Introduction
Auxiliary liver transplantation (ALT) is usually
used for the treatment of acute liver failure and inherited
metabolic liver disease (1,2). The
aim of ALT is to support the patient’s failing liver for a period
of time until the native liver has recovered, or to maintain normal
metabolic functions using a small proportion of the liver mass. ALT
has two main types, namely auxiliary partial orthotopic liver
transplantation (APOLT) and auxiliary partial heterotopic liver
transplantation (APHLT). APOLT is proposed to be a very effective
option (1,2), whereas APHLT has been abandoned
currently due to an increased incidence of primary non-function and
portal vein thrombosis (3).
However, APHLT with portal vein arterialization
(PVA) presents significant advantages, such as that the hilum and
portal vein of the native liver are untouched, the surgical trauma
of more extensive liver dissection of the native liver is avoided,
and adequate portal venous blood flow of the graft liver is
guaranteed. For these reasons, several clinical studies using APHLT
with PVA have been conducted in recent years (4–6);
however, the graft liver has been found to fail in long-term
survival. To solve this problem, Schleimer et al developed a
rat model of APHLT with PVA (7).
In this model, the graft portal vein is connected by a stent with
0.3 mm inner diameter to the recipient’s right renal artery. The
authors declared that the success rate was 90%; however, based on
our experiences, thrombosis occurs quite frequently in the
connected stent, despite the consistent use of systemic
heparinization. This prompted the development of a new procedure to
avoid the thrombosis. In the present study, it was hypothesized
that the splenic artery in the celiac trunk from the graft liver
could be used instead of a stent for PVA.
For this purpose, a new rat model of APHLT with PVA
was developed, based on a modification of the Schleimer model,
using the splenic artery of the graft liver for arterialization of
the portal vein instead of a stent.
Materials and methods
Animals
The experimental protocol was approved by the
Laboratory Animal Ethics Committee of the Inner Mongolia Medical
University (Hohhot, China).
Male Sprague-Dawley (SD) rats, each weighing 300–350
g, were used in this study (obtained from Vital River Laboratory
Animal Technology Co. Ltd, Beijing, China). The body weight of the
donors and recipients were comparable. All procedures were
performed by two persons using a clean but not sterile technique
with a Leica M525 F20 surgical microscope (Leica Microsystems,
Wetzlar, Germany). Anesthesia was induced by inhalation of 3–4%
isoflurane, and maintained by inhalation of 1–2% isoflurane. All
rats were given oxygen at a dose of 0.3–0.5 l/min.
Donor surgery
Preparation of the donor liver
After anesthesia was induced, a crucial incision was
made in the abdomen of the rat. The ligament surrounding the liver
was cut off with an electric coagulation scalpel, the left phrenic
vein and right suprarenal vein were ligated and divided, and the
infrahepatic caval vein was isolated and injected with 2 ml low
molecular weight heparin sodium salt (50 IU/ml; Jiangsu Wanbang
Biochemical Pharmaceutical, Co. Ltd, Xuzhou, China), allowing
systemic heparinization of the rats. The hepatoduodenal ligament
was dissected, and the common bile duct was isolated. The lower
segment of the common bile duct was incised, and a stent catheter
appropriately 1 cm in length (internal diameter, 0.5 mm) was
implanted, followed by double ligature with 8-0 nylon sutures. The
main portal vein was isolated, and the pyloric vein was ligated and
divided. The celiac trunk and its branches were isolated, and the
left gastric artery, splenic artery and gastroduodenal artery were
ligated with 8-0 nylon sutures and divided. The splenic artery
stump was retained at 5 mm in length at least for the subsequent
sleeve anastomosis, and the common hepatic artery and proper
hepatic artery were reserved (Fig.
1).
