Introduction
Matrix metalloproteinase (MMP) is a general term for
the group of proteinases that degrade the extracellular matrix
(ECM). Type IV collagenases consists of two types of MMP, 72-kDa
MMP-2 and 92-kDa MMP-9, which are both synthesized and secreted by
neutrophils and macrophages. The two MMPs are able to degrade the
ECM in the basement membrane. Among multiple types of MMPs, type IV
collagenase is closely associated with diabetes and cardiovascular
disease (1–3). The substrates of type IV collagenases
include type IV and V collagens, fibronectin, laminin, elastin and
denatured collagen matrix (4,5). The
activity of MMPs can be inhibited by the corresponding tissue
inhibitors of metalloproteinases (TIMPs). TIMPs generate MMP-TIMP
complexes through the combination of cysteine residues in the
N-terminus functional region with the active center of the MMP,
thereby blocking the ability of the MMP to bind to substrates. Four
types of TIMP have been identified and are referred to as TIMP-1,
-2, -3 and -4. The specific inhibitors of the type IV collagenases,
MMP-2 and MMP-9, are TIMP-2 and TIMP-1, respectively. By adjusting
the relative concentrations of MMPs and their inhibitors, the body
is able to control the composition of the ECM (6).
Increasing experimental evidence has revealed that
changes in the expression of type IV collagenases and the
corresponding inhibitors, and subsequently the disproportion of the
two, play an important role in the occurrence and development of
atherosclerosis and vascular restenosis (7,8). The
detection of MMP expression in normal human arterial tissue and
atherosclerotic plaque tissue demonstrates that type IV
collagenases, TIMP-1 and TIMP-2 are expressed in normal smooth
muscle cells, but are inactive. However, the increase in type IV
collagenases in the smooth muscle cells and macrophages present in
atherosclerotic plaques indicates their matrix-degrading activity
(3). In addition, endothelial
cells covering the plaque surface, which differ from endothelial
cells in other areas of the body, are able to express active type
IV collagenases (9). In newly
isolated monocytes, the expression of MMP-9 is low; however, a
series of cytokines found in atherosclerotic plaques, including
interleukin (IL)-1β and tumor necrosis factor (TNF)-α, are able to
increase the expression and secretion of MMP-9 (10–12).
MMP-2 and MMP-9 are considered to be the principal MMPs secreted by
monocytes that are capable of degrading type IV collagen, the main
constituent of the basement membrane (13). Furthermore, the expression of MMP-2
and MMP-9 in monocytes is particularly important in penetrating the
first basement membrane barrier of the ECM in the development of
atherosclerosis. Thus, factors that regulate the expression of
MMP-2 and MMP-9 in monocytes may affect the process of
atherosclerosis development.
Monocyte adhesion to endothelial cells and their
subsequent infiltration play an important role in the early onset
of atherosclerosis (14). The
contact adhesion of the two cells is not solely physical and there
is often an interactive dialogue between the cells. In the contact
process, a number of corresponding signaling transduction pathways
are triggered that significantly affect the cell phenotype and
function, including the expression of MMPs (15). For instance, the interaction of T
lymphocytes with endothelial cells may increase the expression of
MMP-2, which is dependent on the mediation of vascular cell
adhesion molecules expressed in the endothelial cells (16). Lee et al demonstrated that
the interaction between monocytes and smooth muscle cells may
induce the expression of MMP-1 and MMP-3 (17). Furthermore, Amorino and Hoover
observed that the direct contact of monocytes with formalin-fixed
human monolayer endothelial cells resulted in an increased
expression of MMP-9 (18).
However, in these studies, the precise mechanism of interaction
between the monocytes and endothelial cells that caused the
increase in MMP expression was not studied in depth. In addition,
the effect of the interaction between monocytes and endothelial
cells on the expression levels of type IV collagenases and their
specific inhibitors in monocytes remains unknown.
In the present study, single and mixed cultures of
monocytes and endothelial cells were established, and changes in
the expression levels of the type IV collagenases, MMP-2 and MMP-9,
as well as their specific inhibitors, TIMP-1 and TIMP-2, were
investigated in the monocytes.
