Introduction
Arrhythmogenic right ventricular cardiomyopathy
(ARVC) is a familial disease that is characterized by right
ventricular fibrofatty degeneration, which promotes right
ventricular dysfunction and arrhythmogenesis (1–3). In
a previous study, we reported the electrocardiogram (ECG) features
of ARVC in China (4). Given that
mutations in desmosomal proteins, including desmin, desmoplakin,
desmoglein, desmocollin, plakoglobin and plakophilin-2 (PKP-2), are
important in the pathogenesis of ARVC, this condition has been
termed a desmosomal disease (5–7).
Cardiomyocytes are held together at so-called
intercalated discs, which are composed of gap junctions, fascia
adherens junctions and desmosomes. Gap junctions are the primary
structures that underlie the electrical conduction between
myocytes. Gap junctions consist of connexons, which are delicate
interdigitating structures composed of hexameric assemblies of
connexins from cells on both sides of each junction (8–10).
The mechanical stability of the gap junction is provided by the
more rigid structure of adherens junctions and desmosomes
co-localized in the intercalated disc region. PKP-2 is an important
desmosomal protein that interacts with plakoglobin (11). In a previous study, we detected a
mutation in the PKP-2 gene in a Chinese patient with ARVC (12).
To evaluate the effect of mechanical coupling on
electrical coupling in ARVC, Oxford et al (13) demonstrated that the inhibition of
PKP-2 expression resulted in reduced expression and abnormal
subcellular localization of connexin43 (Cx43), a typical gap
junction protein. Further to this, two questions were proposed: i)
Whether the expression of ARVC-related PKP-2 mutants or the
silencing of the wild-type protein led to the redistribution of
Cx43, and ii) whether the decrease in Cx43 level was attributable
to changes in gene transcription. The aim of the present study was
to answer these two questions. A novel mutation was detected in a
typical patient with ARVC from our hospital (Guangdong General
Hospital, Guangzhou, China). The mutation was used to study the
association between PKP-2 and Cx43.
Subjects and methods
Clinical data
A 62-year-old male was referred to the hospital due
to recurrent faintness. The patient’s resting ECG showed T-wave
inversion in the V1-4 leads. Sustained ventricular
tachycardia of the left bundle branch block morphology with an
inferior axis was recorded during the inpatient period, and
signal-averaged ECG recordings showed positive late potentials.
Echocardiography revealed an enlarged, hypokinetic right ventricle
with a paper-thin free wall. Following a diagnosis of ARVC, the
patient provided informed consent for genetic analysis. The patient
received radiofrequency current catheter ablation four times, but
the procedures were unsuccessful. He was then instead given an
implantable cardioverter defibrillator (Medtronic, Minneapolis, MN,
USA). This study was conducted in accordance with the Declaration
of Helsinki and with approval from the Ethics Committee of
Guangdong General Hospital. Written informed consent was obtained
from the participant.
Genetic analysis
The genomic DNA and RNA of the patient were
extracted from peripheral blood cells using standard methods. All
14 exonic and adjacent intronic sequences of the PKP-2 gene were
examined using polymerase chain reaction (PCR) amplification
combined with direct sequencing. The primers are listed in Table I (Promega Biotechnology Ltd.,
Shanghai, China). The cDNA of the patient was obtained from the
mRNA by reverse transcription PCR (RT-PCR). RT-PCR combined with
direct sequencing was used to detect the mutation. The primers are
listed in Table II (Promega
Biotechnology Ltd.).
 | Table IPrimers used to amplify the exons of
plakophilin-2. |
Table I
Primers used to amplify the exons of
plakophilin-2.
