Introduction
In general, all chemotherapeutic agents are toxic
for healthy cells, and lower the quality of life due to harmful
side-effects. Furthermore, they induce cellular oxidative stress
(1). Following cancer
chemotherapy, DNA oxidation and lipid peroxidation levels have been
observed to be markedly increased in cancer patients (2). One of the clinical approaches for
reducing the oxidative stress and harmful side-effects in
chemotherapy is to use antioxidant vitamins and ROS scavengers such
as vitamins A, C and E (2–5). Vitamin E derivatives, such as
tocopherols (α, β, γ and δ) and tocotrienols (α, β, γ and δ)
protect the cell membrane against oxidative stress and are used as
adjuvants in cancer treatment. According to Rama and Prasad
(6), high levels of α-tocopherol
and γ-tocopherol in the blood decrease the metastasis risk of
glioma in cancer patients. These substances play important roles in
signal transduction and the regulation of gene expression (7).
Certain epidemiological and clinical studies have
revealed that non-steroidal anti-inflammatory drugs (NSAIDs), which
are used mainly in the treatment of autoimmune diseases, also have
the potential to be used in cancer therapy (8–10).
Indomethacin is a strong NSAID derived from
indolacetic acid. It has demonstrated antiproliferative effects on
colon and breast cancers (11,12).
Similar effects on glioma cells have also been reported (13). It also specifically increases the
efficacy of two chemotherapeutics used in cancer treatment, namely
doxorubicin and vincristine, in T98G human malignant glioma cells
(14). Its antiproliferative and
apoptosis-inducing effects are dependent on the treatment dose and
time. The effects of other types of NSAIDs, such as ibuprofen,
aspirin and naproxen, have also been investigated on glioma cell
lines and some promising results have been obtained (13,15–17).
A common pharmacological property of NSAIDs is the
inhibition of cyclooxygenases (COX1 and COX2). The expression of
COX2 is known to increase markedly in cancer, and its activity is
associated with the histological grade of the tumor and metastasis
of the cancer (18–22). NSAIDs are able to prevent cancer
progression by the inhibition of COXs (23,24).
However, the antiproliferative effect may be independent from the
inhibition of the enzyme (25).
This type of drug may have certain roles in the induction of
apoptosis, control of cell proliferation, invasion and inhibition
of angiogenesis (26); however,
the molecular mechanisms are not fully understood.
In the present study, the aim was to investigate the
effects of α-TOS and indomethacin on oxidative stress parameters,
by determining the intracellular oxidation level, molecular damage
of proteins and lipids, and COX enzyme activity, in order to
predict the possible effects of α-TOS in glioma treatment and/or
prevention.
Materials and methods
Chemicals
α-tocopheryl succinate (α-TOS), indomethacin,
phosphate-buffered saline (PBS) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Dulbecco’s modified Eagle’s medium (DMEM)/Nutrient Mixture F-12 HAM
(F12 HAM) was purchased from Thermo Fischer Scientific Inc.
(Waltham, MA, USA). Fetal bovine serum (FBS) was purchased from
Gibco Life Technologies (Carlsbad, CA, USA). Antibiotic-antimicotic
solution was purchased from Wisent Bioproducts (Quebec, Canada).
Prestained protein molecular weight marker was obtained from
Fermentas (Thermo Fisher Scientific).
Maintenance of the cell line
Rat glioma cells (C6 line) were obtained from
Cerrahpaşa Faculty of Medicine, Histology and Embryology Section
(Istanbul, Turkey) and cultured in the laboratory. The cells were
maintained in DMEM/F12 HAM containing 10% FBS, streptomycin (100
U/ml), penicillin (100 μg/ml) and amphotericin B (0.25 μg/ml) and
were grown in an incubator (Heraeus, Thermo Fisher Scientific) at
37°C with 5% CO2. Stock solutions of α-TOS (25 mM in
ethanol) and indomethacin (100 mM in DMSO) were diluted to the
appropriate concentrations with DMEM/F12 HAM medium. Different
concentrations of α-TOS (10, 25, 50, 100 and 200 μM) and
indomethacin (50, 100, 200, 400, 500 and 600 μM) were tested in
order to determine the CD50 value of each test
material.
Treatment of cells with test
materials
The cultures were started in 96-well microplates
with a cell number of 1×105, with 200 μl cell suspension
contained in each well. The culture media were removed from the
plate at the end of the 24-h incubation period. The experimental
groups were treated with α-TOS (10 μM), indomethacin (200 μM) and a
combined mixture containing 10 μM α-TOS plus 200 μM indomethacin,
whereas the control groups was incubated only with DMEM/F12 HAM,
and the cells were maintained at 37°C and humidified with 5%
CO2 for 48 h.