Materials and methods
Cell culture
A monocyte cell line (U937) and human umbilical vein
endothelial cells (HUVECs) were obtained from the National
Infrastructure of Cell Line Resources (Beijing Union Medical
College, Beijing, China). The cells were maintained in RPMI 1640
medium (Gibco Life Technologies, Carlsbad, CA, USA) supplemented
with 10% calf serum (Huamei Bioengineering Co. Ltd., Shanghai,
China), 20 mM sodium bicarbonate (Sigma-Aldrich, St. Louis, MO,
USA) and 1% penicillin/streptomycin mix (Invitrogen Life
Technologies, Carlsbad, CA, USA). The cells were incubated at 37°C
in a 5% CO2 incubator.
Grouping
Six experimental groups were established as follows:
Endothelial cell and monocyte co-culture group; co-culture group
supplemented with TNF-α monoclonal antibodies (2 μg/ml); co-culture
group supplemented with IL-1β monoclonal antibodies (2 μg/ml);
co-culture group supplemented with TNF-α (2 μg/ml) and IL-1β (2
μg/ml) monoclonal antibodies; single-culture monocyte group; and
cultured monocyte group supplemented with conditioned medium from
the 12 h co-culture of monocytes and endothelial cells. Each group
was cultured serum-free for 24 h post-treatment and subsequently
centrifuged at 800 × g for 3 min at room temperature (20–22°C).
Immunocytochemical staining was performed on the monocytes. In the
five wells of each group, four smears were placed in each well,
which were immunocytochemically stained with monoclonal antibodies
against MMP-2, MMP-9, TIMP-1 and TIMP-2. The MMP-2, MMP-9, TIMP-1,
TIMP-2, TNF-α and IL-1β monoclonal antibodies were purchased from
Shanghai SenXiong Biotech Industry Co., Ltd (Shanghai, China).
Immunocytochemistry and image
analysis
Monocytes were centrifuged at 500 × g to remove the
medium, washed and centrifuged twice at 500 × g at room
temperature, with phosphate-buffered saline (PBS). A monocyte smear
was made on the carrier plate, which was subsequently dried in
shade for 15 min and slowly placed in 4% paraformaldehyde solution
for fixation. Staining was performed using a streptavidin-biotin
complex enzyme immunoassay kit (Wuhan Boster Biological Technology,
Ltd, Wuhan, China), according to the manufacturer’s instructions.
Cells with yellow, brownish-yellow or chocolate-brown colored
particles were considered to be positive cells. QWin image
processing software (Leica Camera AG, Solms, Germany) was used for
image analysis. A view field was randomly selected from each plate.
Based on the number of cells, a total of 30–50 cells were selected
and their average optical density was measured. The average optical
