Introduction
Sepsis is a serious health risk, which may
ultimately lead to the failure of multiple organs, including the
liver, lungs, cardiovascular system, kidneys and gastrointestinal
tract (1–3). In animal models, vascular
hyperactivity to vasoconstrictor agents, induced by bacterial
lipopolysaccharide (LPS), is an important factor contributing to
the eventual circulatory failure (4).
Curcumin, which is derived from the rhizome of the
Curcuma longa (turmeric) plant, has been widely used in
South East Asia as a food component, but also in the treatment of
numerous diseases (5,6). Curcumin has been shown to possesses
anti-inflammatory and antioxidative properties (7). In addition, curcumin has been
reported to inhibit LPS-induced endothelium barrier disruption by
increasing endothelium barrier integrity and inhibiting
LPS-mediated nuclear factor (NF)-κB activation and tumor necrosis
factor (TNF)-α production (8).
However, whether curcumin is able to protect against LPS-induced
vascular hypoactivity has not been fully investigated.
Thrombospondin (TSP)-1 is found in platelet
β-granules and is produced by a number of cell types, including
endothelial cells and vascular smooth muscle cells (9). It has been reported that the
expression of TSP-1 is increased on the platelet surface in
patients suffering from sepsis, and that polymorphisms of the TSP-1
gene are associated with the progression of sepsis-related organ
failure (10). Transforming growth
factor (TGF)-β1 is a cytokine that plays a pivotal role in the
regulation of the immune/inflammatory response and subsequent
tissue repair processes. TGF-β1 has been associated with a number
of human diseases; for example, functioning as a promoter of
fibrosis (11). Numerous studies
have shown that TSP-1 is responsible for a significant proportion
of TGF-β1 activation in a variety of organs, including the lungs,
pancreas, kidneys, liver and heart (12,13).
However, the exact roles of TSP-1 and TGF-β1 in sepsis-associated
vasoconstrictive dysfunction remain unknown. In addition, whether
TSP-1 and TGF-β1 are involved in the protective effect of curcumin
on LPS-induced vasoconstrictive dysfunction is not clear. In the
present study, a LPS-induced sepsis rat model was used to
investigate the association between the protective effects of
curcumin and the alterations in TSP-1 and TGF-β1 expression.
Materials and methods
Reagents
LPS (from Escherichia coli 055:B5), curcumin,
phenylephrine (PE) and acetylcholine (ACh) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). DMSO was purchased from
Jingchun Biological Technology Co., Ltd. (Shanghai, China). TSP-1
and TGF-β1 antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). A rat E-selectin enzyme-linked
immunosorbent assay (ELISA) kit was purchased from Shanghai Yuanye
Biological Technology Co., Ltd. (Shanghai, China). Krebs-Henseleit
(KH) solution compositions were as follows (in mmol/l): NaCl, 120;
NaHCO3, 25; MgSO4, 1.2; CaCl2,
1.25; KCl, 4.5; KH2PO4, 1.2; and glucose,
11.1 (pH 7.4).
Animal preparation
A total of 60 male Sprague-Dawley rats (weight,
220–250 g; age, 40–50 days) were obtained from the Experimental
Animal Center of Zhejiang Academy of Medical Sciences (Zhejiang,
China), and cared for in compliance with the Guide for the Care and
Use of Laboratory Animals (National Institutes of Health, Bethesda,
MA, USA; 14). All experimental protocols were approved by the
Ethics Committee on Animal Experimentation of Zhejiang Academy of
Medical Sciences. The rats were randomly divided into six groups,
including the control, LPS [intraperitoneal (i.p.) injection of 5
mg/kg LPS in sterile saline], LPS + curcumin [i.p. injection of 5
mg/kg LPS and 5, 10 or 20 mg/kg curcumin in 10% dimethyl sulfoxide
(DMSO)] and curcumin groups (i.p. injection of 20 mg/kg curcumin in
10% DMSO). The control and LPS groups also received an equal amount
of 10% DMSO.
Preparation of aortic rings and
measurement of vasoconstriction
Aortic rings were prepared using the method
described by Fang et al (7). Briefly, 24 h after the i.p. injection
of the respective reagents, all the rats were anesthetized with 3%
pentobarbital sodium (60 mg/kg). Then thoracic cavity was opened
and the thoracic aorta was surgically removed. The thoracic aorta
was cut into 2–3-mm rings, and mounted in an organ bath system
(Nanjing MedEase Technology Co., Ltd., Nanjing, China) containing
KH solution (95% O2 and 5% CO2, 37°C).
Isometric tension was recorded using a data-acquisition system
(Nanjing MedEase Technology Co., Ltd.). After 60 min of
equilibration at a passive tension of 2 g, the rings were treated
three times with KCl (60 mmol/l) to evoke maximal excitability. The
integrity of the vascular endothelium was examined by adding PE
(10−6 mol/l) to the organ bath, followed by the addition
of ACh (10−5 mol/l). Only tissues that responded to ACh
with a >70% reduction of PE-induced vasoconstriction were
considered to have an intact endothelium. After washing, PE
(10−8-3×10−6 mol/l)-induced vasoconstriction
was recorded. Contractile responses were expressed as the
percentage of the maximum response induced by KCl initially
(15). The maximum effect
(Emax) and the concentration eliciting 50% of
Emax (EC50) were obtained from the cumulative
concentration-response curve of PE. Subsequently, pD2
was calculated as −logEC50.
