Introduction
Chronic prostatitis/chronic pelvic pain syndrome
(CP/CPPS) is a complex condition, characterized by uncertain
etiology and by limited response to therapy. CP/CPPS affects men of
all ages, and can significantly impair the quality of life (QoL)
and the social functioning of patients.
CP/CPPS is characterized by a wide spectrum of
symptoms, including pain in the pelvic region, irritative and
obstructive voiding symptoms, ejaculatory pain, sexual dysfunction,
depression and psycho-social maladjustment amongst others (1).
The failure to individuate a single etiological
agent has hampered the identification of curative interventions for
CP/CPPS. It has been hypothesized that infection (occult or
non-culturable infection included), as well as genetic, anatomical,
physiological, neurological and immunological factors may be
involved (alone or combined) in the pathogenesis of CP/CPPS. In
this regard, experts consider that different cases of CP/CPPS are
likely to have different etiological determinants and different
disease progression pathways (2).
According to Shoskes et al, the etiological determinants of
CP/CPPS are likely to trigger tissue and cellular responses that
include inflammation and the upregulation of cytokine expression
and release. Inflammatory injury may damage tissue components such
as nerves and blood vessels, in turn causing pain that may produce
contraction of pelvic smooth and skeletal muscles, finally leading
to lower urinary tract symptoms, ejaculatory pain and pain in other
regions, including the lower back and abdomen (3). Prolonged pain may lead to neurogenic
inflammation and peripheral and central sensitization.
It is evident that such a complex network of
etiological factors, signals and cellular responses cannot be
successfully targeted by a single therapeutic agent. Only in very
few cases [reviewed in (4)] can a
single compound attenuate the symptoms of CP/CPPS, and the failure
of single-agent therapy was denounced as early as in the year 2004
by Nickel et al (5). As a
consequence, research efforts have been focused on the design of
new multi-modal therapeutic strategies addressing the wide array of
CP/CPPS signs and symptoms (6).
In order to design optimal symptom-directed
therapeutic protocols, the clinical phenotype of each CP/CPPS
patient should be carefully assessed. A novel algorithm called
UPOINT (an acronym standing for the urinary, psychosocial,
organ-specific, infection, neurological and muscle tenderness
domains involved in the syndrome) has been validated by a number of
independent research groups, and is currently being tested in daily
clinical practice worldwide in its original form, or modified to
include a sexual dysfunction domain (UPOINTS) (7–12).
Following validation of the novel algorithm at the
diagnostic level, a pilot prospective study focusing on therapy
demonstrated that a high fraction (84%) of patients treated by
targeting each positive UPOINT domain had a clinically appreciable
improvement of CP/CPPS symptoms (7).
Since the year 2000 our research group has adopted a
multimodal approach to treat CP/CPPS. α-adrenergic receptor
blockers, antibacterial agents, Serenoa repens extracts and
various supplements active on the prostate gland have been
administered to a large number of patients, whose follow-up data
have been recorded in a database of ~1,600 men affected by
different forms of prostatitis. The present study was aimed at
retrospectively evaluating the long-term effect of combination
therapy on CP/CPPS patients, and to attempt a comparison with other
studies based on UPOINT-driven therapy.
Patients and methods
The present study was performed on patients who were
subjected to diagnostic and therapeutic protocols routinely adopted
in our clinical practice (8).
Patients provided written informed consent to anonymous publication
of their clinical data. According to the Italian regulations
(Determinazione AIFA 20/3/2008, GU 76), the protocol describing the
present observational study was notified to the Ethical Committee
of the Principal Investigator’s hospital (authorization 26/10/2009,
ICP register: 244).
Diagnostic procedures
The clinical data of 914 fully compliant patients,
diagnosed in a single urology outpatient center specialized in
treatment of prostatitis syndromes, and meeting a number of
selective inclusion criteria were retrospectively analyzed.
Patients between 20–59 years were included in this
study if they exhibited at a first visit signs and symptoms of
category III CP/CPPS, according to National Institutes of Health
(NIH) criteria (NIDDK Chronic Prostatitis Workshop, 1995).
History collection, clinical, microscopic,
microbiological and instrumental diagnosis of patients, urological
visits as well as inclusion/exclusion criteria have been described
in detail in a previous report of the present study (8), focusing on the diagnosis and UPOINTS
phenotyping of CP/CPPS patients. Urinary peak flow rate (Qmax) and
the percentage bladder voided volume (%BVV) were assessed in each
patient as previously described (8).
The severity of the chronic prostatitis symptoms was
scored by means of an Italian validated version of the NIH Chronic
Prostatitis Symptom Index (NIH-CPSI), addressing pain and voiding
symptoms, and the impact of the disease on patients’ QoL (13). A reduction of ≥6 points of the
total NIH-CPSI score was considered as a clinically appreciable
improvement of CP/CPPS symptoms (14). All CP/CPPS patients were phenotyped
according to the UPOINTS system, as previously described (8).
The International Index of Erectile Function (IIEF)
questionnaire was adopted to assess the erectile function of
patients (15). Mild to severe
erectile dysfunction (ED) was defined as a sum of the scores for
IIEF questions 1–5 and 15, which in total were inferior to 26
(15).
Study design
At time-point V0 (visit zero), after complete
clinical and microbiological assessments, patients received a full
course of combination pharmacological therapy. Microbiological
eradication of pathogens was assessed at the end of a 4-week cycle
of antibacterial therapy. All other tests were performed after 6
months of continuous combination therapy: at time-point V6 (visit 6
months), patients were subjected to a complete diagnostic protocol,
including microbiological and clinical evaluations. Follow-up
visits, including instrumental assessments, questionnaires and
urological visits, were performed 12 months (time-point V12) and 18
months (time-point V18) after the start of therapy.
