and PLS-DA.
FEBS Lett. 530:191–196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trygg J and Wold S: Orthogonal projections
to latent structures (O-PLS). J Chemom. 16:119–128. 2002.
View Article : Google Scholar
|
|
10
|
Cloarec O, Dumas ME, Trygg J, et al:
Evaluation of the orthogonal projection on latent structure model
limitations caused by chemical shift variability and improved
visualization of biomarker changes in 1H NMR
spectroscopic metabonomic studies. Anal Chem. 77:517–526. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eriksson L, Johansson E, Kettaneh-Wold N
and Wold S: Multi- and Megavariate Data Analysis: Principles and
Applications. Umetrics AB; Umeå: 2001
|
|
12
|
Lauridsen M, Hansen SH, Jaroszewski JW and
Cornett C: Human urine as test material in 1H NMR-based
metabonomics: recommendations for sample preparation and storage.
Anal Chem. 79:1181–1186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ott KH and Aranibar N: Nuclearmagnetic
resonance metabonomics: methods fordrug discovery and development.
Methods Mol Biol. 358:247–271. 2007. View Article : Google Scholar
|
|
14
|
Gu J, Li N, Wu GH, Xie M and Liu FN:
Metabolic effects of administration of branched chain amino acids
on postoperative patients. Parenteral and Enteral Nutrition.
11:93–96. 2004.(In Chinese).
|
|
15
|
Zhou AiRu and Cga Xi Liang: Lipid
Metabolism. Biochemistry. 6. People’s medical publishing house;
Beijing: pp. 558–559. 2004
|
|
16
|
Davis JM, Welsh RS, De Volve KL and
Alderson NA: Effects of branched-chain amino acids and carbohydrate
of fatigue during intermittent, high-intensity running. Int J
Sports Med. 20:309–314. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qian YC: Breathing treatment of the
foundation and clinical. Beijing: People’s Medical Publishing
House, Beijing; pp. 9–92. 2003
|
|
18
|
Zhou AR and Cga XL: Biochemistry. Beijing:
People’s Medical Publishing House, Beijing; pp. 558–559. 2004
|
|
19
|
Han Yifei, Xu Shiqing, Zhu Jiang, Shen
Weide and Zheng Biping: Structure and function of glycoprotein.
Journal of Biology. 2:42–43. 2001.
|