Measurement of serum E-selectin
levels
Peripheral rat blood was collected in a tube with
coagulant, and centrifuged at 2,200 × g for 10 min at 4°C. Serum
supernatant was stored at −80°C until required for analysis. The
levels of E-selectin in the serum samples were quantitated using an
ELISA, according to the manufacturer’s instructions. E-selectin was
determined spectrophotometrically by Tecan Infinite M200 (Tecan
Group Ltd., Mannedorf, Switzerland) at an absorbance wavelength of
450 nm.
Immunohistochemistry and histology
Experiments were performed on slices from 10%
formalin-fixed tissue embedded in paraffin. Slices were incubated
with commercial primary antibodies against TSP-1 (mouse IgG; 1:200;
cat. no. sc-59887; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
or TGF-β1 (rabbit IgG; 1:200; cat. no. sc-146; Santa Cruz
Biotechnology, Inc.) overnight at 4°C, followed by a horseradish
peroxidase-conjugated secondary antibody (1:500; goat anti-mouse
IgG, cat. no. sc-2005 or; goat anti-rabbit IgG, cat. no. sc-2004;
Santa Cruz Biotechnology, Inc.) at room temperature. Peroxidase
activity was visualized with 3,3′-diaminobenzidine and the slices
were subsequently counterstained with hematoxylin. Each slice was
observed in ten randomly selected fields, and the percentage of
TSP-1-positive or TGF-β1-positive cells in the total cells was
calculated. Hematoxylin and eosin (HE) staining was also performed
for morphometric analysis.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean, and were analyzed by two-way analysis of variance (ANOVA)
with a Bonferroni post hoc test or one-way ANOVA with a
Newman-Keuls post hoc test, using Prism 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
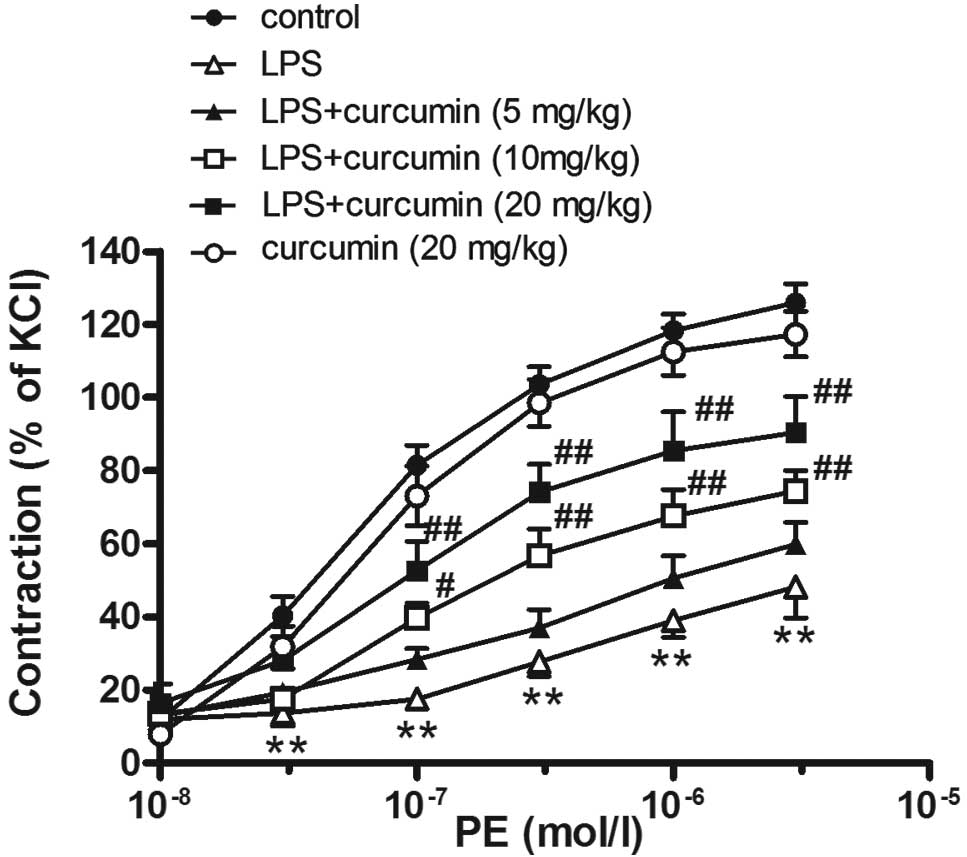
Effects of LPS on PE-induced
vasoconstriction in aortic rings
Compared with the control group, the injection of
LPS decreased the PE-induced vasoconstriction in the thoracic
aortic rings (LPS group: Emax, 54.3±0.8% and
pD2, 6.29±0.29; vs. control group: Emax,
126.0±0.5% and pD2 7.29±0.10; P<0.01; Fig. 1).
Effects of curcumin on LPS-induced
contractile dysfunction in aortic rings
Curcumin (10 or 20 mg/kg) was shown to partly
reverse LPS-induced contractile dysfunction (Emax,
76.4±4.8 and 93.0±6.9%, respectively; pD2, 6.92±0.18 and
7.05±0.23%, respectively; P<0.05). However, a low concentration
of curcumin (5 mg/kg) had no effect on PE-induced vasoconstriction
in sepsis rats. Furthermore, curcumin (20 mg/kg) alone had no
effect on PE-induced vasoconstriction in the aortic rings
(Emax, 119.8±4.7%; pD2, 7.25±0.12; P>0.05,
vs. control group; Fig. 1).