Cytotoxicity tests
Cytotoxic concentrations were determined by a
preliminary cytotoxicity test. The cell numbers were determined
using the MTT assay (27) with
minor modifications. Briefly, the culture media were removed at the
end of 48-h incubation period. Then, 35 μl MTT solution prepared in
PBS was added to each well and the microplates were incubated for 4
h. Following the incubation period, 200 μl DMSO was added to each
well in order to solubilize the formazan crystals. After 15 min,
the absorbance was measured at 570 and 690 nm (reference) using a
microplate reader (μQuant, Bio-Tek Instruments, Inc., Winooski, VT,
USA). The cell viability (%) of each group was calculated from the
following formula: Cell viability (%) =
(Ae/Ac) × 100, where Ae is the
absorbance of the experimental group and Ac is the
absorbance of the control group
Intracellular oxidation level
The intracellular oxidation level was determined on
the basis of spectrophotometric measurement of the fluorescent
product formed by the oxidation of 2′,7′-dichlorofluorescein
diacetate (DCF-DA) (28) When
DCF-DA is normally introduced to the cell, it is reduced by the
cleavage action of esterases. The reduced form (DCF-H) is then
reoxidized to DCF by intracellular reactive oxygen species (ROS)
and fluorescence is increased.
The cells (105 cells) were firstly seeded
onto a 96-well microplate. After incubation at 37°C under 5%
CO2 for 24 h, the experimental groups were treated with
α-TOS and/or indomethacin and incubated for a further 48 h. At the
end of a total 72-h incubation period, the media were excluded and
the wells were washed with PBS. A 200 μl addition of 5 μM DCF-DA
solution was made to each well, and after 15 min the relative
fluorescence was measured using a spectrofluorometer (FLx800;
Bio-Tek), at the excitation and emission wavelengths 485 nm and 530
nm, respectively. Relative fluorescence (F) per minute was
equalized based on the cell number in each well and the
intracellular oxidation level was calculated using the following
equation: Relative intracellular oxidation level (fluorescence % of
control) = (Fe/Fc) × 100, where Fe is the fluorescence in the
experimental group and Fc is the fluoresceence in the control
group.
Lipid peroxidation level
Thiobarbituric acid reactive substances (TBARS) as
the end products of lipid peroxidation were detected by a
spectrophotometric method (29).
Control and experimental cells were transferred to a glass tube,
containing 1 ml trichloroacetic acid (20%) and 0.8% (w/v)
thiobarbituric acid, and boiled for 45 min. The samples were then
cooled to room temperature and centrifuged at 3,000 × g for 5 min.
The absorbance of the supernatant was measured at 535 nm.
Malondialdehyde (MDA) with an extinction coefficient of
1.56×105 M−1cm−1 at 535 nm, was
used as a reference TBARS. The lipid peroxidation level in each
sample was expressed as μM MDA equivalent/mg protein. The protein
concentration of the samples was measured by bicinchoninic acid
(BCA) assay as described previously (30).
Protein carbonyls
OxyBlot kit (Chemicon; EMD Millipore, Billerica, MA,
USA) was used to detect carbonyl groups in oxidatively modified
proteins, as described by the manufacturer. Briefly,
dinitrophenylhydrazine (DNPH) derivatization of 15 μg protein was
carried out at room temperature for 15 min. Derivatized samples
were then separated with 10% SDS-PAGE and transferred onto a
nitrocellulose membrane (31). Red
Ponceau S was used to determine the protein load following the
transfer. The proteins on the membrane were probed with primary
antibody, specific to DNP moieties of the proteins, followed by
horseradish peroxidase-conjugated secondary antibody. Immunoblots
were visualized using an Amersham ECL-Plus Western Blotting
Detection system (GE Healthcare, Chalfont, UK) with an exposure
time 2 min. A second gel containing duplicate samples was run and
stained with Coomassie blue staining (32). Results were qualitatively evaluated
using a prestained protein molecular weight marker.
COX enzyme activity
COX enzyme activity was determined by measuring the
MDA equivalent TBARS produced in the reaction mixture, as
previously described (33).
The reaction mixture contained 100 mM Tris-HCl, pH
8.0, 5 mM reduced glutathione (GSH), 5 μM hemoglobin and soluble
proteins extracted from the cells. Arachidonic acid at a final
concentration of 0.5 mM was then added to the reaction mixture.