density of the five wells was subsequently calculated. The
differences in the optical density values reflected the differences
in the color shades, and also the different concentrations of the
tested substances.
Statistical analysis
Experimental data are presented as the mean ±
standard deviation. Intergroup data processing was based on the
results of the homogeneity test of variance, and the
Student-Newman-Keuls test was used to analyze the differences
between the groups. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS 17.0 statistical software (SPSS, Inc.,
Chicago, IL, USA).
Results
Effect of the different culture
conditions on MMP-2 expression
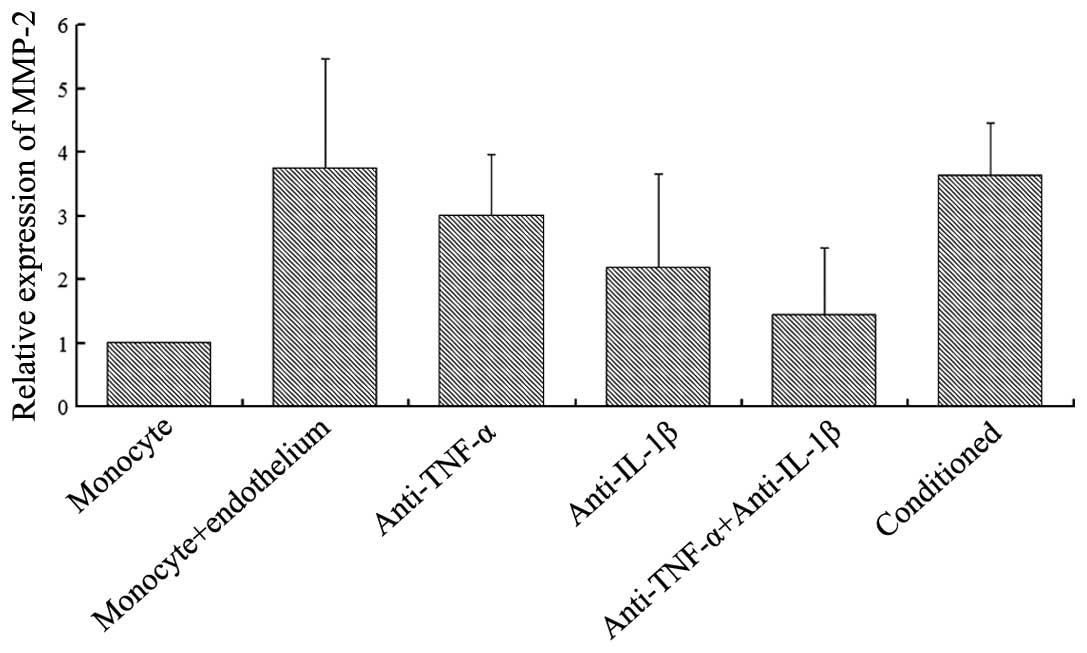
As shown in Fig. 1,
a degree of MMP-2 expression was observed when the monocytes were
single-cultured; however, MMP-2 expression significantly increased
when the monocytes were co-cultured with endothelial cells. In the
co-cultured group, the addition of TNF-α (2 μg/ml) or IL-1β (2
μg/ml) monoclonal antibodies reduced MMP-2 expression, while the
addition of the two cytokine antibodies significantly reduced the
expression of MMP-2. When monocytes were added to the conditioned
medium from the co-culture of monocytes and endothelial cells,
MMP-2 expression increased. A statistically significant difference
was not observed when compared with the two-cell co-cultured group
(P>0.05). However, statistically significant differences were
observed when comparing the conditioned group with the cytokine
antibody groups (P<0.05).
Effect of the different culture
conditions on MMP-9 expression
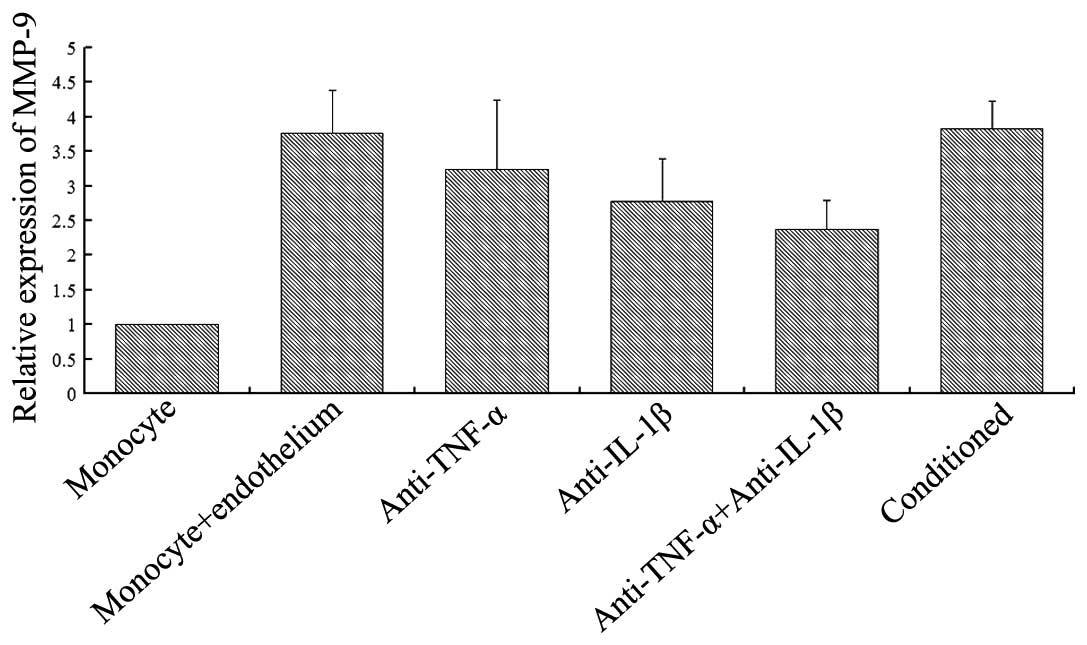
As shown in Fig. 2,
the single culture of monocytes exhibited a certain degree of MMP-9
expression. Following the co-culture of monocytes with endothelial
cells, the expression level of MMP-9 was significantly higher
compared with the single culture. TNF-α (2 μg/ml) or IL-1β (2
μg/ml) monoclonal antibodies were able to inhibit the upregulation
of MMP-9 expression, to a certain extent, observed in the two-cell
co-culture group. However, the addition of the two cytokine
antibodies did not fully inhibit the upregulation of MMP-9
expression, although the level was significantly reduced compared
with the two-cell co-culture group. When monocytes were added to
the conditioned medium from the co-culture of monocytes and
endothelial cells, the MMP-9 expression increased, and the
difference with the two-cell co-cultured group was not
significantly different (P>0.05). However, statistically
significant differences were observed when comparing the
conditioned group with the cytokine antibody groups
(P<0.05).