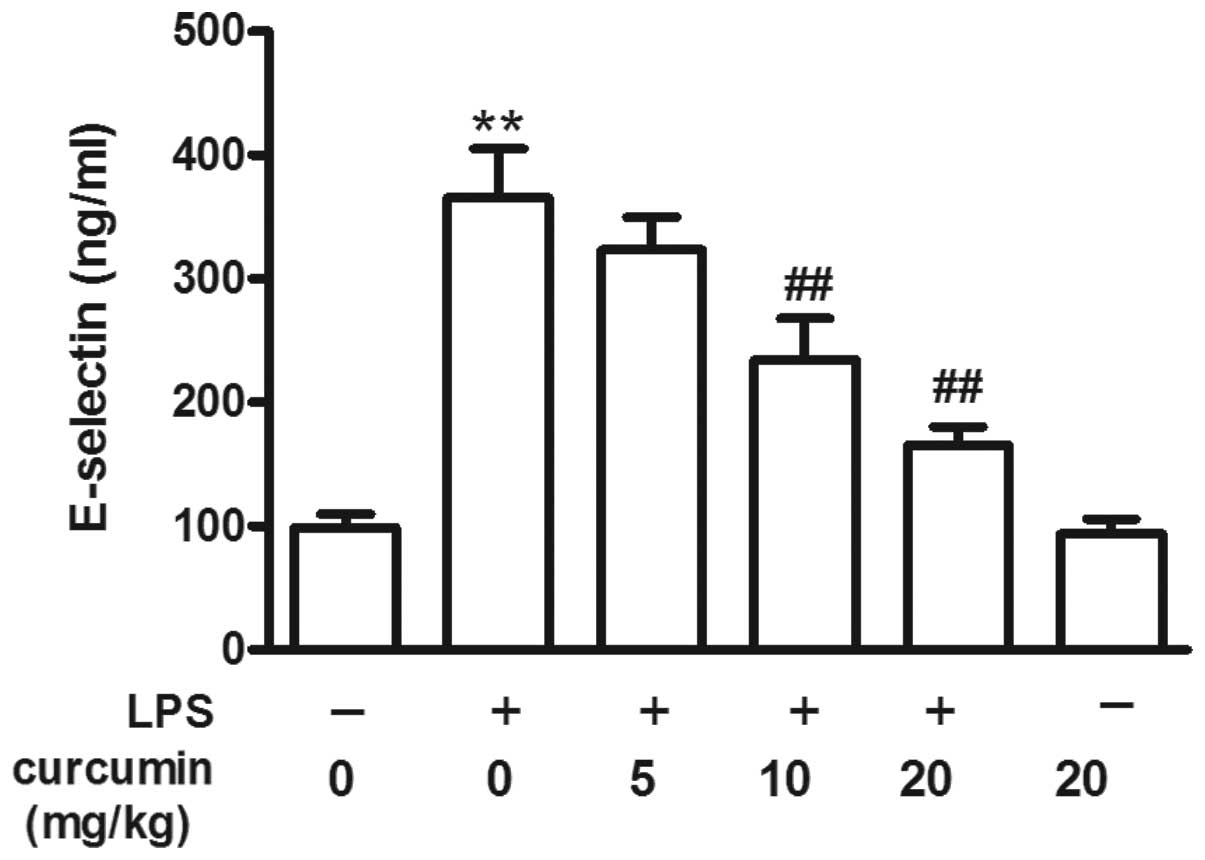
Effects of curcumin on the LPS-induced
increase in E-selectin levels
Compared with the control group, LPS increased the
serum E-selectin levels (P<0.01; Fig. 2). However, curcumin (10 or 20
mg/kg) prevented the LPS-induced increase in serum E-selectin
levels, although low concentrations of curcumin (5 mg/kg) had no
protective effect. Curcumin (20 mg/kg) alone had no effect on serum
E-selectin levels when compared with the control group (P>0.05;
Fig. 2).