Pharmacological treatment
Starting from time-point V0, patients were treated
for 6 months with a combination of drugs, already tested in a
variety of other settings (16).
Combination therapy included a daily dose of the
α-adrenoceptor blocker alfuzosin (10 mg, extended-release
formulation; various brands chosen by the patient or general
practitioner) and a S. repens extract [640 mg/day; from
patient choice of Permixon® (Pierre-Fabre Pharma, Milan,
Italy), SABA® (Lampugnani Farmaceutici, Milan, Italy) or
Serpens® (Laboratorio Italiano Biochimico Farmaceutico
Lisapharma, Como, Italy). The latter was administered alone, or in
the form of a combined tablet preparation (Profluss®;
Konpharma, Rome, Italy) including S. repens (640 mg/day),
lycopene (10 mg/day) and selenium (100 μg/day) (17,18).
The patients for which positive microbiological
cultures of prostate-specific specimens (expressed prostatic
secretions and/or post-massage voided urine) were obtained
(positive UPOINTS infection domain) received in addition an oral
antibacterial therapy with a fluoroquinolone (ciprofloxacin 750
mg/day) and a macrolide (azithromycin 500 mg/day, the first 3
consecutive days of each treatment week) for 4 weeks (16).
Statistical analysis of data
Due to the size of the patient population, and since
the distribution of the NIH-CPSI scores and uroflowmetry data in
the patient population was normal (not shown), the normality
assumption was applied to all datasets. When ordinal scales were
analyzed, both mean and median scores were calculated as measures
of the central tendency of the patient populations, and standard
deviations and interquartile ranges are shown as measures of data
dispersion.
Intergroup differences were calculated by the
Mann-Whitney test (questionnaire scales) or the t-test for unpaired
heteroscedastic samples (continuous variables). Intragroup
differences were analyzed by a paired t-test (continuous variables)
or by the Wilcoxon signed-rank test (ordinal scales).
When two treatment strategies were compared, the
analysis of covariance (two-way ANCOVA) was applied to analyze
inter-arm differences. This test was also applied to comparisons of
questionnaire scores showing non-skewed data distributions,
according to Vickers (19).
Differences in patient proportions at specific study time-points
were analyzed by a two-tailed Z test.
The VassarStats on-line statistics platform
(http://vassarstats.net) and the VassarStats
ANCOVA Excel spreadsheet (http://vassarstats.net/exl/ancovaM.xls) were used for
analysis of data. P<0.05 was considered to indicate a
statistically significant result.
Results
Total patient population
A population of 914 patients, meeting the inclusion
criteria for the present study, was extracted from our clinical
database. Patients were affected by category III CP/CPPS, of the
inflammatory (type IIIa, n=367) or non-inflammatory (type IIIb,
n=547) sub-categories (20).
Patients were phenotyped according to the UPOINTS
system (8). Similarly to a
preliminary report of the present study, and to other international
trials (2,8), 57.60, 33.77, 97.05, 9.94, 46.23,
68.52 and 49.07% of patients exhibited a positive urinary,
psychosocial, organ-specific, infection, neurological,
muscle-tenderness and sexual domain, respectively.
Table I summarizes
the clinical findings of the total patient population at enrollment
(V0), at the end of a 6-month cycle of therapy (V6), and 6 or 12
months after the end of therapy (time-points V12 and V18,
respectively).
 | Table IScores of the NIH-CPSI and IIEF
symptom questionnaires, uroflowmetry data and percentage bladder
voided volume in the total study population. Data are shown at
enrollment (V0), at the end of a 6-month cycle of combination
therapy (V6), and at follow-up 12 months (V12) and 18 months (V18)
after enrollment. |
Table I
Scores of the NIH-CPSI and IIEF
symptom questionnaires, uroflowmetry data and percentage bladder
voided volume in the total study population. Data are shown at
enrollment (V0), at the end of a 6-month cycle of combination
therapy (V6), and at follow-up 12 months (V12) and 18 months (V18)
after enrollment.
| Study
time-point |
|---|
|
|
|---|
| Variable | V0 | V6 | V12 | V18 |
|---|
| NIH-CPSI total
score [mean ± SD, (median, IQR)] | 20.91±7.12
(21,10) | 9.87±5.71
(9,7)a | 8.15±4.52 (8,
4)a,b | 7.62±4.13 (8,
4)a,b |
| NIH-CPSI pain score
[mean ± SD, (median, IQR)] | 9.51±3.57
(9,5) | 4.08±2.67
(4,2)a | 3.35±1.99
(3,1)a,b | 3.09±1.86 (3,
2)a,b |
| NIH-CPSI voiding
symptom score [mean ± SD, (median, IQR)] | 4.01±2.59
(4,4) | 2.01±1.98
(2,3)a | 1.52±1.54
(1,2)a,b | 1.45±1.53
(1,2)a,b |
| NIH-CPSI QoL impact
score [mean ± SD, (median, IQR)] | 7.40±2.81
(8,4) | 3.82±2.28
(3,2)a | 3.23±2.03
(3,2)a,b | 3.02±1.77
(3,2)a,b |
| IIEF, items 1–5 and
15 [mean ± SD, (median, IQR)] | 23.05±5.78
(24,9) | 26.29±4.18
(28,4)a | 26.06±4.92
(28,4)a | 26.23±4.61
(28,4)a |
| Urine peak flow
rate (Qmax, ml/sec) (mean ± SD) | 14.86±6.50 | 18.34±5.25c | 19.02±4.20c,d | 18.89±3.84c |
| Bladder voided
volume (%) (mean ± SD) | 84.53±18.63 | 98.19±7.89c | 99.61±4.38c,d | 99.57±4.03c |
Total NIH-CPSI scores decreased significantly from a
baseline mean value of 20.91 to 9.87 at time-point V6 and to 8.15
and 7.62 at time-points V12 and V18, respectively (P<0.0001 for
all paired comparisons vs. V0, Wilcoxon signed rank test). The
difference between values at V6 and at V12 or V18 was also
significant (P<0.0001, Wilcoxon).