Following incubation at 27°C for 1 min, the reaction was stopped by
the addition of 0.2 ml 100% (w/v) trichloroacetic acid (prepared in
1 M HCl) and keeping the mixture in a boiling water bath for 20
min. The samples were centrifuged (1,000 × g, 5 min) and the
absorbance of the supernatant was measured at 532 nm.
The COX activity was expressed in units of nmol MDA
equivalent/min, using the molar extinction coefficient of MDA
(1.56×105 M−1cm−1).
Statistical analyses
All experiments were carried out at least three
times in triplicate and data were analyzed by unpaired analysis of
variance (ANOVA) using the Graph Pad Prism software package,
version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Cytotoxicity
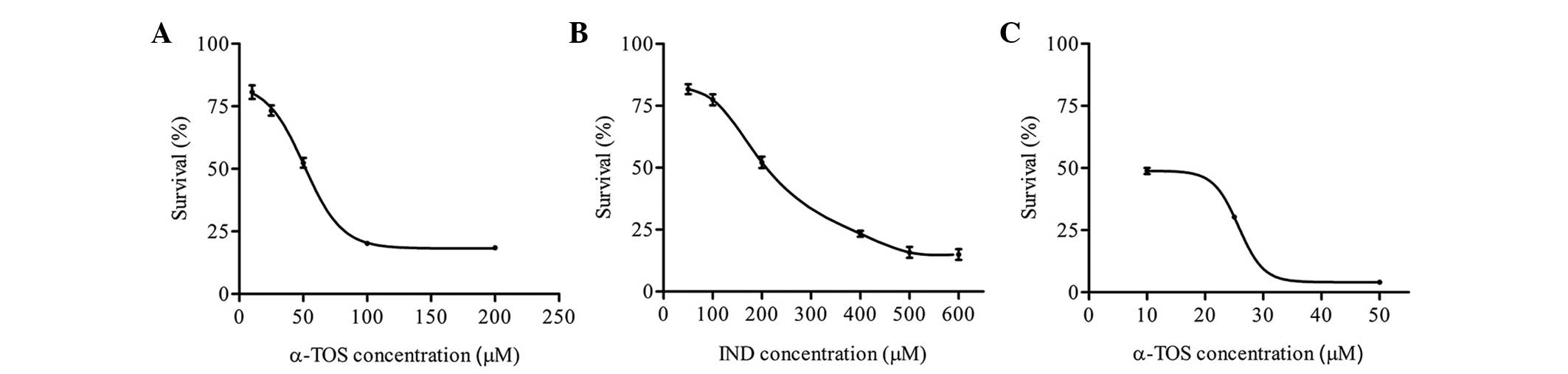
The CD50 values were found to be 50 μM
for α-TOS and 200 μM for indomethacin (Fig. 1A and B). When a combination
containing 50 μM α-TOS and 200 μM indomethacin was applied, the
viability of the cells was only 4% (data not shown). Thus,
different concentrations of α-TOS were added to 200 μM IND and the
cell viability was evaluated. A combination of 10 μM α-TOS and 200
μM indomethacin was used in further experiments, as this
combination caused ~50% survival of the cells.
Oxidative stress parameters
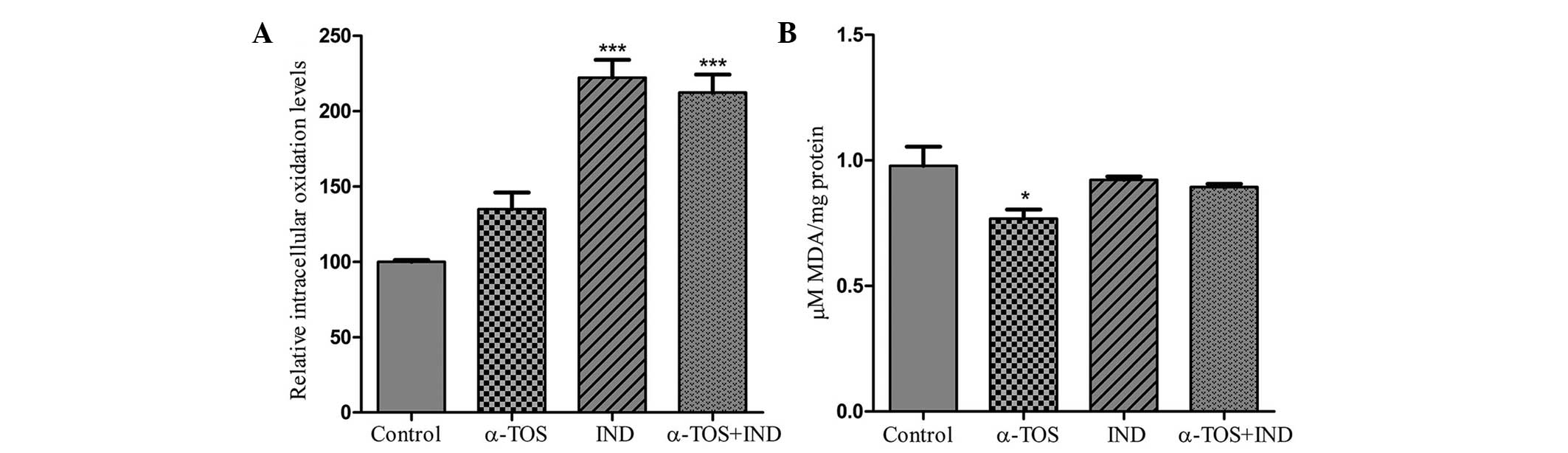
The levels of ROS were increased by 34.6, 122.2 and
112.5%, by the treatment of the cells with α-TOS, IND and α-TOS +
IND, respectively (Fig. 2A).