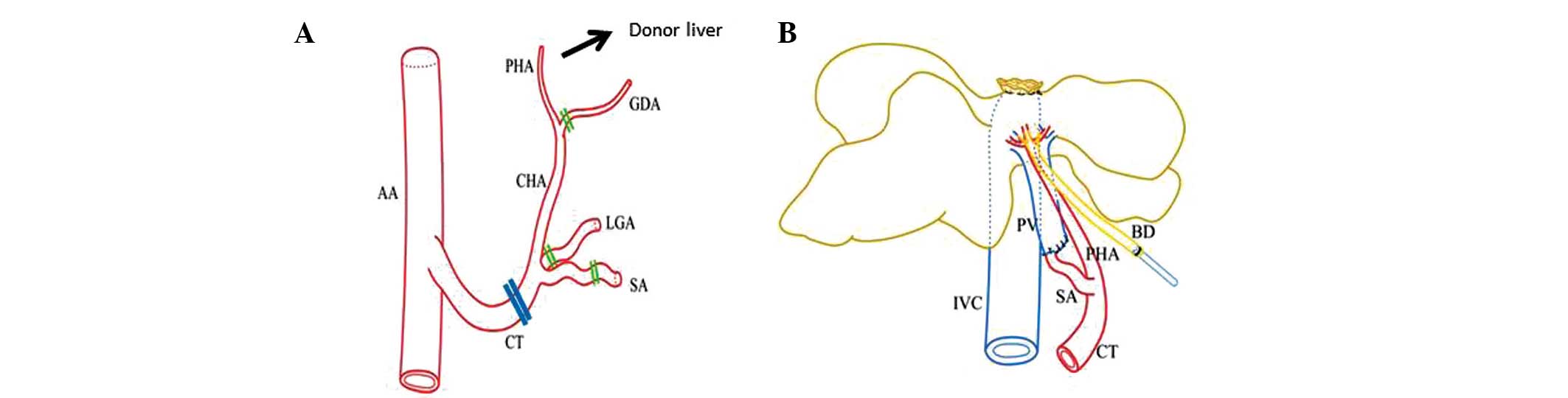 | Figure 1Schematic drawing of donor vessels and
arterial blood supply to the graft liver. (A) Double lines indicate
cutting positions. Note that SA is cut far from the CT. (B) Dual
arterial blood supply of the graft liver. Note that the SA is
connected to the PV. AA, abdominal aorta; PHA, proper hepatic
artery; CHA, common hepatic artery; GDA, gastroduodenal artery;
LGA, left gastric artery; SA, splenic artery; CT, celiac trunk;
IVC, inferior vena cava; BD, bile duct; PV, portal vein. |
Perfusion of the donor liver
The donor liver was perfused through the portal
vein. After clamping the origin of the celiac trunk, the distal
portal vein was clamped and incised, and a perfusion catheter was
implanted. Perfusion was performed with 30 ml lactated Ringer’s
solution containing low molecular weight heparin sodium (12.5
IU/ml), using the gravity perfusion method at a rate of 45
drops/min at 0–4°C. The infrahepatic caval vein was rapidly incised
to allow outflow of the blood and perfusate. The celiac trunk was
transected at its origin and rinsed with lactated Ringer’s solution
containing low molecular weight heparin sodium (12.5 IU/ml). During
the perfusion, the donor liver was persistently rinsed with
physiological saline at 0–4°C until the liver became khaki in
color. The donor liver was completely resected and stored in
lactated Ringer’s solution at 0–4°C.
Trimming of the donor liver
The hepatic pedicle and hepatic vein of the left and
medial liver lobes were ligated with 6-0 nylon sutures, and the
left and medial hepatic lobes accounting for 70% of the liver mass
were removed en bloc and the suprahepatic caval vein was ligated
simultaneously. Thus, 30% of the donor liver was obtained as the
graft. All lumens were rinsed with low molecular weight heparin
sodium salt (50 IU/ml) and trimmed to facilitate the anastomosis. A
portion of the lumen of the portal vein was sutured and closed with
10-0 nylon sutures to make the internal diameter of the portal vein
lumen and external diameter of splenic artery comparable, and then
sleeve anastomosis between the splenic artery and portal vein was
performed (Fig. 2).