A clinically appreciable reduction of ≥6 points of
the NIH-CPSI score (14) was
assessed at the end of therapy in 77.5% of patients (n=708).
A 57% reduction of NIH-CPSI pain scores was
calculated in this population at the end of therapy. Further
significant pain reductions were observed at time-points V12 or V18
(Table I).
The distribution of pain severity according to the
NIH-CPSI pain score cutoffs established by Wagenlehner et al
(21) is shown in Fig. 1A.
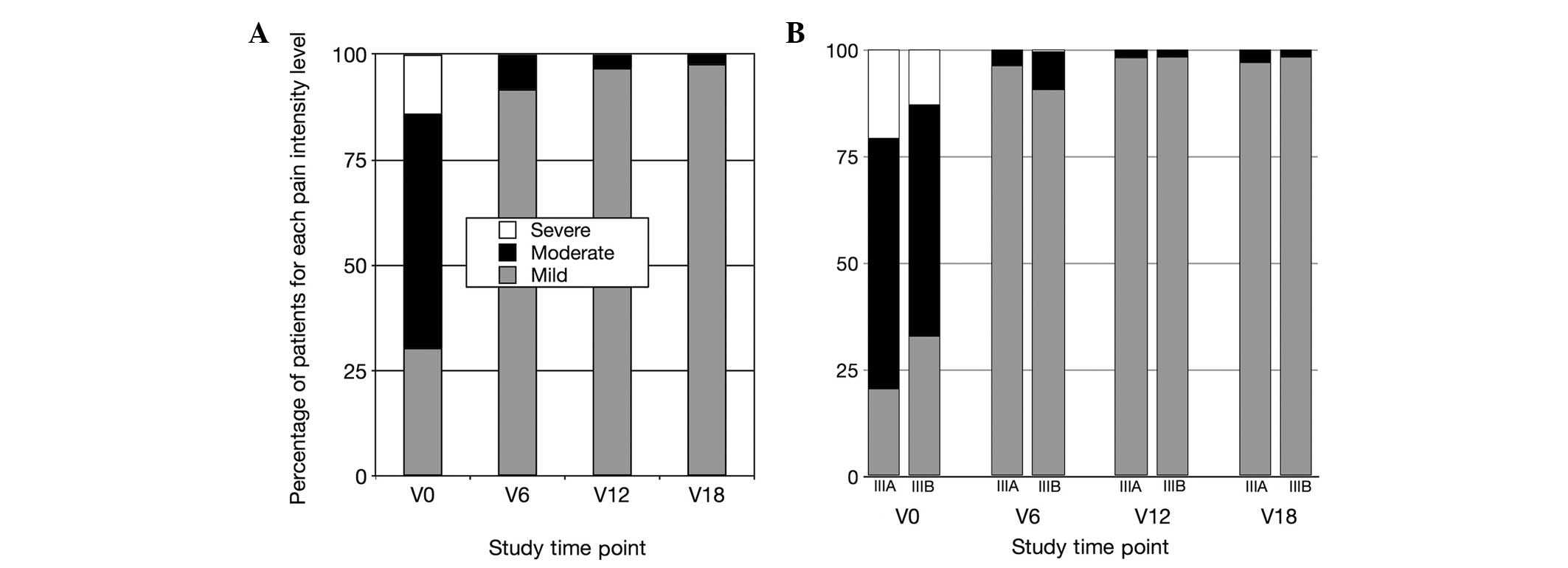 | Figure 1Distribution of pain intensity
scores, assessed in CP/CPPS patients with the NIH-CPSI. NIH-CPSI
sub-scores were assigned to three increasing pain levels, according
to Wagenlehner et al (21)
as follows: Mild pain, 0–7 points; moderate pain, 8–13 points;
severe pain, 14–21 points. (A) Pain intensity distribution in the
total patient population. (B) Pain intensity in patients affected
by the inflammatory (IIIa) or non-inflammatory (IIIb)
sub-categories of CP/CPPS. Data are shown at enrollment (V0), at
the end of therapy (V6), and at follow-up time-points (V12, V18).
CP/CPPS, chronic prostatitis/chronic pelvic pain syndrome;
NIH-CPSI, National Institutes of Health Chronic Prostatitis Symptom
Index. |
Similar to pain, voiding symptom scores decreased
significantly at time-point V6, compared with baseline data
(Table I). An additional
attenuation of voiding symptoms was observed 6 months after the end
of treatment (time-point V12), and was sustained through the entire
follow-up, until the end of the study.
The significant improvement of voiding symptoms
assessed with the NIH-CPSI was confirmed by instrumental
measurement of urinary flow and voiding efficiency; mean peak flow
rates increased from 14.86 ml/sec at V0 to 18.34 ml/sec at V6 and
19.02 or 18.89 ml/sec at V12 or V18, respectively (P<0.0001 for
all comparisons vs. V0, or V6 vs. V12, paired, two-tailed t-test;
Table I). Patients’ percentage
bladder voided volume (%BVV), which was 84.53% of total bladder
content at V0, was also significantly increased at V6 and V12
(98.19% and 99.61%, respectively; P<0.0001 for all comparisons
vs. V0, or V6 vs. V12, paired, two-tailed t-test; Table I); this increase was sustained
through the entire follow-up, until the end of the study.
Symptom relief, assessed with the NIH-CPSI test,
resulted in attenuation of the impact of the disease on the QoL of
patients (Table I).