| Exon (protein
no.) | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| Pkp-2-1 (544) |
GCCCACGAGGCCGAGCTCC |
AGCAAGTCGGTCATACCGAAGA |
| Pkp-2-2 (425) |
TACTTGTTCTTGGCCTTCATTAC |
GCACTAGGATGTAAGAATGTTTC |
| Pkp-2-3 (920) |
TTCAGAGAAACGGACATGTTGG |
AAGGGCTTCCAGAGATAAGTGA |
| Pkp-2-4 (302) |
TAGGCAGGAGGAGGGAGGT |
CCAAAGTGCTGGGAATAT |
| Pkp-2-5 (479) |
CAAGAGCCTCAGTTGTGCTAC |
CCTTCTCTAGCATAACAATGAG |
| Pkp-2-6 (417) |
TAACTATACAGGCTCTTATTTCAG |
CTGGAGTGTAGTGGCACAATC |
| Pkp-2-7 (363) |
CATAGCCCTGGAGTTGATGG |
AAGAACCAAAGGCAGAATATATCC |
| Pkp-2-8 (283) |
ACAAAGACCTGTTGGATACACA |
CTCAGTAAATGAATCAGTGAATAA |
| Pkp-2-9 (431) |
TTCTAGCCATACTCATTGCATTTC |
ACTTGGTATATATCGGCACTATT |
| Pkp-2-10 (481) |
TTCTATTTCAAGGGCTTCTTATG |
AGCCTGACTTGACTTTGCATAA |
| Pkp-2-11 (339) |
TCAACCTCTGGTAATCTACAGA |
CATTGCATTGTATCTTCAGCATG |
| Pkp-2-12 (479) |
AGTGAGCCAAGATGGTGCCA |
CAGCAAACAGGATGTAAAGCC |
| Pkp-2-13 (358) |
GGCCTGACTTCATGGATGGCT |
CCTTTCACGTTTCTGTTTGCTTA |
| Pkp-2-14 (589) |
CTGGGAAGAAATCGCTAAAA |
GCAGAACAATACACTGGAGGC |
 | Table IIPrimers used for the reverse
transcription polymerase chain reaction. |
Table II
Primers used for the reverse
transcription polymerase chain reaction.
| No. | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| 1 |
ATGGCAGCCCCCGGCGCCCC |
GCCTGGCCGACAGTCAAGTG |
| 2 |
GTGGATTCCAGCGGGAGGAG |
GAGAGGTTATGAAGAATGCACACA |
| 3 |
ACCATTGCAGATTACCAGCCAGA |
TCAGTCTTTAAGGGAGTGGTAGG |
Construction of the plasmids
The cDNA for the wild-type and mutant PKP-2 was
obtained in our laboratory by RT-PCR using the mRNA derived from
the peripheral blood cells of normal controls and from the patient
with ARVC. There were 30 normal, healthy controls, all of which
provided informed consent. The primers were as follows: Forward,
5′-GAAGGAATTCGG TACATGGCAGCCCCCGGCGCCCCAGC-3′ and reverse,
5′-TCGCGATCGCCCGGGCTCTTCTAGTCT TTAAGGGAGTGGTAGGCTT-3′. The cDNA
clone nucleotides of the wild-type and mutant PKP-2 were inserted
in a pReversied-M-29 vector (Promega Biological Products Ltd.,
Shanghai, China) carrying green fluorescent protein by digestion
with the enzymes EcoRI and AsiSI, respectively. The
nucleotides were then verified by sequencing.
Cell culture and transfections
Rat mesenchymal stem cell (rMSC) lines expressing
Cx43 were provided by Dr Xiaohong Li (Medical Research Center,
Guangdong Cardiovascular Institute, Guangdong General Hospital,
Guangzhou, China). The cell lines were maintained at 37°C in a
humidified atmosphere of 5% CO2 and 95% air. The cell
lines were cultured in Dulbecco’s Minimal Essential Medium with
penicillin/streptomycin and 10% fetal bovine serum (Promega
Biotechnology Ltd.). The wild-type and mutant PKP-2 plasmids were
transfected into rMSC lines using Lipofectamine® 2000
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s protocol. rMSC RNA and protein were extracted
48 h after transfection.