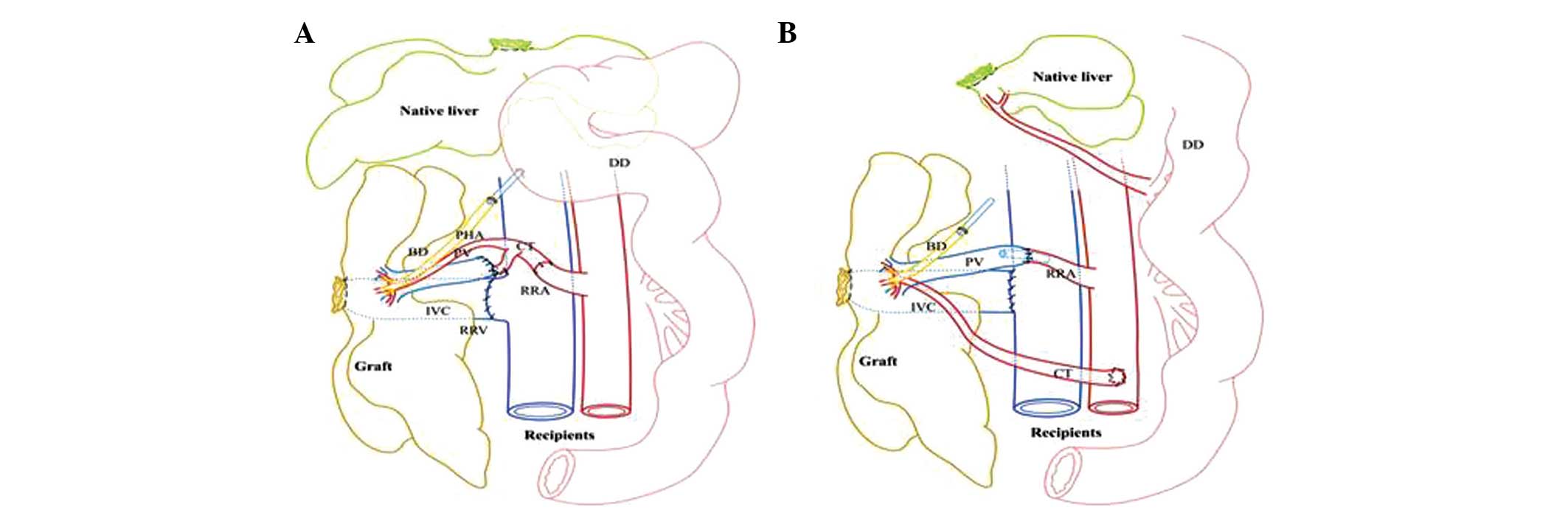 | Figure 2Comparison of vascular reconstruction
in APHLT between this study and the Schleimer model. (A) Schematic
drawing of LDABS in the current study. Note that the PV is
connected to the SA directly and the CT is connected to the RRA
without any stent. (B) Schematic drawing of the Schleimer model.
Note that the PV is connected directly to the right renal artery
using a stent, whereas the CT is connected to the AA by end-to-side
anastomosis. APHLT, auxiliary partial heterotopic liver
transplantation; LDABS, liver dual arterial blood supply; AA,
abdominal aorta, PHA, proper hepatic artery; CHA, common hepatic
artery; GDA, gastroduodenal artery; LGA, left gastric artery; SA,
splenic artery; CT, celiac trunk; IVC, inferior vena cava; RRA,
right renal artery; RRV, right renal vein; BD, bile duct; DD,
duodenum; PV, portal vein. |
Recipient surgery
Implantation of the donor liver
Following the successful induction of anesthesia, a
median incision was made into the abdomen. The right renal artery
and vein were isolated, and the origin of the right renal artery
and the site for the right renal vein entry into the infrahepatic
caval vein were clamped. The right kidney was then resected. The
graft was implanted into the right renal fossa, and an inverted
continuous all-layer suture was performed between the infrahepatic
caval vein of the graft and the right renal vein of the recipient.