The IIEF (items 1–5 plus 15) was used to assess ED
in the study population. A score of 26 was used as a cutoff value
to evidence mild to severe ED (15). At baseline (time-point V0) 56.9% of
patients showed ED (a score <26). This proportion decreased at
V6 (26.06%; P<0.0002 vs. V0, two-tailed Z test), and remained
steady at subsequent time-points (V12, 26.6%; V18, 26.4%). Mean
IIEF values at V0, V6, V12 and V18, and the statistical
significance of intragroup comparisons, are shown in Table I.
Subgroup analysis based on CP/CPPS
sub-categories (IIIa vs. IIIb)
The study population included patients affected by
either the inflammatory (IIIa) or the non-inflammatory (IIIb)
sub-categories of CP/CPPS.
A comparative analysis was performed to assess any
differential response to therapy between these cohorts. Table II summarizes the clinical findings
of IIIa and IIIb patient cohorts at baseline, at the end of a
6-month cycle of therapy, and 6 or 12 months after the end of
therapy (time-points V12 and V18, respectively). Intragroup paired
analysis showed that both IIIa and IIIb patients underwent
significant reductions of NIH-CPSI total scores at the end of
therapy (P<0.0001 vs. V0 for both cohorts, Wilcoxon signed rank
test; Table II), further
improving at the V12 or V18 follow-up time-points (P<0.0001 vs.
V0 and V6 for both cohorts, Wilcoxon).
 | Table IIIntragroup and intergroup analysis of
NIH-CPSI total score and symptom domain subscores, of IIEF erectile
dysfunction scores, of mean urinary peak flow levels and percentage
bladder voided volumes in patients affected by the inflammatory
(IIIa) and non-inflammatory (IIIb) sub-categories of CP/CPPS,
assessed at time-points V0, V6, V12 and V18. |
Table II
Intragroup and intergroup analysis of
NIH-CPSI total score and symptom domain subscores, of IIEF erectile
dysfunction scores, of mean urinary peak flow levels and percentage
bladder voided volumes in patients affected by the inflammatory
(IIIa) and non-inflammatory (IIIb) sub-categories of CP/CPPS,
assessed at time-points V0, V6, V12 and V18.
| Study
time-point |
|---|
|
|
|---|
| V0 | V6 | V12 | V18 |
|---|
|
|
|
|
|
|---|
| Variable | IIIa | IIIb | IIIa | IIIb | IIIa | IIIb | IIIa | IIIb |
|---|
| NIH-CPSI total
score [mean ± SD, (median, IQR)] | 22.22±6.99
(22,10) | 19.99±7.03e (20,10) | 8.99±5.10
(8,5)a | 10.49±6.03g (9,8)a | 7.86±4.39
(8,4)a,b | 8.35±4.62i (8,5)a,b | 7.55±4.29
(7,4)a,b | 7.66±4.01
(8,5)a,b |
| NIH-CPSI pain score
[mean ± SD, (median, IQR)] | 10.22±3.60
(10,5) | 9.02±3.46e (9,6) | 3.73±2.21
(3,2)a | 4.32±2.92g (4,3)a | 3.24±1.80
(3,2)a,b | 3.43±2.12
(3,1)a,b | 3.11±1.79
(3,2)a,b | 3.07±1.92
(3,2)a,b |
| NIH-CPSI voiding
symptom score [mean ± SD, (median, IQR)] | 4.12±2.52
(4,4) | 3.91±2.64
(4,4) | 1.61±1.67
(2,2)a | 2.28±2.13g (2,4)a | 1.38±1.52
(1,2)a,b | 1.63±1.56i (2,2)a,b | 1.32±1.48
(1,2)a,b | 1.54±1.56
(1,2)a,b |
| NIH-CPSI QoL impact
score [mean ± SD, (median, IQR)] | 7.90±2.86
(8,4) | 7.05±2.73e (7,4) | 3.58±2.02
(3,2)a | 3.99±2.43g (4,3)a | 3.13±1.81
(3,2)a,b | 3.31±2.18i (3,2)a,b | 3.04±1.79
(3,2)a,b | 3.01±1.76
(3,2)a,b |
| IIEF, items 1–5+15
[mean ± SD, (median, IQR)] | 23.57±5.26
(24,8) | 22.71±6.11
(24,10) | 26.52±3.81
(28,3)a | 26.15±4.44
(28,4)a | 26.39±4.81
(28,3)a | 25.87±5.04
(28,4)a | 26.32±4.70
(28,3)a | 26.02±5.66
(28,4)a |
| Urine peak flow
rate (Qmax, ml/sec) (mean ± SD) | 13.76±5.30 | 15.60±7.11f | 17.92±3.94c | 18.62±5.97c | 18.29±3.55c | 19.55±4.55c,d | 18.27±3.39c | 19.37±4.10c |
| Bladder voided
volume (%) (mean ± SD) | 83.25±18.96 | 85.37±18.37 | 98.95±6.00c | 97.65±8.97c,h | 99.78±3.83c | 99.48±4.75c,d | 99.33±5.01c | 99.76±3.10c |
Intragroup analysis of CPSI subscores showed that in
both IIIa and IIIb patients pain, voiding symptoms and the impact
of the disease on the QoL decreased markedly and significantly at
time-points V6 (P<0.0001 vs. V0 for both IIIa and IIIb cohorts,
Wilcoxon signed rank test; Table
II), and decreased further at V12 or V18 (P<0.0001 vs. V0
and V6 for both cohorts, Wilcoxon).
Figure 1B shows the
distribution of pain severity scores in IIIa and IIIb patients. The
two groups showed a marked improvement of pain symptoms at 6
months, with no patient showing severe pain at this time-point. A
small fraction of patients had moderate pain symptoms at time-point
V6 (IIIa, 3.73%; IIIb, 8.78%), further decreasing at follow-up (at
V12: IIIa, 1.86%; IIIb, 1.69%; at V18: IIIa, 2.98%; IIIb,
1.69%).