Effect of the different culture
conditions on TIMP-1 expression
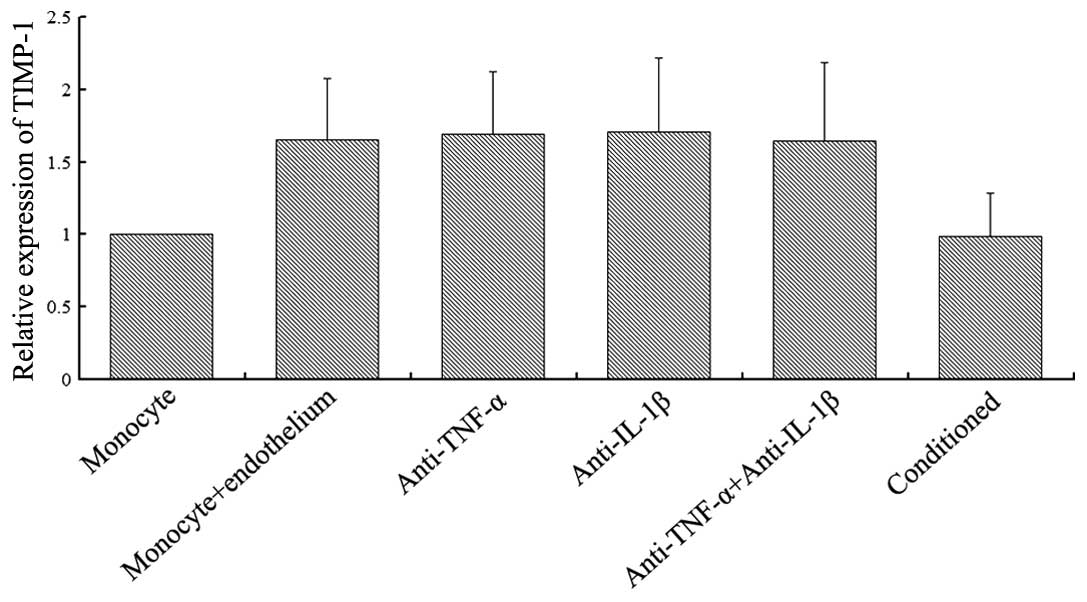
As shown in Fig. 3,
TIMP-1 expression was observed in the monocyte single-culture. When
the monocytes were co-cultured with endothelial cells, the
expression levels of TIMP-1 increased significantly (P<0.05),
but the enhanced amplitude was less than two times the monocyte
single-culture. The addition of TNF-α (2 μg/ml) and/or IL-1β (2
μg/ml) monoclonal antibodies to the co-cultured group revealed no
effect on TIMP-1 expression (P>0.05). Furthermore, the addition
of conditioned medium from the co-culture of endothelial cells and
monocytes to the cultured monocytes demonstrated no effect on
TIMP-1 expression when compared with the single-culture monocytes
(P>0.05).