PKP-2 and Cx43 expression
mRNA was purified and quantified from the rMSC lines
48 h after transfection. The expression of PKP-2 and Cx43 was
observed at the transcriptional level using RT-PCR and SYBR green
dye (Promega Biotechnology Ltd.). The primers used were as follows:
i) PKP-2 (120 bp) forward, 5′-GCTGCTTCCGTCCTTCTGTA-3′ and reverse,
5′-GGAGTGGTAGGCTTTGGCA-3′; Cx43 (111 bp) forward,
5′-AGAATGTAGCAGTAATCGCCA-3′ and reverse, 5′-TGA
CTAAAGCCCGTTTACAGTAC-3′; β-actin (155bp) (internal reference)
forward, 5′-TGTTGCCCTAGACTTCGAGCA-3′ and reverse,
5′-GGACCCAGGAAGGAAGGCT-3′. A mixture of all reagents was prepared
as follows: 10 μl SYBR green reaction buffer, 7 μl sterile purified
water, 1 μl 20 μM forward primer, 1 μl 20 μM reverse primer and l
μl purified mRNA (≤250 ng/reaction). Samples were run in triplicate
on a 7300 Real-Time PCR System in 96-well MicroAmp®
optical plates (both Applied Biosystems, Foster City, CA, USA). RT
was performed at 50°C for 30 min, followed by inactivation of RT at