Uroflowmetry parameters (Qmax and %BVV) improved
significantly in both groups (P<0.0001 vs. V0 for both IIIa and
IIIb cohorts, paired t-test; Table
II). This effect was sustained throughout the follow-up period
(Table II).
Compared with IIEF average baseline values (<26
points in both IIIa and IIIb cohorts), at time-point V6 average
scores were above the 26 point threshold (P<0.0001 vs. V0 for
both IIIa and IIIb cohorts, Wilcoxon signed rank test; Table II). Recovery from ED was sustained
throughout the follow-up period (Table II).
Intergroup analysis (CP/CPPS IIIa vs. IIIb) showed
significant differences of baseline values of the NIH-CPSI total
score and of pain and QoL subscores, with IIIa patients showing
more severe symptoms (P<0.001 vs. IIIb for all comparisons at
time-point V0, Mann-Whitney test; Table II). The ANCOVA test was used to
analyze differential responses to therapy between IIIa and IIIb
cohorts, as it corrects baseline imbalances and is also suitable
for nonparametric analysis of data (19). As shown in Table II, analysis of symptom improvement
at the end of therapy and follow-up (time-points V6 and V12/V18)
evidenced significant intergroup differences. At time-point V6,
pain symptoms improved more markedly in IIIa patients (mean
reduction, 6.48 points), compared with IIIb patients (mean
reduction, 4.69 points; P<0.0001, ANCOVA). Similarly, voiding
symptoms and QoL scores improved more markedly in IIIa patients
(voiding score mean reduction: IIIa, 2.5 points; IIIb, 1.63 points;
impact on QoL mean reduction: IIIa, 4.32 points; IIIb, 3.06 points;
P<0.0001 for both comparisons, ANCOVA). These differences
concurred to generate a mean reduction of 13.23 and 9.5 points of
the total NIH-CPSI score in IIIa and IIIb cohorts, respectively.
Also in this case, intergroup differences were significant
(P<0.0001, ANCOVA). A reduction of ≥6 points was observed at the
end of therapy in 88.43% of IIIa patients and in 72.93% of IIIb
patients (P<0.001, two-tailed Z-test).
Intergroup Qmax values differed at baseline, with
IIIa patients showing lower peak urinary flows compared with IIIb
(1.9 ml difference between IIIa and IIIb; P<0.001, unpaired
t-test). At time-point V6, both groups showed a significant
improvement of mean Qmax values (mean increase: IIIa, 4.15 ml/sec;
IIIb, 3.01 ml/sec), but no intergroup difference was determined
(P=0.48, ANCOVA). The percentage bladder voided volume, which was
not different at baseline (P=0.09, unpaired t-test), increased more
markedly in IIIa patients (15.69% increase in mean voided bladder
volume), compared with IIIb patients (12.28% increase in mean
voided bladder volume; P<0.001, ANCOVA).
Analysis of sexual dysfunction (IIEF ED scores)
showed no baseline imbalances and no intergroup differences at
time-point V6 (Table II). At
baseline, 58.7 and 55.7% of IIIa and IIIb patients, respectively,
had an IIEF ED score <26. These percentages decreased to 24.0%
(IIIa) and 27.4% (IIIb) at time-point V6. Intergroup analysis
showed no significant differences (P=0.6, two-tailed Z-test).
Subgroup analyses based on differential
treatment
Antibacterial agents
At enrollment, patients were assigned to different
CP/CPPS cohorts (IIIa vs. IIIb) on the basis of the
presence/absence of inflammatory findings in post-massage urine or
expressed prostatic secretions. However, each study arm comprised
patients subjected to different protocol treatments. All patients
received α-adrenoceptor blockers and S. repens extracts.
Patients affected by IIIb CP-CPPS not showing evidence of infection
were not treated with antibacterial agents (NO-AB cohort). Patients
with evidence of infection (in either the IIIa or IIIb group) were
treated with antibacterial agents (AB cohort). In addition,
antibacterial therapy was administered to patients with
inflammatory IIIa CP/CPPS in the absence of infection, since the
presence of pyuria is often suggestive of an underlying occult
infection. In this respect, experts suggest empirical antibacterial
therapy if infection is suspected in CP/CPPS patients (22).
Intergroup analysis limited to pre- and post-therapy
data was performed to assess any differential response to
combination therapy between the AB and NO-AB cohorts. Total
NIH-CPSI scores decreased more markedly in the AB cohort (mean
reduction, 8.51 points) compared with the NON-AB cohort (mean
reduction, 4.25 points). Comparison by ANCOVA evidenced a
significant intergroup difference (P=0.027). NIH-CPSI pain symptoms
and the impact of the disease on QoL decreased markedly and
significantly at time-point V6; however, intergroup analysis showed
no significant differences between cohorts (pain, P=0.23; QoL,
P=0.81, ANCOVA; data not shown).
NIH-CPSI voiding symptom scores decreased more
markedly in the AB cohort (mean reduction, 2.6 points) than in the
NO-AB cohort (mean reduction, 1.48 points). This intergroup
difference was highly significant (P<0.0001, ANCOVA). The
percentage bladder voided volume increase was significantly more
pronounced in the AB cohort (mean increase, 15.83%) compared with
the comparator NO-AB cohort (mean increase, 12.03%; P=0.0029,
ANCOVA). Intergroup analysis of pre- and post-therapy urinary peak
flow rates (AB cohort, 4.25 ml/sec increase; NO-AB cohort, 2.83
ml/sec increase) lacked statistical significance (P=0.11,
ANCOVA).
Intergroup analysis of IIEF ED scores identified no
significant differences between cohorts (P=0.48, ANCOVA; data not
shown).
Phytotherapy and antioxidant
supplements
CP/CPPS patients were treated with different oral
preparations of S. repens. One preparation was based on the
sole plant extract (S cohort), whereas another preparation
contained the same dose of S. repens extract, combined with
lycopene and selenium (SLS cohort). To assess any differential
response to the two different S. repens preparations, an
intergroup comparison was performed between the S and SLS cohorts.