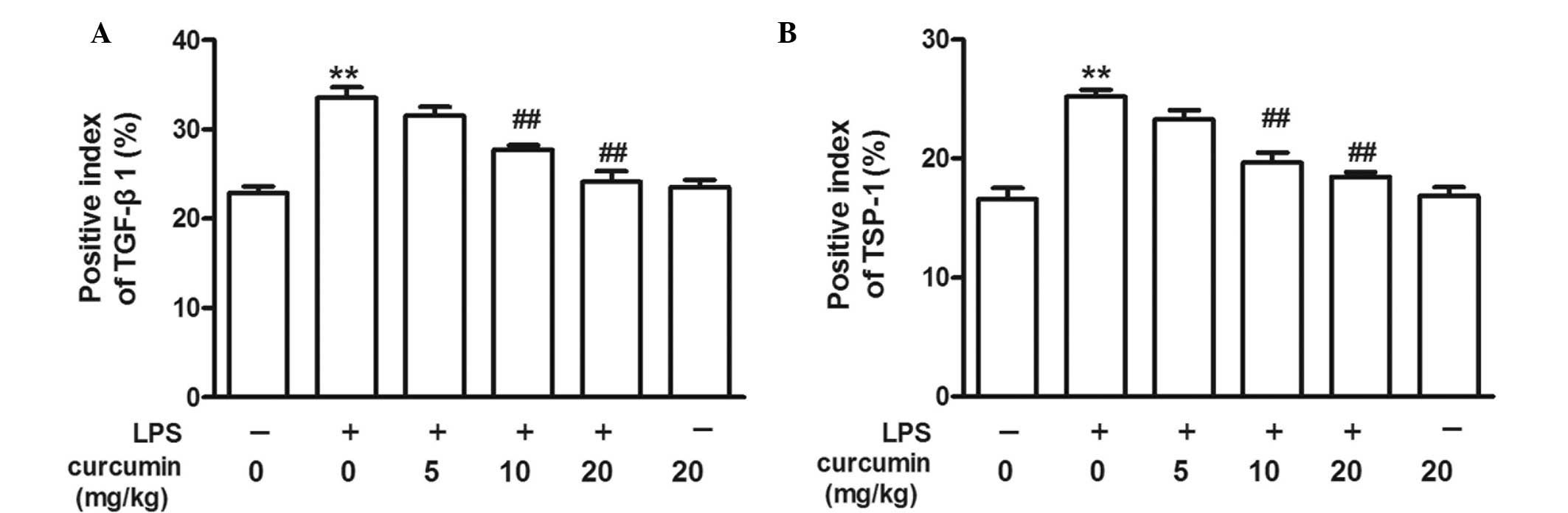
Effects of curcumin on LPS-induced TSP-1
and TGF-β1 overexpression
TSP-1 and TGF-β1 immunoreactivity were observed as a
granular immunostaining pattern in the vascular tissues. In the
control group, TSP-1- and TGF-β1-positive cells were observed in
the vascular endothelium and subendothelial layer, but not in the
smooth muscle cells. LPS increased the percentage of TSP-1- and
TGF-β1-positive cells in the vascular endothelium and
subendothelial layer, and positive cells were also observed in the
smooth muscle cells. Thus, treatment with curcumin (10 or 20 mg/kg)
inhibited LPS-induced TSP-1 and TGF-β1 overexpression (Figs. 3–5).
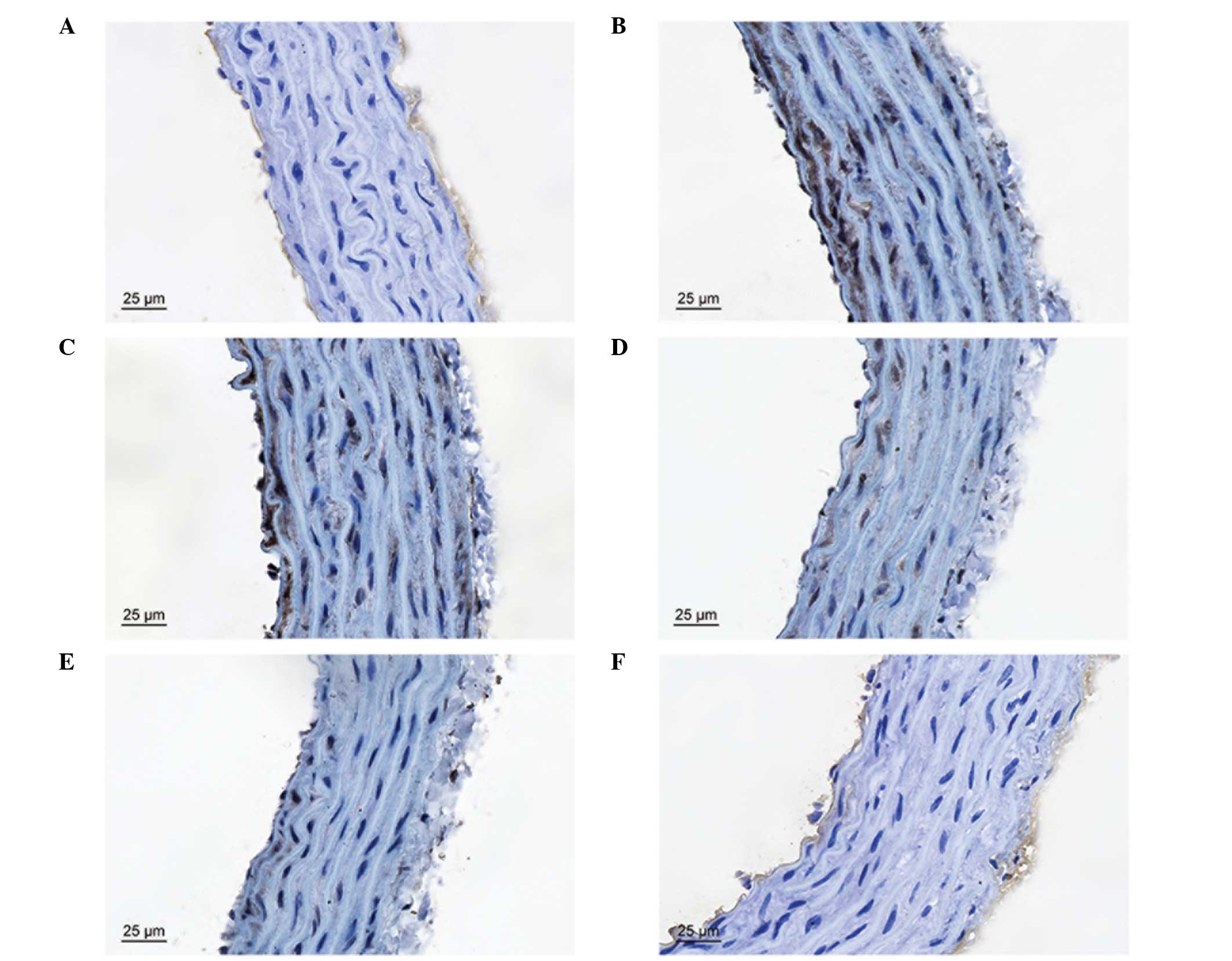
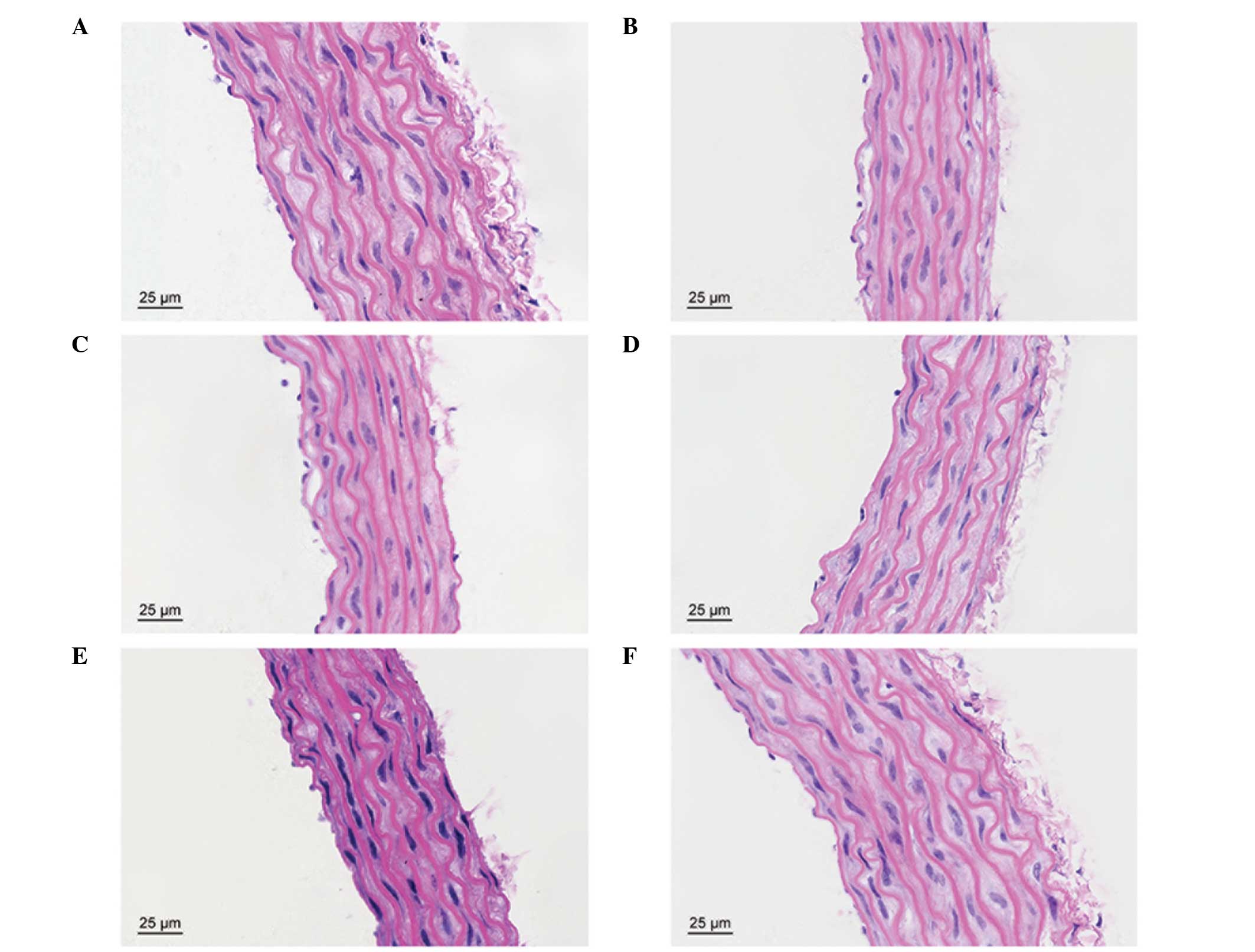
Effects of curcumin on LPS-induced
vascular injury
In the control group, the vascular structure of each
layer was clear and complete. However, the thoracic aortic
structure was evidently damaged by LPS, particularly in the
endothelium, subendothelial layer and elastic membrane. Treatment
with curcumin was shown to protect against LPS-induced vascular
injury (Fig. 6).
Discussion
Previous studies have shown that patients who
survive severe sepsis have an increased risk of cardiovascular
events (16), and sepsis-induced
cardiovascular dysfunction is one of the major causes of mortality
in sepsis patients (17,18). However, whether aortic contractile
function is injured during sepsis remains unclear. The present
study demonstrated that PE-induced vasoconstriction of the aortic
rings exhibited a significant decline following treatment with LPS
for 24 h, suggesting that sepsis may cause aortic vasoconstrictive
dysfunction. Previous studies have indicated that a K+
channel abnormality may be an underlying mechanism (4,18).