95°C for 15 min, and 40 cycles of 95°C (30 sec), 54°C (30 sec), and
72°C (40 sec). Relative quantities were calculated using Stratagene
instrument software (Agilent Technologies, Santa Clara, CA,
USA).
Statistical analysis
Differences in mRNA content among the control,
wild-type and mutant groups were evaluated using two-way analysis
of variance, with α of ≤0.05 considered significant. All results
are expressed as the mean ± standard error.
Results
Genetic analysis
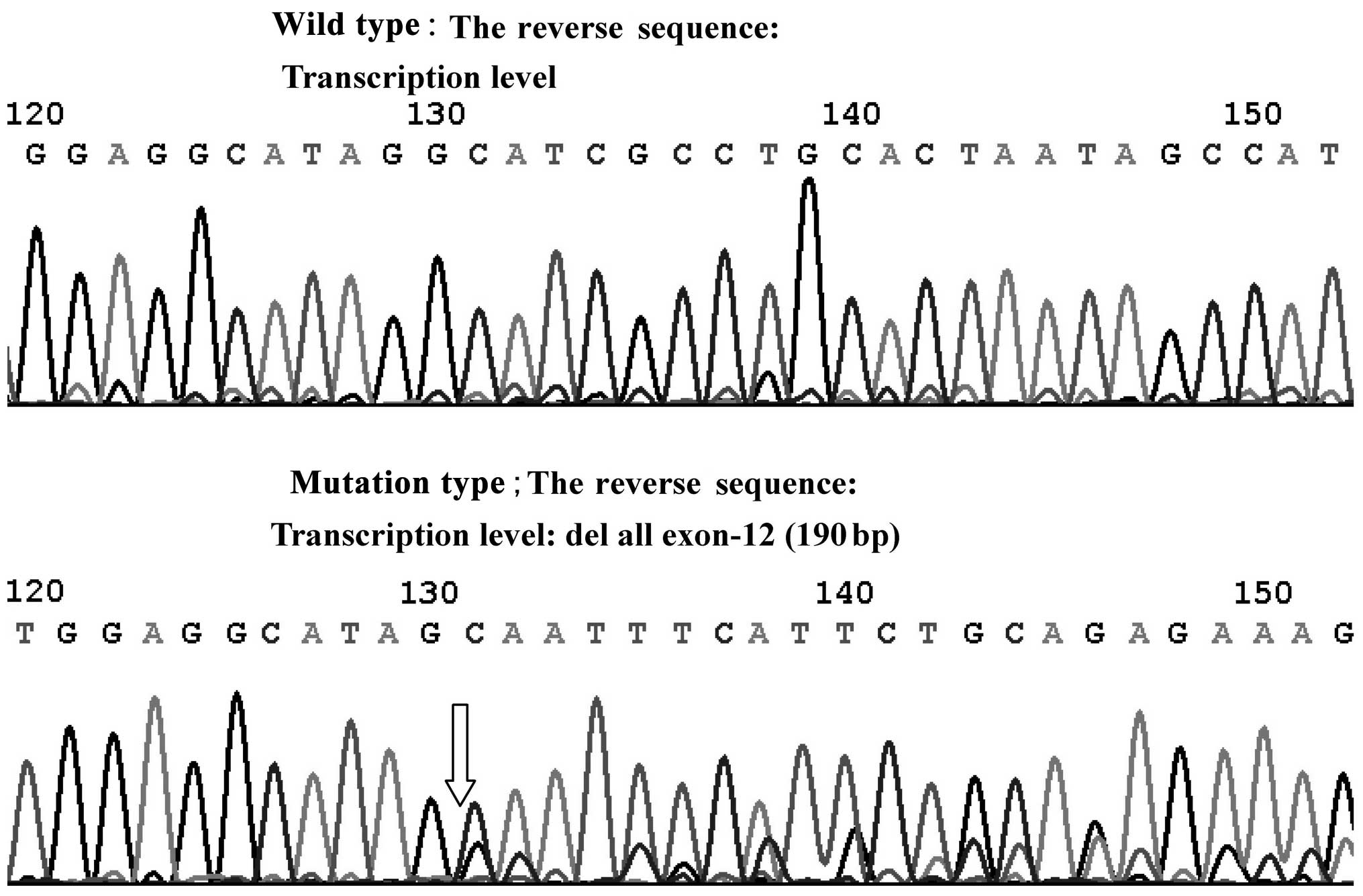
The junction of intron-10 and exon-11 in PKP-2
showed the deletion mutation (2 bp) (c.2146-1-2146 Del GA) at the
DNA level by direct sequencing. Direct sequencing also revealed the
deletion of exon-12 (190 bp) at the transcriptional level (Fig. 1).
PKP-2 expression
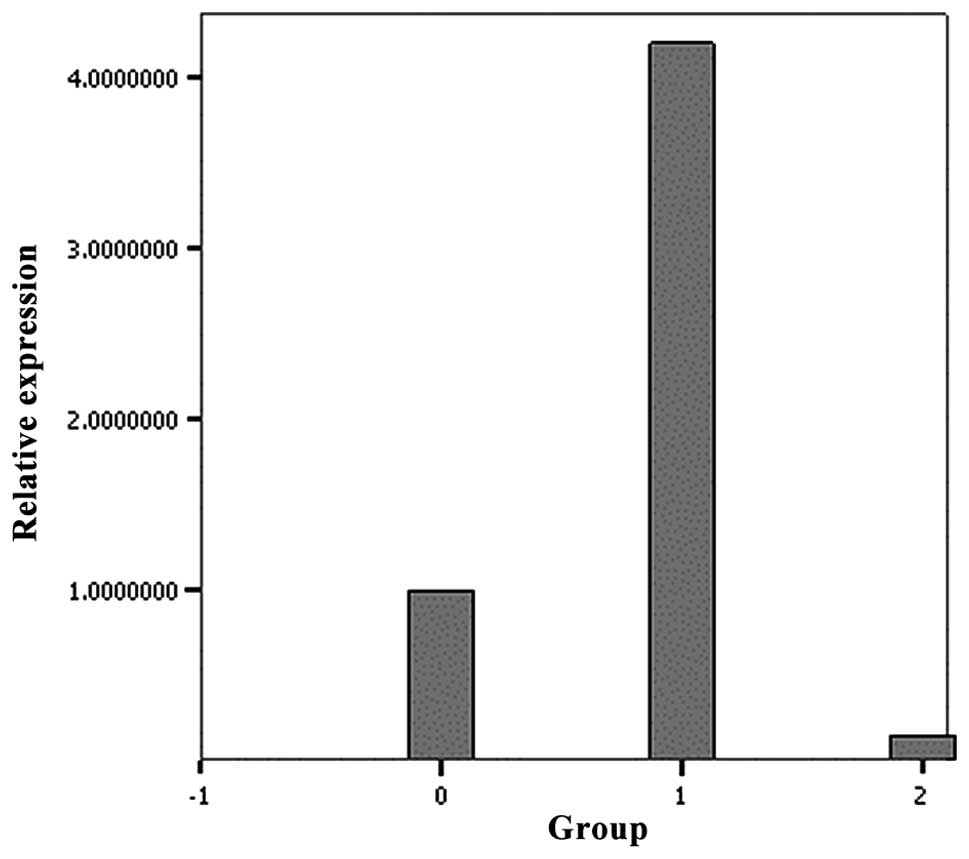
RT-PCR was used to detect the differential
expression of PKP-2 mRNA. The level of mRNA expression observed in
the mutant PKP-2 group was significantly lower than that in the
wild-type group (Fig. 2).