Patients treated with antibacterial agents were not included in
this subgroup comparison due to their unbalanced presence in the S
and SLS cohorts; analysis was limited to IIIb patients treated with
α-adrenoceptor blockers and one of the two alternative S.
repens preparations.
In the S and SLS cohorts, combination treatment
induced a marked and significant improvement of CP/CPPS signs and
symptoms, assessed at time-point V6 with the NIH/CPSI and IIEF
questionnaires, or measured by uroflowmetry (Qmax and %BVV).
Intragroup analysis revealed significant reductions
of NIH-CPSI scores in both groups (P<0.0001 for CPSI total and
pain, void, and QoL subscores, Wilcoxon signed rank test), as well
as highly significant improvements of the Qmax and %BVV parameters
(P<0.0001 for both comparisons, paired t-test; data not
shown).
Intergroup analysis, limited to pre- and
post-therapy data (V0 and V6) evidenced that the arm treated with
the preparation of S. repens combined with lycopene and
selenium showed a significantly improved relief from voiding
symptoms, compared with that in patients treated with S.
repens alone. The mean improvements of voiding scores, assessed
with the NIH-CPSI test, were 0.72 points for the S cohort and 1.14
points for the SLS cohort (P=0.047, ANCOVA). Average increases of
Qmax at time-point V6 were 1.8 ml/sec in the S cohort and 2.6
ml/sec in the SLS cohort (P=0.019, ANCOVA). The average bladder
voided volume increased by 9.2% in the S cohort and by 12.9% in the
SLS cohort (P=0.011, ANCOVA).
The mean reduction of the impact of the disease on
the QoL of patients, assessed with the NIH-CPSI test, was 2.21
points in the S cohort and 2.66 points in the SLS cohort. This
differential response was significant (P=0.049, ANCOVA). Intergroup
analysis showed that the reductions of NIH-CPSI pain and total
scores were not different at the statistical level (data not
shown).
Discussion
In a prospective trial by Shoskes et al, it
was demonstrated that 84% of patients treated with a multimodal
therapy strategy addressing all six phenotypic domains of UPOINT
had a reduction of ≥6 points in the total score of the NIH-CPSI
symptom questionnaire (7). This
important result confirmed the validity and applicability of the
UPOINT algorithm for the patient-tailored diagnosis and therapy of
CP/CPPS.
In the present study, a reduction of ≥6 points of
the total NIH-CPSI score was achieved in 77.5% of patients
subjected to combination therapy for a period of 6 months. Notably,
this value is higher than the placebo effect of ~64%, demonstrated
in long-term studies (23). This
result shows that a clinically appreciable improvement may be
achieved in a considerable fraction of patients treated with a
fixed combination of agents targeting the urinary (α-adrenoceptor
blockers and S. repens), organ-specific (S. repens,
antioxidant/anti-inflammatory supplements and anti-inflammatory
macrolides) and infection (antibacterial quinolones and macrolides,
anti-biofilm and immune-modulating macrolides) domains of
UPOINTS.
Acknowledging the limitations of a retrospective
study design, certain comparisons and considerations may be
attempted.
If an appreciable improvement can be potentially
achieved in >70% of patients treated with a combination of
α-blockers, antibacterial agents, plant extracts and supplements,
and given that drugs targeting the psychological (antidepressants
and anxiolytics), neurological (amitriptyline and pregabalin) and
muscle-tenderness domains (myorelaxants) of UPOINTS can induce
considerable side-effects, it could be advisable to design a simple
two-step algorithm for each patient. As a first step, a combination
similar to the one adopted in the present study may be
administered. In instances of an unsatisfactory response (a
reduction of the NIH-CPSI score <6), second-level agents such as
antidepressants, anxiolytics, pregabalin, myorelaxants and others
may be added to the therapeutic protocol in a second phase.
Phosphodiesterase-5 inhibitors may also be
administered to patients, to address the sexual dysfunction domain
of UPOINTS (8,10,12,24).
Also in this case, these drugs may be administered in instances of
failure of first-line agents, since the present study documents
that the simple combination therapy proposed above resulted in an
improvement of erectile function in approximately half (54.2%) of
the patients, although phosphodiesterase-5 inhibitors were not
administered during the present study. Therefore, a therapy
initially targeting only the U, O and I domains of UPOINTS may in
turn result in improvements of other domains, such as sexual
function, in a relevant fraction of patients. This is in agreement
with the evidence emerging from a previous study focusing on
chronic bacterial prostatitis, a condition related to CP/CPPS, in
which it was shown that combination therapy with antibacterial
agents, α-blockers and S. repens extracts had a positive
effect on the sexual function of a substantial proportion of
patients (16).
Furthermore, it can be hypothesized that attenuation
of the symptoms of CP/CPPS and improvement of the sexual function
may have a beneficial effect on the psychological domain of
UPOINTS, and in particular on disease-related anxiety and
depression, potentially limiting the administration of psychoactive
drugs to a smaller number of patients. Research is in progress to
investigate this hypothesis.
The cohort of patients analyzed in the present study
included subjects affected by the inflammatory (IIIa) and
non-inflammatory (IIIb) sub-categories of CP/CPPS. This
sub-classification has always been controversial, and many experts
have reputed the IIIa and IIIb variants of CPPS to be equivalent if
not identical conditions, based on the demonstration that the
presence of leukocytes, and hence the extent of inflammation, does
not correlate with the severity of symptoms of CP/CPPS, and that
variable amounts of leukocytes are also retrieved in post-massage
specimens of healthy/asymptomatic subjects (25,26).
In order to explore any difference in the clinical
presentation and in the response to therapy of IIIa and IIIb
CP/CPPS, subgroup analysis of the patient cohort was performed in
the present study.