Treatment with 200 μM indomethacin alone or in combination with
α-TOS (10 μM) induced intracellular oxidation by at least
three-fold more than treatment with α-TOS alone.
The level of TBARS generated as a result of lipid
peroxidation was 0.978±0.133 μM MDA/mg protein in control cells.
Following treatment with 10 μM α-TOS, lipid peroxidation was found
to decrease to 0.768±0.0626 μM MDA/mg protein (Fig. 2B), while in the IND and α-TOS + IND
groups, the TBARS level was almost the same as that in the control
group, at IND 0.922±0.0241 and 0.894±0.0215 μM MDA/mg protein,
respectively.
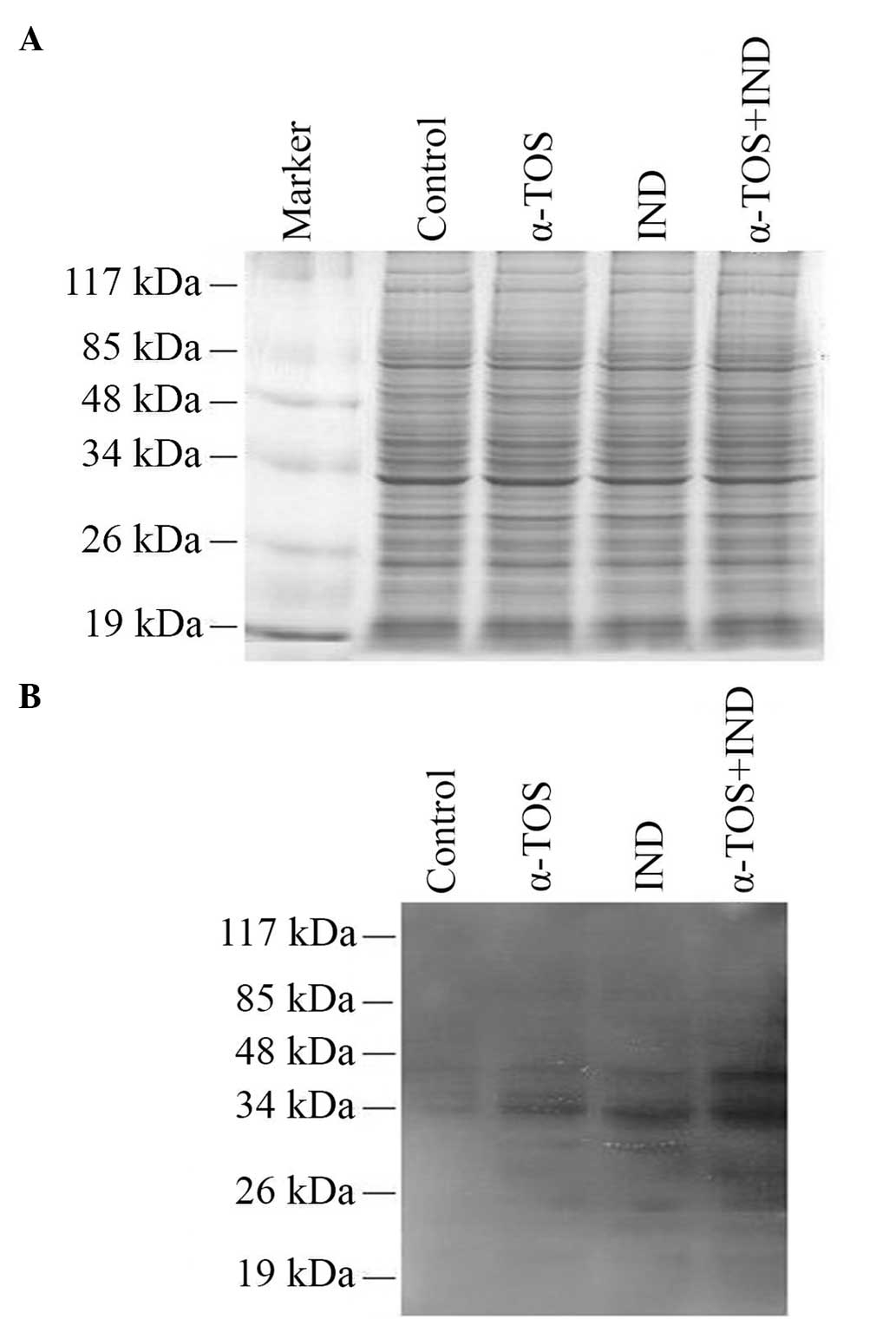
Protein damage was evaluated by the analysis of
carbonylated proteins on a nitrocellulose membrane by western
blotting. All three treatments induced protein carbonylation
(Fig. 3B). At least 10
polypeptides were detected as carbonylated in all samples. The most
intense carbonyl bands were observed for the α-TOS + IND group.
COX enzyme activity
The COX enzyme activities were measured, as it is
known that IND acts as an inhibitor of this enzyme. IND treatment
lowered the enzyme activity by 39.17%. Notably, 10 μM α-TOS also
decreased COX activity by 22.7%. Thus α-TOS appeared to inhibit COX
(Table I). The combination of
α-TOS + IND reduced the COX activity by 46.39% compared with that
in the control.
 | Table ICyclooxgenase activity in C6 glioma
cells. |
Table I
Cyclooxgenase activity in C6 glioma
cells.
| Group | Cyclooxgenase
activity (nmol MDA equivalent/min) |
|---|
| Control | 97±6.76 |
| α-TOS | 75±4.82 |
| IND | 59±3.56 |
| α-TOS + IND | 52±4.16 |
Discussion
The main problems in the treatment of brain cancers
by chemotherapy, radiotherapy and surgery are the side-effects,
such as pain, vomiting and damage to healthy cells and tissues that
results in several complications. Due to the feelings of sadness,