In the present study, LPS was also demonstrated to increase the
level of serum E-selectin, which is consistent with results
reported by Kim et al (8).
E-selectin, which can be synthesized by vascular endothelial cells,
has the role of promoting leukocyte rolling along the vascular
endothelium. Under normal physiological conditions, there is very
little or almost no expression of E-selectin in vascular
endothelial cells. LPS is known to activate innate immune
responses, resulting in the production of a great variety of
inflammatory cytokines (19).
Particular in vitro and in vivo stimuli, including
inflammatory cytokines (TNF-α) or activated transcription factors
(NF-κB), can induce vascular endothelial cells to overexpress
E-selectin (20,21). E-selectin molecules on the cell
membrane surface may detach or dissolve, becoming soluble (22). Serum soluble E-selectin is an
important index of vascular endothelial cell injury (23). Thus, the results of the present
study indicate that LPS may lead to aortic endothelial injury.
Curcumin, a natural yellow pigment, is derived from
the rhizome of Curcuma longa (24). The compound has been used in
traditional Chinese medicine for centuries to treat a variety of
diseases, including pulmonary fibrosis, diabetic nephropathy and
cervical cancer (25–27). The results of the present study
demonstrated that curcumin may have a preventative effect against
sepsis-induced vasoconstrictive dysfunction and the increase in
serum E-selectin levels. Furthermore, the results of HE staining
indicated that curcumin decreased the pathological changes of the
aortic vascular tissues induced by sepsis; however, the specific
mechanism underlying these effects remains unknown.
TSP-1 is a matrix glycoprotein with diverse roles in
various cellular and physiological processes (28). Sources of TSP-1 include activated
platelets, leukocytes, endothelial cells, vascular smooth muscle
cells and fibroblasts (29–31).
The expression of TSP-1 is usually increased at sites of
inflammation and is hypothesized to play an important role in
multiple biological processes, including thrombosis, wound healing
and the immune response (32–36).
In animal models of diabetes, TSP-1 expression was found to
increase significantly in the cavernous tissue (37). TSP-1 expression may impact the
prognosis of patients with severe sepsis by inhibiting innate
immune function. Furthermore, TSP-1 deficiency has been reported to
have a protective effect in cecal ligation puncture and
Escherichia coli injection (i.p.) models of peritoneal
sepsis (38). The present study
showed that the protein expression of TSP-1 increased in the aortic
tissues of LPS-treated rats, while curcumin was able to inhibit
this overexpression.
TGF-β1 is generally considered to be involved in the
regulation of inflammation and tissue remodeling following injury
(39–41). In normal blood vessels, TGF-β1
inhibits endothelial and vascular smooth muscle cell proliferation
(42,43). TSP-1 has been reported as an
important activator of TGF-β1 in diabetic nephropathy. KRFK
sequences in TSP-1 have been shown to combine with the
latency-associated peptide (LAP) region of latent TGF-β1, inducing
changes in the spatial configuration of the LAP. Subsequently, the
receptor binding site on TGF-β1 is exposed and the TSP-1/TGF-β1
complex is activated (13). In
addition, TSP-1 has been shown to upregulate latent TGF-β1
activation, and in turn, TGF-β1 may facilitate TSP-1 synthesis
(44). LPS can strongly induce an
increase in the expression of TGF-β1 and acute phase proteins in
TGF-β1-transgenic mice (45).
Sepsis promotes the release and activity of TGF-β1, thereby causing
alveolar epithelial cell dysfunction (46). Furthermore, previous studies have
reported that curcumin protects against sepsis-induced acute lung
injury by inhibiting the expression of molecules involved in the
TGF-β1/SMAD3 pathway (47,48). In keloid fibroblast cells, the
excessive production of extracellular matrix may be blocked and/or
rapidly decreased by curcumin through depression of the TGF-β1/SMAD
pathway (49). In the present
study, the protein expression of TGF-β1 increased in the aortic
tissues during sepsis and curcumin was shown to inhibit this
sepsis-induced overexpression, indicating that TGF-β1 may be
involved in the protective effect of curcumin against
sepsis-induced vasoconstriction injury.
In summary, the present study has provided
experimental evidence demonstrating that curcumin alleviates
LPS-induced vasoconstrictive dysfunction in rat thoracic aortas. In
addition, inhibition of TSP-1 and TGF-β1 expression may be involved
in this protective effect.
Acknowledgements
This study was supported by the Zhejiang Provincial
Applied Research Fund of Technology for Public Welfare (no.
2014C37027).
References
|
1
|
Vandijck D, Decruyenaere JM and Blot SI:
The value of sepsis definitions in daily ICU-practice. Acta Clin
Belg. 61:220–226. 2006. View Article : Google Scholar
|
|
2
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Felbinger TW, Suchner U and Goetz AE:
Treating patients with severe sepsis. N Engl J Med. 341:56–57.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen SJ, Wu CC, Yang SN, Lin CI and Yen
MH: Hyperpolarization contributes to vascular hyporeactivity in
rats with lipopolysaccharide-induced endotoxic shock. Life Sci.
68:659–668. 2000. View Article : Google Scholar
|
|
5
|
Jurenka JS: Anti-inflammatory properties
of curcumin, a major constituent of Curcuma longa: a review of
preclinical and clinical research. Altern Med Rev. 14:141–153.