Effect of the different culture
conditions on TIMP-2 expression
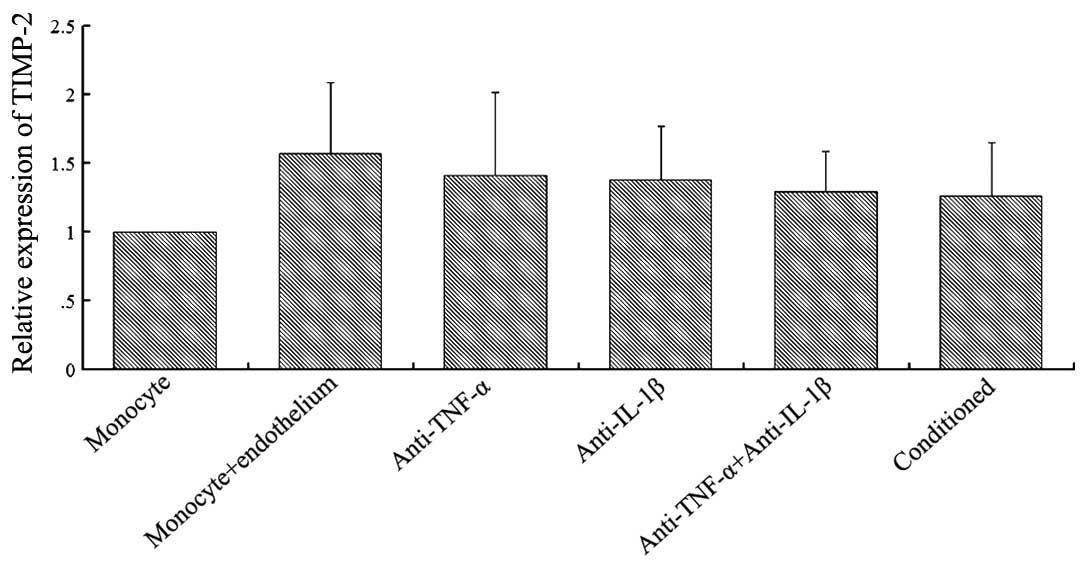
As shown in Fig. 4,
TIMP-2 was expressed when the monocytes were single-cultured, but a
significantly increased expression was observed when the monocytes
were co-cultured with endothelial cells (P<0.05). The addition
of TNF-α (2 μg/ml) or IL-1β (2 μg/ml) monoclonal antibodies to the
co-culture group partially inhibited the upregulation of TIMP-2
expression observed in the two-cell co-culture group. However,
simultaneous addition of the two cytokine antibodies did not
completely inhibit the upregulation of TIMP-2 expression. The
addition of conditioned medium from the co-culture of endothelial
cells and monocytes to the cultured monocytes caused an increase in
TIMP-2 expression when compared with the single-cultured monocytes
(P<0.05). A significant difference was also observed between the
conditioned cultured group and the monocyte-endothelium co-cultured
group (P<0.05; n=5).
Discussion
The adhesion of monocytes to endothelial cells and
secondary subendothelial migration play important roles in the
development of atherosclerosis. Furthermore, monocyte regulation of
type IV collagenase expression is particularly important for the
decomposition of the ECM during infiltration (19). However, there have been no definite
results on whether the adhesion of endothelial cells and monocytes
has an effect on the expression levels of type IV collagenases and
their specific inhibitors. The present study observed that when
monocytes were cultured alone, the type IV collagenases MMP-2 and
MMP-9, and their specific inhibitors, TIMP-1 and TIMP-2, were
expressed to a certain extent. However, following co-culture with
endothelial cells, the expression levels of type IV collagenases
were significantly increased, while the expression levels of their
specific inhibitors increased to a lesser degree. Following the
adhesion of monocytes to endothelial cells, a functional change
occurs where the ability to decompose the matrix becomes stronger.
This change is beneficial to the migration of the monocytes and
their ingestion of the lipids in the arterial wall, which causes
the transformation into foam cells. The current experiments also
demonstrated that TNF-α and IL-1β partially mediated the expression
levels of the monocyte type IV collagenases, MMP-2 and MMP-9. These
observations indicate that the functional change of type IV
collagenases in monocytes during the adhesion of monocytes to
endothelial cells plays an important role in the occurrence of
atherosclerosis. Thus, the regulation of MMP expression may become
an intervention target in the treatment of atherosclerotic vascular
disease (19,20).