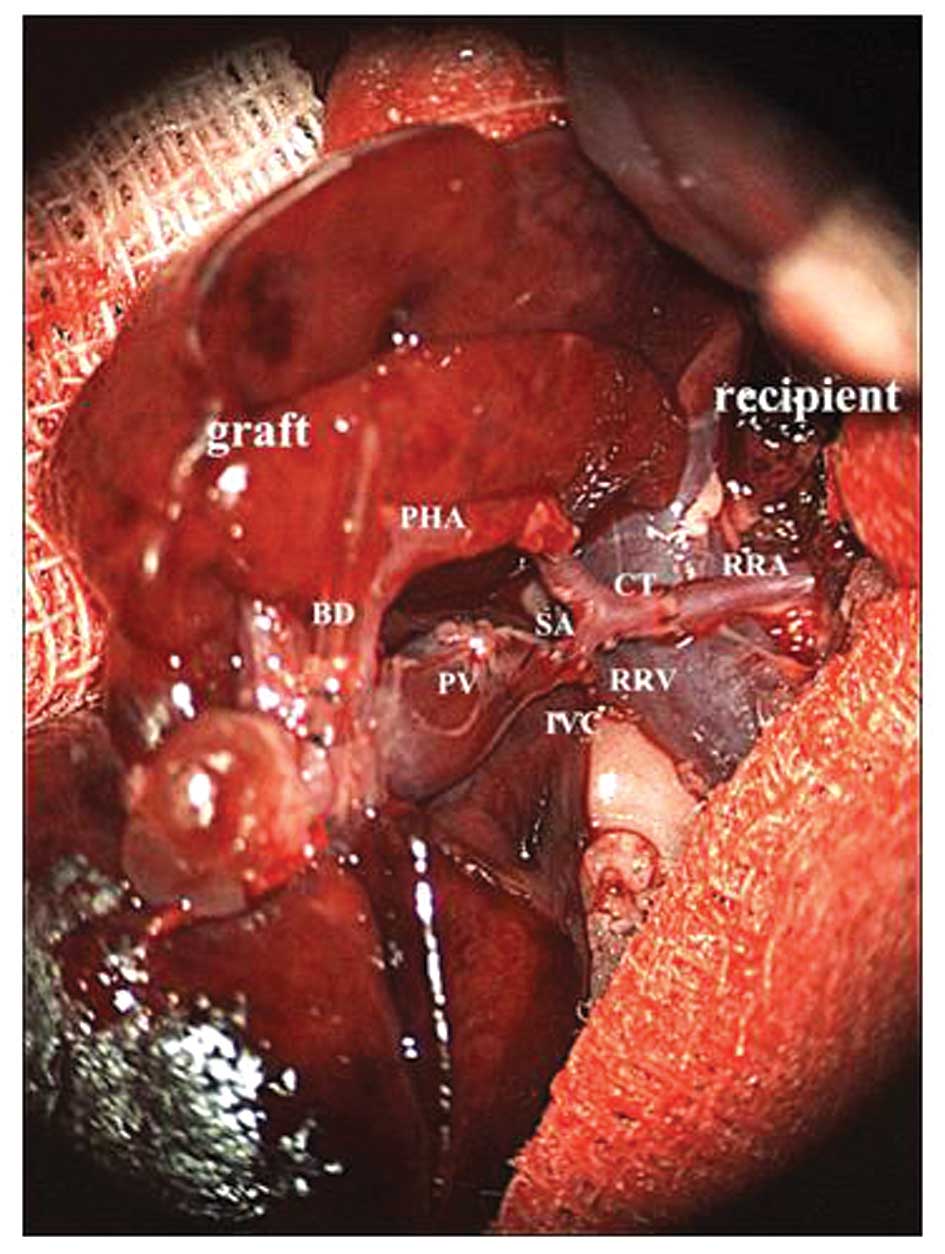
Sleeve anastomosis was performed between the celiac trunk of the
graft and the right renal artery of the recipient. Following
successful anastomosis, the right renal venous clamp and right
renal arterial clamp were removed successively to allow
reperfusion; rapid recovery of the color of the graft, pulsation in
the proper hepatic artery and celiac trunk, and cystic dilatation
of the proximal portal vein were observed. The graft was warmed by
rinsing with warm physiological saline, and bile was found to be
discharged from the biliary stent catheter. Then, a stent catheter
was implanted into the duodenum of the recipient, followed by
purse-string suturing with 8-0 nylon sutures. To avoid dislocation
and blood flow disturbance of the graft, the donor’s ligated
suprahepatic caval vein was sutured and fixed with the right
lateral abdominal wall (Fig.