2009.PubMed/NCBI
|
|
6
|
Bengmark S, Mesa MD and Gil A:
Plant-derived health: the effects of turmeric and curcuminoids.
Nutr Hosp. 24:273–281. 2009.PubMed/NCBI
|
|
7
|
Fang XD, Yang F, Zhu L, Shen YL, Wang LL
and Chen YY: Curcumin ameliorates high glucose-induced acute
vascular endothelial dysfunction in rat thoracic aorta. Clin Exp
Pharmacol Physiol. 36:1177–1182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim DC, Ku SK, Lee W and Bae JS: Barrier
protective activities of curcumin and its derivative. Inflamm Res.
61:437–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stein JJ, Iwuchukwu C, Maier KG and Gahtan
V: Thrombospondin-1-induced vascular smooth muscle cell migration
and proliferation are functionally dependent on microRNA-21.
Surgery. 155:228–233. 2014. View Article : Google Scholar
|
|
10
|
Gawaz M, Dickfeld T, Bogner C,
Fateh-Moghadam S and Neumann FJ: Platelet function in septic
multiple organ dysfunction syndrome. Intensive Care Med.
23:379–385. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
August P and Suthanthiran M: Transforming
growth factor beta signaling, vascular remodeling, and
hypertension. N Engl J Med. 354:2721–2723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crawford SE, Stellmach V, Murphy-Ullrich
JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP and Bouck N:
Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell.
93:1159–1170. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yevdokimova N, Wahab NA and Mason RM:
Thrombospondin-1 is the key activator of TGF-beta1 in human
mesangial cells exposed to high glucose. J Am Soc Nephrol.
12:703–712. 2001.PubMed/NCBI
|
|
14
|
Guide for the Care and Use of Laboratory
Animals. National Research Council Committee for the Update of the
Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press; Washington DC, USA: 2011
|
|
15
|
Farmer MR, Roberts RE, Gardiner SM and
Ralevic V: Effects of in vivo lipopolysaccharide infusion on
vasoconstrictor function of rat isolated mesentery, kidney, and
aorta. J Pharmacol Exp Ther. 306:538–545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yende S, Linde-Zwirble W, Mayr F,
Weissfeld LA, Reiss S and Angus DC: Risk of cardiovascular events
in severe sepsis survivors. Am J Respir Crit Care Med.
189:1065–1074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coquerel D, Neviere R, Delile E, Mulder P,
Marechal X, Montaigne D, Renet S, Remy-Jouet I, Gomez E, Henry JP,
do Rego JC, Richard V and Tamion F: Gene deletion of protein
tyrosine phosphatase 1B protects against sepsis-induced
cardiovascular dysfunction and mortality. Arterioscler Thromb Vasc
Biol. 34:1032–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sorrentino R, d’Emmanuele di Villa Bianca
R, Lippolis L, Sorrentino L, Autore G and Pinto A: Involvement of
ATP-sensitive potassium channels in a model of a delayed vascular
hyporeactivity induced by lipopolysaccharide in rats. Br J
Pharmacol. 127:1447–1453. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Sun W, Yang CH, Hu HZ and Jiang YH:
Tanshinone II a protects against lipopolysaccharides-induced
endothelial cell injury via Rho/Rho kinase pathway. Chin J Integr
Med. 20:216–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higai K, Shimamura A and Matsumoto K:
Amadori-modified glycated albumin predominantly induces E-selectin
expression on human umbilical vein endothelial cells through NADPH
oxidase activation. Clin Chim Acta. 367:137–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kneuer C, Ehrhardt C, Radomski MW and
Bakowsky U: Selectins - potential pharmacological targets? Drug
Discov Today. 11:1034–1040. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leeuwenberg JF, Smeets EF, Neefjes JJ,
Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ and Buurman WA:
E-selectin and intercellular adhesion molecule-1 are released by
activated human endothelial cells in vitro. Immunology. 77:543–549.
1992.PubMed/NCBI
|
|
23
|
Roldán V, Marín F, Lip GY and Blann AD:
Soluble E-selectin in cardiovascular disease and its risk factors.
A review of the literature. Thromb Haemost. 90:1007–1020.
2003.PubMed/NCBI
|
|
24
|
Gerczuk PZ, Breckenridge DG, Liles JT,
Budas GR, Shryock JC, Belardinelli L, Kloner RA and Dai W: An
apoptosis signal-regulating kinase 1 inhibitor reduces
cardiomyocyte apoptosis and infarct size in a rat
ischemia-reperfusion model. J Cardiovasc Pharmacol. 60:276–282.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang H, Yu T, Wen L, Wang H, Fei D and
Jin C: Curcumin enhances the effectiveness of cisplatin by
suppressing CD133+ cancer stem cells in laryngeal
carcinoma treatment. Exp Ther Med. 6:1317–1321. 2013.PubMed/NCBI
|
|
26
|
de Pablo R, Monserrat J, Reyes E, Díaz D,
Rodríguez-Zapata M, la Hera Ad, Prieto A and Alvarez-Mon M:
Sepsis-induced acute respiratory distress syndrome with fatal
outcome is associated to increased serum transforming growth factor
beta-1 levels. Eur J Intern Med. 23:358–362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roy M and Mukherjee S: Reversal of
resistance towards cisplatin by curcumin in cervical cancer cells.