In general, patients affected by the IIIa
inflammatory sub-category of CP/CPPS exhibited more severe signs
and symptoms (for example, in NIH-CPSI scores and Qmax) at baseline
when compared with IIIb patients. However, the improvement of
symptoms was significantly more pronounced in IIIa patients than in
IIIb patients. For example, a reduction of ≥6 points of the total
NIH-CPSI score (14) was assessed
at the end of therapy in 88.4 or 72.9% of patients affected by
CP/CPPS IIIa or IIIb, respectively.
These data are not in agreement with a recent
retrospective Korean study, performed on ~100 subjects, showing no
baseline imbalances and no differential response to combination
therapy (alfuzosin plus levofloxacin) between IIIa and IIIb CP/CPPS
patients (27). Although baseline
NIH-CPSI symptom scores and voiding parameters are almost identical
between the Korean and the present study, comparison of the results
is difficult, as in the Korean study therapy courses were shorter
(6 weeks vs. 6 months in the present study), and efficacy
assessments were performed at earlier time-points (6 weeks vs. 6
months in the present study).
In contrast to the Korean study and to our own
previous view, the evidence emerging from the present investigation
suggests that the inflammatory and non-inflammatory sub-categories
of CP/CPPS may indeed represent two distinct pathological
conditions or, alternatively, two different stages of the same
condition. In the latter case, CP/CPPS IIIb might represent a later
stage of the disease, less responsive to treatment, less prone to
improvement, and characterized by a less pronounced inflammatory
profile.
The differential response to therapy between IIIa
and IIIb cohorts might also be due to the fact that all IIIa
patients were treated with two combined antibacterial agents,
whereas the vast majority (>90%) of IIIb patients did not
receive antibacterial treatment. Antibacterial agents were
administered to IIIb patients showing evidence of infection, and to
all IIIa patients. The rationale for administering antibacterial
agents to non-infected IIIa patients is based on the hypothetical
presence of undetected or difficult-to-culture pathogens in
prostate ducts (22).
In addition to their antibacterial activity,
macrolides and, to a lesser extent, fluoroquinolones might have
concurred to more marked symptom improvement in IIIa patients
through their potent intrinsic anti-inflammatory properties
(28,29).
Although the addition of antibacterial agents to the
α-blocker/S. repens regime might have concurred to the
improved response to therapy in IIIa patients, more severe symptoms
at baseline point to a different clinical presentation of these
patients, compared with subjects affected by the non-inflammatory
form of the disease.
To further investigate the impact of antibacterial
agents on symptom remission, the present study population was
divided in two cohorts. The cohort treated with antibacterial
agents included all IIIa subjects, as well as IIIb patients showing
evidence of infection, whereas the remaining IIIb patients received
α-blockers and S. repens extracts. Briefly, differential
treatment resulted in different intergroup responses. The cohort
treated with antibacterial agents had a more marked improvement of
NIH-CPSI voiding and total scores. Voiding symptom relief was
reflected by a significant improvement of peak urinary flow and
bladder voiding capacity. This result supports the use of
antibacterial agents in the frame of multimodal treatment of
CP/CPPS, although, in contrast with published recommendations
(22), our clinical group is
increasingly reluctant to administer antibiotics empirically in
daily practice, in the absence of documented evidence of
infection.
CP/CPPS patients were treated over time with
different oral preparations of S. repens. One kind of
preparation, based on the sole plant extract, was administered in
earlier years in our clinical practice. Subsequently, a preparation
containing a S. repens extract combined with lycopene and
selenium was adopted, on the basis of published evidence showing
increased efficacy of this combination (17,18).
The results of the present study suggest that the
addition of supplements characterized by a marked antioxidant
activity may contribute to the improvement of voiding symptoms and
QoL in CP/CPPS patients. These results are in agreement with the
outcome of two recent randomized trials showing that a preparation
of S. repens extracts, combined with lycopene and selenium,
is more active than the plant extract alone as a symptom reliever
and as a negative modulator of inflammation in CP/CPPS patients
(18,19).
Within the limits of a retrospective observational
study, the present results document the efficacy of the multimodal
administration of diverse agents in the improvement of signs and
symptoms of CP/CPPS. A reduction of ≥6 points of the NIH-CPSI score
was achieved in >70% of patients, phenotyped with the novel
UPOINTS system and treated with a fixed combination of
α-adrenoceptor blockers, S. repens extracts and antioxidant
supplements, to which antibacterial agents were added, in cases
with evidence of prostatic infection or in the presence of
inflammatory findings strongly suggestive of an ongoing occult
infective process.
References
|
1
|
Habermacher GM, Chason JT and Schaeffer
AJ: Prostatitis/chronic pelvic pain syndrome. Annu Rev Med.
57:195–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nickel JC and Shoskes DA: Phenotypic
approach to the management of the chronic prostatitis/chronic
pelvic pain syndrome. BJU Int. 106:1252–1263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shoskes DA, Nickel JC, Rackley RR and
Pontari MA: Clinical phenotyping in chronic prostatitis/chronic
pelvic pain syndrome and interstitial cystitis: a management
strategy for urologic chronic pelvic pain syndromes. Prostate
Cancer Prostatic Dis. 12:177–183. 2009. View Article : Google Scholar
|
|
4
|
Nickel JC, Shoskes DA and Wagenlehner FM:
Management of chronic prostatitis/chronic pelvic pain syndrome
(CP/CPPS): the studies, the evidence and the impact. World J Urol.