3).
Blood flow control of arterialized
transplanted hepatic portal vein
Following completion of the vascular reconstruction
of the graft, the blood flow of the arterialized portal vein was
measured using an ultrasonic flowmeter (Transonic Systems, Inc.,
Ithaca, NY, USA), and controlled within the physiological range
(from 0.8±0.2 to 2.4±0.8 ml/min/g liver weight) (8) through suturing and narrowing the
origin of the right renal artery of the recipient.
Recipient hepatectomy
A 70% section of the native liver (left and medial
hepatic lobes) was resected en bloc by bloodless hepatectomy. The
Glisson’s system of two hepatic lobes was ligated with 6-0 nylon
sutures and then divided. The proximal portal vein was punctured
and slowly infused with 3 ml lactated Ringer’s solution (37°C,
similar to autotransfusion). Then, the hepatic vein was wrapped and
ligated with 6-0 nylon sutures, and the left and medial hepatic
lobes were resected successively in a clockwise direction. The
abdominal cavity was infused with 5 ml warm lactated Ringer’s
solution, and two-layer interrupted suture of the abdominal wall
was performed with 3-0 silk sutures.
Preoperative preparation
All rats were fasted for 24 h prior to the
transplantation, and were given free access to water. During the
transplantation, a heating blanket was applied to the recipient
rats to keep the body temperature at 36°C. The recipient rats were
awake within 3 min after the transplantation, and then were able to
perform turn-over activities. A high post-operative body
temperature was maintained, and all rats had free access to food
and water. In addition, 2 ml low molecular weight heparin sodium
salt (50 IU/ml) was injected subcutaneously daily following the
surgery. Antibiotics and analgesics were not administered
generally.
Results
In total, 20 rats were used to establish the rat
models of APHLT (10 as donors and 10 as recipients). The mean
operative duration was 154.5±16.4 min, and the warm and cold
ischemia times of the graft were 8.1±1.1 min and 64.5±6.6 min,
respectively. The blood flow of the arterialized portal vein to the
graft was 1.8±0.3 ml/min/g liver weight, as measured by the
ultrasonic flowmeter, which was within the physiological range.
The blood flow at all anastomotic stomas was smooth
following transplantation. Waking with a post-surgical survival of
>24 h was defined as successful model establishment. In this
study, the success rate of model establishment was 70% (7/10), and
three rats died due to long operative duration, large trauma and
bleeding of the anastomotic stoma in the 24 h after surgery. The
rat had free access to food and water following the surgery. All
rats were able to perform normal activities, had glossy hair, and
were alert. All rats that were successfully modeled survived 7 days
post-surgery (100%; 7/7). The graft was found to be soft in texture
and bright red in color following exploratory laparotomy.
Discussion
In the present study, a new rat model of APHLT with
PVA was developed, characterized by: i) the graft splenic artery is
reserved on the celiac trunk from the donor; ii) the graft portal
vein is directly sleeve anastomosed to the graft splenic artery for
arterialization of the portal vein; iii) the graft celiac trunk is
sleeve anastomosed with the right kidney artery of the recipient;
iv) the graft infrahepatic caval vein is end-to-end anastomosed
with the right renal vein of the recipient. Furthermore, the study
introduces a new term instead of PVA to describe this vascular
reconstruction method; since the blood supplies of the graft liver
in both the hepatic artery and portal vein are from same source
(arterial blood from the graft celiac artery), the term is liver
dual arterial blood supply (LDABS). LDABS differs from
arterioportal fistula (9), partial
portal vein arterialization (PPVA) (10) or arterialized portal vein blood
supply alone during which the hepatic artery blood supply ceased
owing to thrombosis, surgical ablation or injury (11,12).