fear, anxiety and anger that patients experience, and as a result
of potential for mortality, patients often turn to complementary
and alternative therapies (2).
NSAIDs have been found to inhibit the progression and invasion of
various glioma tumors (17,34)
Indomethacin is an NSAID that is able to pass through the
blood-brain barrier and inhibit COX enzymes irreversibly (35). Thus, indomethacin has been proposed
as a potential drug for use in glioma therapy (14).
The administration of antineoplastic agents during
cancer chemotherapy results in a much greater degree of oxidative
stress than is induced by the cancer itself (36). The high level of oxidative stress
during chemotherapy may be overcome by the body’s oxidative defense
systems, using antioxidants specialized mainly to reduce lipid
peroxidation. In addition, complementary nutritional therapy with
antioxidants such as vitamin E (mixed tocopherols and
tocotrienols), β-carotene (natural mixed carotenoids), vitamin C
(ascorbic acid) and vitamin A (retinoic acid) during chemotherapy
may inhibit the effect of oxidative stress on healthy cells and
tissues, and the development of multidrug resistance in cancer
cells (1). Antioxidant supplements
are also a common choice for patients who try complementary and
alternative methods in addition to conventional therapies. While it
is accepted that antioxidants are useful in the reduction of the
adverse effects of chemotherapy, the prevailing opinion is that
they may reduce the effectiveness of chemotherapy (37,38).
Vitamin E is a remarkable supplement for cancer
therapy (2), due to its potential
effect in the reduction of oxidative stress during chemotherapy
(39). However, its effects on
drug metabolism as well as on the stress response of cancer cells
are not yet fully documented.