Cx43 expression
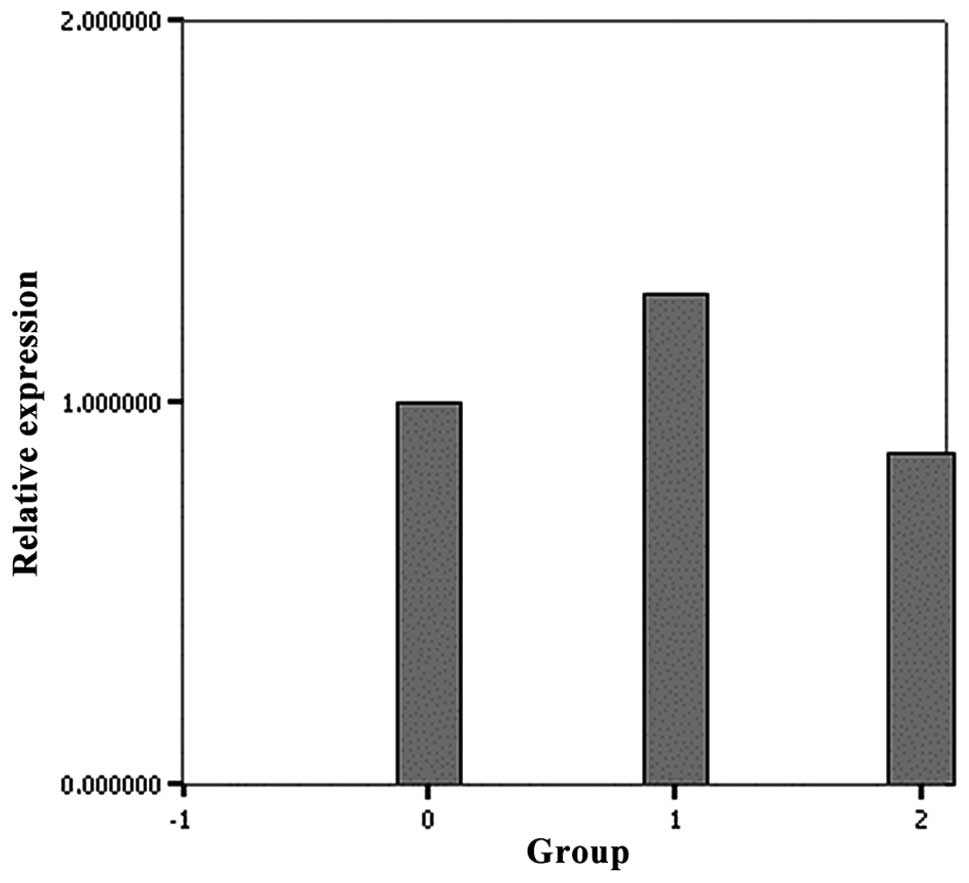
RT-PCR of the mutant PKP-2 group showed a
significantly lower level of Cx43 mRNA compared with the wild-type
PKP-2 (Fig. 3). The mRNA
expression of Cx43 in the wild-type group and control were not
significantly different (P=0.085).