In atherosclerotic vascular disease caused by
diabetes and hypertension, an important part of the pathogenesis
process is the aggregation of leukocytes in the circulatory system
and their adherence locally in endothelial cells through adhesion
molecules (21). Following
adhesion, the leukocytes penetrate the endothelial cells and the
corresponding basement membrane; however, the exact mechanism
remains unclear. A previous study indicated that MMPs facilitate
this process (1). The direct
contact of cytokine-stimulated monocytes with the human endothelial
cell monolayer has been shown to result in increased MMP-9
expression in monocytes (22);
however, the precise mechanism is yet to be elucidated. During the
infiltration of the artery wall by inflammatory cells, the
degradation of the basement membrane of the endothelial cells by
MMPs damages the endothelial barrier function, resulting in an
increase in the inflow of plasma proteins. Once the inflammatory
cells in the vessel wall are infiltrated, they are able to interact
with the ECM and oxidized low-density lipoproteins, further
promoting the expression of MMPs in macrophages (23). Macrophages are able to secrete
stimulatory factors that induce the secretion of MMPs and promote
their activation. In the development of atherosclerosis, the
increased activity of MMPs leads to changes in the structure of the
vessel wall, resulting in the remodeling of the blood vessel wall
(23,24). Thus, the effective mechanism of the
monocyte-endothelium interaction on MMP expression in monocytes is
of great significance for the study of atherosclerosis
incidence.
The present study observed that when monocytes were
cultured alone, the expression levels of MMP-2 and MMP-9 were low,
while the expression levels of TIMP-1 and TIMP-2 were relatively
high. This observation indicates that for type IV collagenases,
under normal conditions, the potentially stronger control mechanism
of TIMPs is able to regulate the activity of the enzymes. Since
MMPs have numerous degradable substrates and are able to degrade a
wide range of ECM proteins, such as collagen, elastin and
glycoproteins, controlling the activity of MMPs under physiological
conditions is important. In monocytes, the basal expression levels
of TIMP-1 and TIMP-2 are high and the potential secretion is large,
which is beneficial to achieving the timely control of MMPs and
maintaining a stable organizational structure under physiological
conditions. Under certain pathological conditions, the expression
levels of MMPs and TIMPs are often in disorder. For example, during
the occurrence and development of atherosclerosis, smooth muscle
cells proliferate and migrate to the tunica intima, forcing the
cells to pass through the ECM of the vessel wall and the basement
membrane. Previous experiments have demonstrated that the
upregulation of MMP expression is closely associated with the
migration of smooth muscle cells (3). Thus, the use of MMP inhibitors may
significantly reduce the migration and proliferation of smooth
muscle cells (25).
In the early stage of atherosclerosis development, a
number of monocytes penetrate the vascular endothelium to
infiltrate the vessel wall. During the process, the basement
membrane and the blood vessel walls must be penetrated. Therefore,
the expression of MMPs in monocytes, particularly the expression of
the type IV collagenases, which are able to degrade type IV
collagen in the basement membrane, plays an important role in the
onset of atherosclerosis. Hojo et al (26), Matías-Román et al (27) and Mostafa Mtairag et al
(28) studied the effects of the
monocyte-endothelium interaction on MMP secretion and confirmed
that co-culture of the two cells promoted the expression of MMPs in
leukocytes; however, the studies did not conduct a thorough
investigation of the specific mechanisms underlying this process.
The experimental results of the current study are consistent with
the conclusions of the aforementioned studies. Furthermore, the
present study conducted an in-depth investigation on the underlying
mechanism, and revealed that TNF-α and IL-1β play important roles.
However, these cytokines are not the only stimulatory factors, as
the addition of TNF-α and IL-1β antibodies did not completely
inhibit the increase in the expression levels of MMP-2 and MMP-9,
indicating that a number of other cytokines are involved in the
endothelial cell-stimulated upregulation of MMP-2 and MMP-9
expression in monocytes.
With regard to whether endothelial cell and monocyte
adhesion may directly activate the gene signaling transduction
pathways of type IV collagenases and their inhibitors by adhesion
molecules, the present study revealed that in the regulation of
MMP-2 and MMP-9, this direct activation pathway may not exist or
that such a direct regulatory role is very small. However, for
TIMP-1 and TIMP-2, this direct activation process may exist. When
compared with the conditioned culture group, in the monocytes of
the two-cell co-culture group, relatively high expression levels of
TIMP-1 and TIMP-2 were observed. This increase in the expression
levels of the two types of inhibitor may be due to the contact of
the two cells directly inducing the expression of TIMP-1 and
TIMP-2. Through the corresponding combination of adhesion molecules
with their ligands, the signaling transduction pathways of TIMP-1
and TIMP-2 genes may be activated, causing an increase in
expression levels. The current study revealed that when compared
with the single-cultured monocyte group, the expression levels of
MMP-2 and MMP-9 in the conditioned group were significantly higher.