In the present study, the aim was to elucidate the
effects of α-TOS, a water-soluble vitamin E derivative, on glioma
cells treated with indomethacin, a potential chemotherapeutic
agent. It was found that 50% of C6 glioma cells survived in the
presence of 50 μM α-TOS or 200 μM indomethacin in the medium. α-TOS
was also implemented in combination with indomethacin. In the
combination, the concentration of the components was selected as 10
μM for α-TOS and 200 μM for indomethacin. The antiproliferative
effect of NSAIDs may also be explained by the direct inhibition of
COX-2 (40). However, this
mechanism is not eligible for C6 glioma cells, since these cells
are not able to express COX-2 (41). Therefore, the data obtained in the
present study may be associated with COX-1 inhibition by
indomethacin.
At the end of the 48-h implementation period, the
oxidative stress as well as molecular damage that occurred in the
cells were analyzed by measuring the intracellular oxidation level,
lipid peroxidation and protein carbonyls. In addition, COX activity
was measured in order to determine the degree of inhibition of this
enzyme by α-TOS, alone or along with indomethacin.
In this study, all treatments induced intracellular
ROS production (Fig. 2A). Although
α-TOS has been reported to be a strong antioxidant (42), a pro-oxidant effect of α-TOS was
exhibited for the concentration that was used in this study.
Similar results have been reported in murine melanoma, malignant
mesothelioma and breast cancer cells (22,43,44).
Indomethacin and α-TOS induced ROS production in the reaction
system of the present study. ROS production by indomethacin is well
documented in gastric mucosal cells (45,46);
however, to the best of our knowledge, the present study is the
first to report on indomethacin-induced ROS production in C6 glioma
cells. Although, ROS generation was detected in the cells treated
with α-TOS and/or indomethacin, lipid peroxidation was not induced.
Moreover, lipid damage was reduced in the α-TOS group compared with
that in the control (Fig. 2B).
Similar results were obtained in a previous study in which a murine
melanoma cell line was treated with 0.15 μM indomethacin plus 1–10
μg/ml α-TOS (43). This protective
effect may result from an inhibitory effect of α-TOS on lipid
peroxidation (7).
The oxidative modifications of proteins
(carbonylation) may lead to a loss of specific function (47,48)
and contribute to neuronal cell injury and death. Accordingly, the
levels of protein carbonyls following α-TOS and/or indomethacin
treatment were also measured in the present study. The induction of
protein carbonylation by all treatments indicates that ROS produced
by α-TOS and indomethacin interacted with cellular proteins.
The present study briefly demonstrates that α-TOS
and/or indomethacin induce protein damage, but inhibit lipid
peroxidation in C6 glioma cells. In particular, indomethacin
induces the generation of ROS and causes molecular damage while
exhibiting its effects. The results of the study indicate that
α-TOS does not have a negative effect on the action of
indomethacin. Further studies are required with animal models to
observe the efficacy of α-TOS more clearly. These results, in
combination with the documented literature, may be useful in the
development of new glioma therapies.
Acknowledgements
This study was supported by the Istanbul University
Research Foundation (Project No: 4120 and Project No: 2675).
References
|
1
|
Block IK, Koch CA, Mead NM, et al: Impact
of antioxidant supplementation on chemotherapeutic efficacy: A
systematic review of the evidence from randomized controlled
trials. Cancer Treat Rev. 33:407–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Drisko AJ, Chapman J and Hunter JV: The
use of antioxidant therapies during chemotherapy. Gynecol Oncol.
88:434–439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teicher BA, Schwartz JL, Holden SA, Ara G
and Northey D: In vivo modulation of several anticancer agents by
β-carotene. Cancer Chemoth Pharmacol. 34:235–241. 1994. View Article : Google Scholar
|
|
4
|
Mackerras D, Irwig L, Simpson JM, et al:
Randomized double-blind trial of beta-carotene and vitamin C in
women with minor cervical abnormalities. Brit J Cancer.
79:1448–1453. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamson DW and Brignall MS: Antioxidants
and cancer therapy II: quick reference guide. Altern Med Rev.
5:152–163. 2000.PubMed/NCBI
|
|
6
|
Rama B and Prasad KN: Study on the
specificity of α-tocopheryl (vitamin E) acid succinate effects on
the melanoma, glioma and neuroblastoma cells in culture. Proc Soc
Exp Biol Med. 174:302–307. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azzi A, Gysin R, Kempná P, et al: The role
of α-tocopherol in preventing disease: from epidemiology to
molecular events. Mol Aspects Med. 24:325–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiff SJ, Koutsos MI, Qiao L and Rigas B:
Nonsteroidal antiinflammatory drugs inhibit the proliferation of
colon adenocarcinoma cells: effects on cell cycle and apoptosis.