Asian Pac J Cancer Prev. 15:1403–1410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Esemuede N, Lee T, Pierre-Paul D, Sumpio
BE and Gahtan V: The role of thrombospondin-1 in human disease. J
Surg Res. 122:135–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baenziger NL, Brodie GN and Majerus PW: A
thrombin-sensitive protein of human platelet membranes. Proc Natl
Acad Sci USA. 68:240–243. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DiPietro LA, Nissen NN, Gamelli RL, Koch
AE, Pyle JM and Polverini PJ: Thrombospondin 1 synthesis and
function in wound repair. Am J Pathol. 148:1851–1860.
1996.PubMed/NCBI
|
|
31
|
Iruela-Arispe ML, Bornstein P and Sage H:
Thrombospondin exerts an antiangiogenic effect on cord formation by
endothelial cells in vitro. Proc Natl Acad Sci USA. 88:5026–5030.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hugo C: The thrombospondin 1-TGF-beta axis
in fibrotic renal disease. Nephrol Dial Transplant. 18:1241–1245.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adams JC and Lawler J: The
thrombospondins. Cold Spring Harb Perspect Biol. 3:a0097122011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen H, Herndon ME and Lawler J: The cell
biology of thrombospondin-1. Matrix Biol. 19:597–614. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brunner G and Blakytny R: Extracellular
regulation of TGF-beta activity in wound repair: growth factor
latency as a sensor mechanism for injury. Thromb Haemost.
92:253–261. 2004.PubMed/NCBI
|
|
36
|
Saltzman AK, Olson TA, Mohanraj D, Carson
LF and Ramakrishnan S: Prevention of postoperative adhesions by an
antibody to vascular permeability factor/vascular endothelial
growth factor in a murine model. Am J Obstet Gynecol.
174:1502–1506. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang XM, Shi PH, Cao SH, Yu HJ, Azad J
and Ling SC: Expression changes of transforming growth factor-beta1
and thrombospondin-1 in cavernous tissues of diabetic rats. Urol
Int. 84:221–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McMaken S, Exline MC, Mehta P, Piper M,
Wang Y, Fischer SN, Newland CA, Schrader CA, Balser SR, Sarkar A,
Baran CP, Marsh CB, Cook CH, Phillips GS and Ali NA:
Thrombospondin-1 contributes to mortality in murine sepsis through
effects on innate immunity. PLoS One. 6:e196542011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bone RC: Sir Isaac Newton, sepsis, SIRS,
and CARS. Crit Care Med. 24:1125–1128. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Daniel C, Wiede J, Krutzsch HC, Ribeiro
SM, Roberts DD, Murphy-Ullrich JE and Hugo C: Thrombospondin-1 is a
major activator of TGF-beta in fibrotic renal disease in the rat in
vivo. Kidney Int. 65:459–468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim HJ, Park SY, Park OJ and Kim YM:
Curcumin suppresses migration and proliferation of Hep3B
hepatocarcinoma cells through inhibition of the Wnt signaling
pathway. Mol Med Rep. 8:282–286. 2013.PubMed/NCBI
|
|
42
|
Redondo S, Santos-Gallego CG, Ganado P,
García M, Rico L, Del Rio M and Tejerina T: Acetylsalicylic acid
inhibits cell proliferation by involving transforming growth
factor-beta. Circulation. 107:626–629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ruiz E, Redondo S, Gordillo-Moscoso A and
Tejerina T: Pioglitazone induces apoptosis in human vascular smooth
muscle cells from diabetic patients involving the transforming
growth factor-beta/activin receptor-like kinase-4/5/7/Smad2
signaling pathway. J Pharmacol Exp Ther. 321:431–438. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murphy-Ullrich JE and Poczatek M:
Activation of latent TGF-beta by thrombospondin-1: mechanisms and
physiology. Cytokine Growth Factor Rev. 11:59–69. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garcia-Lazaro JF, Thieringer F, Lüth S,
Czochra P, Meyer E, Renteria IB, Galle PR, Lohse AW, Herkel J and
Kanzler S: Hepatic over-expression of TGF-beta1 promotes
LPS-induced inflammatory cytokine secretion by liver cells and
endotoxemic shock. Immunol Lett. 101:217–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bechara RI, Pelaez A, Palacio A, Joshi PC,
Hart CM, Brown LA, Raynor R and Guidot DM: Angiotensin II mediates
glutathione depletion, transforming growth factor-beta1 expression,
and epithelial barrier dysfunction in the alcoholic rat lung. Am J
Physiol Lung Cell Mol Physiol. 289:L363–L370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu F, Lin SH, Yang YZ, Guo R, Cao J and
Liu Q: The effect of curcumin on sepsis-induced acute lung injury
in a rat model through the inhibition of the TGF-beta1/SMAD3
pathway. Int Immunopharmacol. 16:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yoshida H, Okumura N, Nishimura Y,
Kitagishi Y and Matsuda S: Turmeric and curcumin suppress
presenilin 1 protein expression in Jurkat cells. Exp Ther Med.
2:629–632. 2011.PubMed/NCBI
|
|
49
|
Hsu YC, Chen MJ, Yu YM, Ko SY and Chang
CC: Suppression of TGF-β1/SMAD pathway and extracellular matrix
production in primary keloid fibroblasts by curcuminoids: its
potential therapeutic use in the chemoprevention of keloid. Arch
Dermatol Res. 302:717–724. 2010. View Article : Google Scholar : PubMed/NCBI
|