However, when compared with simple co-cultured group, no
statistically significant difference was observed, indicating that
the increased secretion of type IV collagenases caused by the
contact adhesion of the endothelial cells and monocytes plays a
major role in affecting the expression levels of MMP-2 and MMP-9 in
monocytes. The expression levels of MMP-2 and MMP-9 significantly
increased following exposure of the monocytes to endothelial cells.
However, the expression level increases in the corresponding
inhibitors, TIMP-1 and TIMP-2, were lower. This observations
indicates that under physiological conditions, TIMP-1 and TIMP-2
are able to regulate MMP activity; however, during the contact
adhesion of endothelial cells and monocytes, the upregulation of
MMPs and their inhibitors is not synchronized. The original
dominant position of the TIMPs has changed, and the balance between
the two has been lost, which may be deduced from the reduced
expression levels of the inhibitors. Subsequently, the expression
and activity of type IV collagenases increase and the ability to
decompose the basement membrane collagen and vascular wall matrix
is enhanced.
In conclusion, the present study revealed that the
interaction of monocytes and endothelial cells may stimulate the
functional transformation of monocytes, resulting in an increase in
the expression levels of the type IV collagenases, MMP-2 and MMP-9,
and the destruction of the balance between them and their specific
TIMPs. The interaction of monocytes and endothelial cells induces
functional changes in the monocytes, which contribute to the
occurrence and development of atherosclerosis.
Acknowledgements
The study was supported by grants from the Research
Fund for the Doctoral Program of Higher Education (no.
20100201120065) and the National Natural Science Foundation of
China (no. 81100210).
References
|
1
|
Elgebaly MM, Prakash R, Li W, et al:
Vascular protection in diabetic stroke: role of matrix
metalloprotease-dependent vascular remodeling. J Cereb Blood Flow
Metab. 30:1928–1938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niu W and Qi Y: Matrix metalloproteinase
family gene polymorphisms and risk for coronary artery disease:
systematic review and meta-analysis. Heart. 98:1483–1491. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pasterkamp G, Schoneveld AH, Hijnen DJ, et
al: Atherosclerotic arterial remodeling and the localization of
macrophages and matrix metalloproteases 1, 2 and 9 in the human
coronary artery. Atherosclerosis. 150:245–253. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baramova E and Foidart JM: Matrix
metalloproteinase family. Cell Biol Int. 19:239–242.
1995.PubMed/NCBI
|
|
5
|
Romanic AM, White RF, Arleth AJ, et al:
Matrix metalloproteinase expression increases after cerebral focal
ischemia in rats: inhibition of matrix metalloproteinase-9 reduces
infarct size. Stroke. 29:1020–1030. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matrisian LM: Metalloproteinases and their
inhibitors in matrix remodeling. Trends Genet. 6:121–125. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Skjøt-Arkil H, Barascuk N, Register T and
Karsdal MA: Macrophage-mediated proteolytic remodeling of the
extracellular matrix in atherosclerosis results in neoepitopes: a
potential new class of biochemical markers. Assay Drug Dev Technol.