Exp Cell Res. 222:179–188. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith ML, Hawcroft G and Hull MA: The
effect of non-steroidal anti-inflammatory drugs on human colorectal
cancer cells: evidence of different mechanisms of action. Eur J
Cancer. 36:664–674. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cuzick J, Otto F, Baron JA, et al: Aspirin
and non-steroidal anti-inflammatory drugs for cancer prevention: an
international consensus statement. Lancet Oncol. 10:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta RA, Tan J, Krause WF, et al:
Prostacyclin-mediated activation of peroxisome peroxiome
proliferator-activated receptor delta in colorectal cancer. Proc
Natl Acad Sci USA. 97:13275–13280. 2000. View Article : Google Scholar
|
|
12
|
Yasumaru M, Tsuji S, Tsujii M, et al:
Inhibition of angiotensin II activity enhanced the antitumor effect
of cyclooxygenase-2 inhibitors via insulin-like growth factor I
receptor pathway. Cancer Res. 63:6726–6734. 2003.PubMed/NCBI
|
|
13
|
Bernardi A, Jacques-Silva MC,
Delgado-Cañedo A, Lenz G and Battastini AMO: Nonsteroidal
anti-inflammatory drugs inhibit the growth of C6 and U138-MG glioma
cell lines. Eur J Pharmacol. 532:214–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amin R, Kamitani H, Sultana H, et al:
Aspirin and indomethacin exhibit antiproliferative effects and
induce apoptosis in T98G human glioblastoma cells. Neurol Res.
25:370–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holt S and Fowler CJ: Anandamide
metabolism by fatty acid amide hydrolase in intact C6 glioma cells.
Increased sensitivity to inhibition by ibuprofen and flurbiprofen
upon reduction of extra but not intracellular pH. Naunyn
Schmiedebergs Arch Pharmacol. 367:237–244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ribeiro G, Benadiba M, Colquhoun A and
Silva DO: Diruthenium (II, III) complexes of ibuprofen, aspirin,
naproxen and indomethacin non-steroidal anti-inflammatory drugs:
Synthesis, characterization and their effects on tumor-cell
proliferation. Polyhedron. 27:1131–1137. 2008. View Article : Google Scholar
|
|
17
|
Lee BS, Nalla AK, Stock IR, et al:
Oxidative stimuli-responsive nanodrug of camptothecin kills
gliobalstoma cells. Bioorg Med Chem Lett. 20:5262–5268. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shono T, Tofilon PJ, Bruner JM, Owolabi O
and Lang FF: Cyclooxygenase-2 expression in human gliomas:
prognostic significance and molecular correlations. Cancer Res.
61:4375–4381. 2001.PubMed/NCBI
|
|
19
|
Kürzel F, Hagel Ch, Zapf S, et al:
Cyclo-oxygenase inhibitors and thromboxane synthase inhibitors
differentially regulate migration arrest, growth inhibition and
apoptosis in human glioma cells. Acta Neurochir (Wien). 144:71–87.
2002. View Article : Google Scholar
|
|
20
|
Nathoo N, Barnett GH and Golubic M: The
eicosanoid cascade: possible role in gliomas and meningiomas. J
Clin Pathol. 57:6–13. 2004. View Article : Google Scholar
|
|
21
|
New P: Cyclooxygenase in the treatment of
glioma: its complex role in signal transduction. Cancer Control.
11:152–164. 2004.PubMed/NCBI
|
|
22
|
Wang M, Yoshida D, Liu S and Teramoto A:
Inhibition of cell invasion by indomethacin on glioma cell lines:
in vitro study. J Neurooncol. 72:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grubbs CJ, Lubet RA, Koki AT, et al:
Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced
urinary bladder cancers in male B6D2F1 mice and female Fischer-344
rats. Cancer Res. 60:5599–5602. 2000.PubMed/NCBI
|
|
24
|
Williams CS, Watson AJM, Sheng H, et al:
Celecoxib prevents tumor growth in vivo without toxicity to normal
gut: lack of correlation between in vitro and in vivo models.
Cancer Res. 60:6045–6051. 2000.PubMed/NCBI
|
|
25
|
Baek JS, Wilson CL, Lee CH and Eling TE:
Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs):
inhibition of cyclooxygenase and induction of NSAID-activated gene.
J Pharmocol Exp Ther. 301:1126–1131. 2002. View Article : Google Scholar
|
|
26
|
Shiff SJ, Shivaprasad P and Santini DL:
Cyclooxygenase inhibitors: drugs for cancer prevention. Curr Opin
Pharmacol. 3:352–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival application to proliferation and
cytotoxicity assay. J Immunol Methods. 65:551983. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Negre-Salvayre A, Augé N, Duval C, et al:
Detection of intracellular reactive oxygen species in cultured
cells using fluorescent probes. Method Enzymol. 352:62–71. 2002.