Rat models have been widely used in experimental
liver transplantation studies, including transplantation immunity,
liver graft preservation and liver regeneration, since rats are
cheap, and easy to model, as well as having immunological tolerance
(13). The first ALT model in rats
was reported by Lee and Edgington in 1966 (14). Subsequent to this, various ALT
models in rats have been developed (15–17).
Of these models, the APHLT with PVA model in rats was developed by
Schleimer et al (7); one of
advantages of this model is that portal venous blood flow is
controlled by the connected stent. This model is simple and easy to
perform. However, our experiments demonstrated that this method
suffers from the following problems: i) the stent that joins the
portal vein of the graft to the right renal artery of the recipient
is extremely likely to undergo thrombosis, even if systemic
heparinization is used following the surgery; ii) the abdominal
aorta is required to be clamped during the end-to-side anastomosis
between the celiac trunk of the graft and the recipient’s abdominal
aorta for reconstruction of the hepatic artery in the graft liver.
This procedure not only affects the recipient’s internal
environment, but also is challenging to perform; iii) it is
necessary to clamp the recipient’s infrahepatic caval vein during
the end-to-side anastomosis with the infrahepatic caval vein of the
graft, which further affects the rat’s systemic state.
To overcome these challenges, a new method for
establishing the rat model of APHLT with LDABS was developed in the
present study. This provided an experimental basis to investigate
the feasibility of using LDABS to reconstruct the blood flow of the
graft in heterotopic auxiliary liver transplantation and explore
the molecular mechanism in the regulatory effect of LDABS on graft
liver regeneration. The following aspects should be noted during
the surgery: i) during the preparation of the donor liver, the
isolation is difficult to perform due to the thin diameter of the
celiac trunk and its branches, and the isolation may stimulate
spasm of the proper hepatic artery, leading to the possible effect
of transient ischemia on the donor liver; ii) the large
differentiation in the diameter between the portal vein and splenic
artery (~3-fold) leads to difficulties in anastomosis. In this
study, a portion of the portal venous lumen was sutured and closed
and then sleeve anastomosed with the splenic artery. The blood flow
of the portal vein was 1.8±0.3 ml/min/g liver weight, as measured
by the ultrasonic flowmeter, which was within the physiological
range. However, the anastomosis is challenging to perform, and the
operator should have a high-level microvascular anastomosis
technique; iii) the hepatic artery and portal vein of the graft
receive the same arterial blood supply from the recipient’ right
renal artery via the celiac trunk of the graft, which avoids
vascular reconstruction using the recipient’s abdominal aorta, and
causes minor trauma to the recipient; and iv) end-to-end
anastomosis of the infrahepatic caval vein of the graft and the
recipient’s right renal vein is used to build the blood reflux
pathway. This method may avoid the blockage of the recipient’s
infrahepatic caval vein during the anastomosis, and reduce the
effect on the recipient’s systemic state; however, this pathway is
likely to develop venous return obstruction owing to vascular
distortion. Therefore, the length of the infrahepatic caval vein of
the graft and the recipient’s right renal vein should be shortened
as much as possible so as to widen the venous outflow of the graft.
Exploratory laparotomy one week after the surgery revealed that the
graft was soft in texture and bright red in color.
In future, if the vascular reconstruction developed
in this model is considered for application to large animals or
even to human subjects, the iliac artery could be used instead of
the renal artery, such as described by Fernández-Rodríguez et
al in pigs (18).
This study has a limitation, which is that no
histological analysis or serum tests of liver enzyme activity were
performed to evaluate the functioning of the graft liver. Future
studies to perform these are planned.