8:542–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeh JL, Hsu JH, Liang JC, et al:
Lercanidipine and labedipinedilol - a attenuate
lipopolysaccharide/interferon-γ-induced inflammation in rat
vascular smooth muscle cells through inhibition of HMGB1 release
and MMP-2, 9 activities. Atherosclerosis. 226:364–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagareddy PR, Rajput PS, Vasudevan H, et
al: Inhibition of matrix metalloproteinase-2 improves endothelial
function and prevents hypertension in insulin-resistant rats. Br J
Pharmacol. 165:705–715. 2012. View Article : Google Scholar :
|
|
10
|
Robinson SC, Scott KA and Balkwill FR:
Chemokine stimulation of monocyte matrix metalloproteinase-9
requires endogenous TNF-alpha. Eur J Immunol. 32:404–412. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vaday GG, Hershkoviz R, Rahat MA, et al:
Fibronectin-bound TNF-alpha stimulates monocyte matrix
metalloproteinase-9 expression and regulates chemotaxis. J Leukoc
Biol. 68:737–747. 2000.PubMed/NCBI
|
|
12
|
Zhang Y, McCluskey K, Fujii K and Wahl LM:
Differential regulation of monocyte matrix metalloproteinase and
TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and
IL-1 beta through prostaglandin-dependent and -independent
mechanisms. J Immunol. 161:3071–3076. 1998.PubMed/NCBI
|
|
13
|
Galis ZS and Khatri JJ: Matrix
metalloproteinases in vascular remodeling and atherogenesis: the
good, the bad, and the ugly. Circ Res. 90:251–262. 2002.PubMed/NCBI
|
|
14
|
Shagdarsuren E, Djalali-Talab Y,
Aurrand-Lions M, et al: Importance of junctional adhesion
molecule-C for neointimal hyperplasia and monocyte recruitment in
atherosclerosis-prone mice-brief report. Arterioscler Thromb Vasc
Biol. 29:1161–1163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mestas J and Ley K: Monocyte-endothelial
cell interactions in the development of atherosclerosis. Trends
Cardiovasc Med. 18:228–232. 2008. View Article : Google Scholar
|
|
16
|
Romanic AM and Madri JA: The induction of
72-kD gelatinase in T cells upon adhesion to endothelial cells is
VCAM-1 dependent. J Cell Biol. 125:1165–1178. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee E, Grodzinsky AJ, Libby P, et al:
Human vascular smooth muscle cell-monocyte interactions and
metalloproteinase secretion in culture. Arterioscler Thromb Vasc
Biol. 15:2284–2289. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amorino GP and Hoover RL: Interactions of
monocytic cells with human endothelial cells stimulate monocytic
metalloproteinase production. Am J Pathol. 152:199–207.
1998.PubMed/NCBI
|
|
19
|
Benjamin MM and Khalil RA: Matrix
metalloproteinase inhibitors as investigative tools in the
pathogenesis and management of vascular disease. EXS. 103:209–279.
2012.PubMed/NCBI
|
|
20
|
Mannello F, Medda V, Ligi D and Raffetto
JD: Glycosaminoglycan sulodexide inhibition of MMP-9 gelatinase
secretion and activity: possible pharmacological role against
collagen degradation in vascular chronic diseases. Curr Vasc
Pharmacol. 11:354–365. 2013. View Article : Google Scholar
|
|
21
|
Legein B, Temmerman L, Biessen EA and
Lutgens E: Inflammation and immune system interactions in
atherosclerosis. Cell Mol Life Sci. 70:3847–3869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Desai A, Darland G, Bland JS, et al:
META060 attenuates TNF-α-activated inflammation,
endothelial-monocyte interactions, and matrix metalloproteinase-9
expression, and inhibits NF-κB and AP-1 in THP-1 monocytes.
Atherosclerosis. 223:130–136. 2013. View Article : Google Scholar
|
|
23
|
Roycik MD, Myers JS, Newcomer RG and Sang
QX: Matrix metalloproteinase inhibition in atherosclerosis and
stroke. Curr Mol Med. 13:1299–1313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilson HM, Barker RN and Erwig LP:
Macrophages: promising targets for the treatment of
atherosclerosis. Curr Vasc Pharmacol. 7:234–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong YJ, Cho HJ, Whang K, et al: Melittin
has an inhibitory effect on TNF-α-induced migration of human aortic
smooth muscle cells by blocking the MMP-9 expression. Food Chem
Toxicol. 50:3996–4002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hojo Y, Ikeda U, Takahashi M, et al:
Matrix metalloproteinase-1 expression by interaction between
monocytes and vascular endothelial cells. J Mol Cell Cardiol.
32:1459–1468. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matías-Román S, Gálvez BG, Genís L, et al:
Membrane type 1-matrix metalloproteinase is involved in migration
of human monocytes and is regulated through their interaction with
fibronectin or endothelium. Blood. 105:3956–3964. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mostafa Mtairag E, Chollet-Martin S,
Oudghiri M, et al: Effects of interleukin-10 on
monocyte/endothelial cell adhesion and MMP-9/TIMP-1 secretion.
Cardiovasc Res. 49:882–890. 2001. View Article : Google Scholar : PubMed/NCBI
|