31:747–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nickel JC, Downey J, Ardern D, Clark J and
Nickel K: Failure of a monotherapy strategy for difficult chronic
prostatitis/chronic pelvic pain syndrome. J Urol. 172:551–554.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shoskes DA, Hakim L, Ghoniem G and Jackson
CL: Long-term results of multimodal therapy for chronic
prostatitis/chronic pelvic pain syndrome. J Urol. 169:1406–1410.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shoskes DA, Nickel JC and Kattan MW:
Phenotypically directed multimodal therapy for chronic
prostatitis/chronic pelvic pain syndrome: a prospective study using
UPOINT. Urology. 75:1249–1253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Magri V, Wagenlehner F, Perletti G,
Schneider S, Marras E, Naber KG and Weidner W: Use of the UPOINT
chronic prostatitis/chronic pelvic pain syndrome classification in
European patient cohorts: sexual function domain improves
correlations. J Urol. 184:2339–2345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Samplaski MK, Li J and Shoskes DA:
Clustering of UPOINT domains and subdomains in men with chronic
prostatitis/chronic pelvic pain syndrome and contribution to
symptom severity. J Urol. 188:1788–1793. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Z, Zhang J, He J and Zeng G: Clinical
utility of the UPOINT phenotype system in Chinese males with
chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a
prospective study. PLoS One. 8:e520442013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shoskes DA and Nickel JC: Classification
and treatment of men with chronic prostatitis/chronic pelvic pain
syndrome using the UPOINT system. World J Urol. 31:755–760. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davis SN, Binik YM, Amsel R and Carrier S:
Is a sexual dysfunction domain important for quality of life in men
with urological chronic pelvic pain syndrome? Signs ‘UPOINT’ to
yes. J Urol. 189:146–151. 2013. View Article : Google Scholar
|
|
13
|
Giubilei G, Mondaini N, Crisci A, Raugei
A, Lombardi G, Travaglini F, et al: The Italian version of the
National Institutes of Health Chronic Prostatitis Symptom Index.
Eur Urol. 47:805–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Propert KJ, Litwin MS, Wang Y, Alexander
RB, Calhoun E, Nickel JC, et al: Responsiveness of the National
Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI).
Qual Life Res. 15:299–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosen RC, Cappelleri JC and Gendrano N:
The International Index of Erectile Function (IIEF): a
state-of-the-science review. Int J Impot Res. 14:226–244. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Magri V, Montanari E, Škerk V, Markotić A,
Marras E, Restelli A, Naber KG and Perletti G:
Fluoroquinolone-macrolide combination therapy for chronic bacterial
prostatitis: retrospective analysis of pathogen eradication rates,
inflammatory findings and sexual dysfunction. Asian J Androl.
13:819–827. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morgia G, Cimino S, Favilla V, Russo GI,
Squadrito F, Mucciardi G, et al: Effects of Serenoa repens,
selenium and lycopene (Profluss®) on chronic
inflammation associated with benign prostatic hyperplasia: results
of ‘FLOG’ (Flogosis and Profluss in Prostatic and Genital Disease),
a multicentre Italian study. Int Braz J Urol. 39:214–221.
2013.PubMed/NCBI
|
|
18
|
Morgia G, Mucciardi G, Galì A, Madonia M,
Marchese F, Di Benedetto A, et al: Treatment of chronic
prostatitis/chronic pelvic pain syndrome category IIIa with Serenoa
repens plus selenium and lycopene (Profluss) versus S. repens
alone: an Italian randomized multicenter-controlled study. Urol
Int. 84:400–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vickers AJ: Parametric versus
non-parametric statistics in the analysis of randomized trials with
non-normally distributed data. BMC Med Res Methodol. 5:352005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krieger JN, Nyberg L Jr and Nickel JC: NIH
consensus definition and classification of prostatitis. JAMA.
282:236–237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wagenlehner FM, van Till JW, Magri V,
Perletti G, Houbiers JG, Weidner W and Nickel JC: National
Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI)
symptom evaluation in multinational cohorts of patients with
chronic prostatitis/chronic pelvic pain syndrome. Eur Urol.
63:953–959. 2013. View Article : Google Scholar
|
|
22
|
Wagenlehner FM, Weidner W and Naber KG:
Therapy for prostatitis, with emphasis on bacterial prostatitis.
Expert Opin Pharmacother. 8:1667–1674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wagenlehner FM, Ballarini S and Naber KG:
Immunostimulation in chronic prostatitis/chronic pelvic pain
syndrome (CP/CPPS): a one-year prospective, double-blind,
placebo-controlled study. World J Urol. 32:1595–1603. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tran CN and Shoskes DA: Sexual dysfunction
in chronic prostatitis/chronic pelvic pain syndrome. World J Urol.
31:741–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schaeffer AJ, Knauss JS, Landis JR,
Propert KJ, Alexander RB, Litwin MS, et al: Leukocyte and bacterial
counts do not correlate with severity of symptoms in men with
chronic prostatitis: the National Institutes of Health Chronic
Prostatitis Cohort Study. J Urol. 168:1048–1053. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nickel JC, Alexander RB, Schaeffer AJ,
Landis JR, Knauss JS, Propert KJ, et al: Leukocytes and bacteria in
men with chronic prostatitis/chronic pelvic pain syndrome compared
to asymptomatic controls. J Urol. 170:818–822. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sung YH, Jung JH, Ryang SH, Kim SJ and Kim
KJ: Clinical significance of national institutes of health
classification in patients with chronic prostatitis/chronic pelvic
pain syndrome. Korean J Urol. 55:276–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parnham MJ, Haber VE,
Giamarellos-Bourboulis EJ, Perletti G, Verleden GM and Vos R:
Azithromycin: Mechanisms of action and their relevance for clinical
applications. Pharmacol Ther. 143:225–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perletti G, Skerk V, Magri V, Markotic A,
Mazzoli S, Parnham MJ, Wagenlehner FM and Naber KG: Macrolides for
the treatment of chronic bacterial prostatitis: an effective
application of their unique pharmacokinetic and pharmacodynamic
profile. Mol Med Rep. 4:1035–1044. 2011.PubMed/NCBI
|