In conclusion, a new rat model of APHLT with LDABS
without stenting for vascular reconstruction was developed. This is
a feasible and reliable rat model for liver transplantation
study.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (grant no. 81260073). Chunlei Han was
funded by Centre of Excellence in Molecular Imaging in
Cardiovascular and Metabolic Research, supported by the Academy of
Finland.
References
|
1
|
Faraj W, Dar F, Bartlett A, et al:
Auxiliary liver transplantation for acute liver failure in
children. Ann Surg. 251:351–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohno Y, Mita A, Ikegami T, et al:
Temporary auxiliary partial orthotopic liver transplantation using
a small graft for familial amyloid polyneuropathy. Am J Transplant.
12:2211–2219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jaeck D, Pessaux P and Wolf P: Which types
of graft to use in patients with acute liver failure? (A) Auxiliary
liver transplant (B) Living donor liver transplantation (C) The
whole liver (A) I prefer auxiliary liver transplant. J Hepatol.
46:570–573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Charco R, Margarit C, López-Talavera JC,
et al: Outcome and hepatic hemodynamics in liver transplant
patients with portal vein arterialization. Am J Transplant.
1:146–151. 2001. View Article : Google Scholar
|
|
5
|
Erhard J, Lange R, Rauen U, et al:
Auxiliary liver transplantation with arterialization of the portal
vein for acute hepatic failure. Transpl Int. 11:266–271. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Margarit C, Bilbao I, Charco R, et al:
Auxiliary heterotopic liver transplantation with portal vein
arterialization for fulminant hepatic failure. Liver Transpl.
6:805–809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schleimer K, Stippel DL, Tawadros S, et
al: Improved technique of heterotopic auxiliary rat liver
transplantation with portal vein arterialization. Langenbecks Arch
Surg. 391:102–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schleimer K, Stippel DL, Kasper HU, et al:
Physiologic microcirculation of the heterotopically transplanted
rat liver with portal vein arterialization depending on optimal
stent diameter. Med Sci Monitn. 12:BR140–BR145. 2006.
|
|
9
|
Iwaki T, Miyatani H, Yoshida Y, et al:
Gastric variceal bleeding caused by an intrahepatic arterioportal
fistula that formed after liver biopsy: a case report and review of
the literature. Clin J Gastroenterol. 5:101–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsivian M, Neri F, Prezzi D, et al: Portal
vein arterialization in hepatobiliary surgery and liver
transplantation. Transplant Proc. 39:1877–1878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melandro F, Lai Q, Levi Sandri GB, et al:
A case of portal vein arterialization after a liver transplant. Exp
Clin Transplant. 11:287–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu J, Wu H, Prasoon P and Zeng Y: Portal
vein arterialization in hilar cholangiocarcinoma: one case report
and literature review. Eur J Gastroenterol Hepatol. 24:229–232.
2012. View Article : Google Scholar
|
|
13
|
Spiegel HU and Palmes D: Surgical
techniques of orthotopic rat liver transplantation. J Invest Surg.
11:83–96. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee S and Edgington TS: Liver
transplantation in the rat. Surg Forum. 17:220–222. 1966.PubMed/NCBI
|
|
15
|
Muller G: A simple technique for
heterotopic auxiliary liver transplantation in the rat.
Transplantation. 36:221–222. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan YD, Praet M, Vanzieleghem B, et al:
Effects of re-arterialization on early graft function and
regeneration in the rat model of heterotopic auxiliary liver
transplantation. Eur Surg Res. 32:11–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rong XD, Hishikawa S, Sato M, et al: Rat
auxiliary liver transplantation without portal vein reconstruction:
comparison with the portal vein-arterialized model. Microsurgery.
21:189–195. 2001. View Article : Google Scholar
|
|
18
|
Fernández-Rodríguez OM, Ríos A, Montoya M,
et al: Description of a new auxiliary heterotopic partial liver
transplantation technique with portal vein arterialization of
applicability in heterotopic liver xenotransplantation. Transplant
Proc. 35:2051–2053. 2003. View Article : Google Scholar
|