Discussion
The main clinical feature of ARVC is ventricular
tachycardia, which originates from the right ventricle and can lead
to syncope or sudden cardiac death (14–16).
Ventricular arrhythmia is attributed to disordered electrical
signals; however, the factor inducing the gap junction to affect
the conduction of electrical signals remains unknown. We propose
that one of the mechanisms is the effect of PKP-2 mutation on the
decrease in the expression of Cx43, an important part of the gap
junction.
Cx43 expression has also been found to be
significantly reduced in patients with Naxos disease. Naxos disease
is characterized by ARVC, palmoplantar keratoderma and wooly hair,
which suggests that the integrity of the desmosome is a
prerequisite for the normal functions of the gap junction (17,18).
In the present study, a novel PKP-2 mutation was
detected in a patient with ARVC; this mutation not only appeared at
the DNA and RNA levels, but also induced changes in the amino acid
sequence (Table III). The
changes to the base pairs and amino acids occurred when exon-12 was
spliced. The amino acid sequence of protein number 767 changed from
GCC to GCU; however, the protein remained as alanine. The next
protein along, protein number 768, changed from lysine to
methionine, due to the amino acid sequence change from AAA to AUG.
In answer to the questions raised by Oxford et al (13), it was found that the expression of
ARVC-related PKP-2 mutations could lead to a similar redistribution
of Cx43. In addition, it was demonstrated that the decrease in Cx43
content was attributable to changes observed at the level of gene
transcription.
 | Table IIIChanges in the base pairs and amino
acids when exon-12 is spliced. |
Table III
Changes in the base pairs and amino
acids when exon-12 is spliced.
| WT base | WT amino acid | MT base | MT amino acid | Protein number |
|---|
| AAU | N | AAU | N | 764 |
| GAA | E | GAA | E | 765 |
| AUU | I | AUU | I | 766 |
| GCC | A | GCU | A | A767A |
| AAA | K | AUG | M | K768M |
| GAA | E | CCU | P | E769P |
| ACU | T | CCA | P | T770P |
| CUC | L | ACA | T | L771T |
| CCU | P | AAG | K | P772K |
In a previous study, Fidler et al (19) performed endomyocardial biopsies of
27 patients with ARVC with mutated PKP-2 genes, as confirmed by
sequencing. The biopsied tissues were then assessed by
immunofluorescence to visualize intercalated disc proteins. Reduced
Cx43 expression and localization to the intercalated disc were
observed in heterozygous human PKP-2 volunteers, which potentially
explains the delayed conduction and propensity to develop
arrhythmia in this disease.
The mechanisms by which mutations in mechanical
junctions affect the rhythm of the heart remain unknown (8). The results of the present study
indicate that mutations in PKP-2, an important structural protein
that stabilizes cardiac gap junctions, contribute to the
pathophysiology underlying AVRC. It is believed that the effect of
mutant PKP-2 may be a consequence of alterations in the electrical
conductions in the heart. The potential effect of PKP-2 mutations
on mitochondrial function and myocyte apoptosis merits further
study.
In conclusion, this study is the first to
investigate the pathophysiological processes of ARVC using the
PKP-2 mutation, which was screened in a clinical setting, to
determine whether the expression of Cx43 has a function in the
development of ARVC. In future studies we aim to collect more data
from patients with ARVC and detect the same mutation to further
reveal the nature of this disease.
Acknowledgements
The present study was supported by the Guangdong
Province Science and Technology Program (grant no.
2011B031800008).
References
|
1
|
Murray B: Arrhythmogenic right ventricular
dysplasia/cardiomyopathy (ARVD/C): a review of molecular and
clinical literature. J Genet Couns. 21:494–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
John RM, Tedrow UB, Koplan BA, et al:
Ventricular arrhythmias and sudden cardiac death. Lancet.