View Article : Google Scholar
|
|
29
|
Draper HH and Hadley M: Malondialdehyde
determination as an index of lipid peroxidation. Methods Enzymol.
186:421–431. 1990. View Article : Google Scholar
|
|
30
|
Stoscheck CM: Quantitation of protein.
Methods Enzymol. 182:50–69. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gravel P: Protein Blotting by the Semidry
method. The Protein Protocols Handbook. 2nd edition. Smith Bryan
John: Humana Press; Totowa, NJ: pp. 321–334. 2002, View Article : Google Scholar
|
|
32
|
Walker JM: Quantification of Proteins on
Polyacrylamide Gels. The Protein Protocols Handbook. 2nd edition.
Smith Bryan John: Humana Press; Totowa, NJ: pp. 237–243. 2002
|
|
33
|
Flower RJ, Cheung HS and Cushman DW:
Quantitative determination of prostaglandins and malondialdehyde
formed by the arachidonate oxygenase (prostaglandin synthetase)
system of bovine seminal vesicle. Prostaglandins. 4:325–341. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sivak-Sears NR, Schwartzbaum JA, Miike R,
Moghadassi M and Wrensch M: Case-control study of use of
nonsteroidal antiinflammatory drugs and glioblastoma multiforme. Am
J Epidemiol. 159:1131–1139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Courad JP, Besse D, Delchambre C, et al:
Acetaminophen distribution in the rat central nervous system. Life
Sci. 69:1455–1464. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rao P and Knaus EE: Evolution of
nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX)
inhibition and beyond. J Pharm Pharm Sci. 11:81s–110s. 2008.
|
|
37
|
Conklin KA: Dietary antioxidants during
cancer chemotherapy: impact on chemotherapeutic effectiveness and
development of side effects. Nutr Cancer. 37:1–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weijl NI, Cleton FJ and Osanto S: Free
radicals and antioxidants in chemotherapy-induced toxicity. Cancer
Treat Rev. 23:209–240. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Salganik IR, Albright DC, Rodgers J, et
al: Dietary antioxidant depletion: enhancement of tumor apoptosis
and inhibition of brain tumor growth in transgenic mice.
Carcinogenesis. 21:909–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ristimäki A, Sivula A, Lundin J, et al:
Prognostic significance of elevated cyclooxygenase-2 expression in
breast cancer. Cancer Res. 62:632–635. 2002.PubMed/NCBI
|
|
41
|
Ishibashi M, Bottone FG Jr, Taniura S, et
al: The cyclooxygenase inhibitor indomethacin modulates gene
expression and represses the extracellular matrix protein laminin
gamma1 in human glioblastoma cells. Exp Cell Res. 302:244–252.
2005. View Article : Google Scholar
|
|
42
|
Constantinou C, Papas A and Constantinou
AI: Vitamin E and cancer: An insight into the anticancer activities
of vitamin E isomers and analogs. Int J Cancer. 123:739–752. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ottino P and Duncan JR: Effect of vitamin
E succinate on free radical formation, lipid peroxidation levels
and cyclooxygenase activity in murine melanoma cells. Nutr Res.
17:661–676. 1997. View Article : Google Scholar
|
|
44
|
Stapelberg M, Gellert N, Swettenham E, et
al: Alpha-tocopheryl succinate inhibits malignant mesothelioma by
disrupting the fibroblast growth factor autocrine loop: mechanism
and the role of oxidative stress. J Biol Chem. 280:25369–25376.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Naito Y and Yoshikawa T: Oxidative stress
involvement and gene expression in indomethacin-induced
gastropathy. Redox Rep. 11:243–253. 2006. View Article : Google Scholar
|
|
46
|
Maity P, Bindu S, Dey S, et al:
Indomethacin, a non-steroidal anti-inflammatory grug, develops
gastropathy by inducing reactive oxygen species-mediated
mitochondrial pathology and associated apoptosis in gastric mucosa.
J Biol Chem. 284:3058–3068. 2009. View Article : Google Scholar
|
|
47
|
O’Callaghan JP: Neurotypic and gliotypic
proteins as biochemical markers of neurotoxicity. Neurotoxicol
Teratol. 10:445–452. 1988. View Article : Google Scholar
|
|
48
|
Hrkal Z: On the way to functional
genomics. Sb Lek. 104:217–222. 2003.PubMed/NCBI
|