380:1520–1529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiene G, Rigato I, Pilichou K, Corrado D
and Basso C: Arrhythmogenic right ventricular cardiomyopathy. What
is needed for a cure? Herz. 37:657–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu S, Wang P, Hou Y, Yang P, Xiao Y and
Zhan X: Epsilon wave in arrhythmogenic right ventricular
dysplasia/cardiomyopathy. Pacing Clin Electrophysiol. 32:59–63.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Tavora F, Li L, Fowler D, Zhao Z
and Burke A: Arrhythmogenic right ventricular cardiomyopathy:
reassessing the link with the desmosome. Pathology. 44:596–604.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cox MG, van der Zwaag PA, van der Werf C,
et al: Arrhythmogenic right ventricular dysplasia/cardiomyopathy:
pathogenic desmosome mutations in index-patients predict outcome of
family screening: Dutch arrhythmogenic right ventricular
dysplasia/cardiomyopathy genotype-phenotype follow-up study.
Circulation. 123:2690–2700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sen-Chowdhry S, Syrris P and McKenna WJ:
Genetics of right ventricular cardiomyopathy. J Cardiovasc
Electrophysiol. 16:927–935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delmar M: Desmosome-ion channel
interactions and their possible role in arrhythmogenic
cardiomyopathy. Pediatr Cardiol. 33:975–979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paul M, Wichter T, Gerss J, et al:
Connexin expression patterns in arrhythmogenic right ventricular
cardiomyopathy. Am J Cardiol. 111:1488–1495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oxford EM, Danko CG, Kornreich BG, et al:
Ultrastructural changes in cardiac myocytes from Boxer dogs with
arrhythmogenic right ventricular cardiomyopathy. J Vet Cardiol.
13:101–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirchner F, Schuetz A, Boldt LH, et al:
Molecular insights into arrhythmogenic right ventricular
cardiomyopathycaused by plakophilin-2 missense mutations. Circ
Cardiovasc Genet. 5:400–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu SL, Wang PN, Hou YS, et al: The
mutation of plakophilin-2 gene in arrhythmogenic right ventricular
cardiomyopathy. Chin Med J (Engl). 122:403–407. 2009.
|
|
13
|
Oxford EM, Musa H, Maass K, Coombs W,
Taffet SM and Delmar M: Connexin43 remodeling caused by inhibition
of plakophilin-2 expression in cardiac cells. Circ Res.
101:703–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marcus FI and Abidov A: Arrhythmogenic
right ventricular cardiomyopathy 2012: diagnostic challenges and
treatment. J Cardiovasc Electrophysiol. 23:1149–1153. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Philips B, Madhavan S, James C, et al:
Outcomes of catheter ablation of ventricular tachycardia in
arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ
Arrhythm Electrophysiol. 5:499–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Irie T, Kaneko Y, Nakahara T and
Kurabayashi M: Right bundle branch block morphology of ventricular
tachycardia in arrhythmogenic right ventricular cardiomyopathy. J
Cardiovasc Electrophysiol. 21:712–713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaplan SR, Gard JJ, Protonotarios N, et
al: Remodeling of myocyte gap junctions in arrhythmogenic right
ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos
disease). Heart Rhythm. 1:3–11. 2004. View Article : Google Scholar
|
|
18
|
Ortaç R, Tavlı V, Diniz G, Yılmazer MM and
Demirpençe S: Naxos-Carvajal disease: a rare cause of
cardiomyopathy with woolly hair and palmoplantar hyperkeratosis.
Anadolu Kardiyal Derg. 11:E17–E18. 2011.
|
|
19
|
Fidler LM, Wilson GJ, Liu F, et al:
Abnormal connexin43 in arrhythmogenic right ventricular
cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med.
13:4219–4228. 2009. View Article : Google Scholar
|