Introduction
Stroke is a serious threat to human health, and is
recognized as the most important factor resulting in nerve injury
(1,2). Evidence suggests that nerve injury
occurs following ischemia in susceptible brain regions, such as the
hippocampus and the cerebral cortex. Neuronal cell death has been
identified by morphological analysis of the ischemic brain, and
genetic and biochemical evidence further support the association of
neuronal cell death with ischemia (3,4).
Angelicae sinensis is a traditional Chinese
medicinal herb that has long been used to treat ischemic stroke and
anemia (5,6). The chemical constituents of the
extract of the roots of Angelica sinensis are classified
into essential oil- and water-soluble materials (7). Ferulic acid (FA) is the main
water-soluble component of the roots of Angelica sinensis.
Previous studies have demonstrated that FA is able to enhance
hematopoietic progenitor cell activity resulting in accelerated
blood cell recovery by stimulating erythropoietin (EPO) and
granulocyte colony-stimulating factor (G-CSF) expression (8,9). A
further study demonstrated that FA was able to reduce cerebral
infarction in a model of transient middle cerebral artery occlusion
(10).
EPO and G-CSF were originally recognized as humoral
mediators involved in the maturation and proliferation of
hematopoietic progenitor cells (11), and their neuroprotective effects
were subsequently identified. In vitro and in vivo
studies in animal models revealed that exogenously administered EPO
and G-CSF are neuroprotective (12,13).
Endogenous EPO and G-CSF have also demonstrated beneficial effects
in experimental stroke (14,15).
Although FA has been shown to reduce cerebral
infarct secondary to transient focal cerebral ischemia, little data
is available regarding its neuroprotective effect on nerve injury
and the related protective factors EPO and G-CSF. Thus, the aim of
this study was to investigate the protective effect of FA on nerve
injury induced by cerebral ischemia and whether the
cerebroprotective effect of FA is associated with EPO and G-CSF
induction in the rat brain.
Materials and methods
Animals and drugs
Male Sprague-Dawley rats weighing 220±20 g were
obtained from Experimental Animal Center of Shandong University of
Chinese Traditional Medicine (Jinan, China). They were kept in
air-conditioned rooms (temperature, 23±2°C) on a 12 h light-dark
cycle, with free access to food and water. Animal experimental
procedures were carried out in strict accordance with the
guidelines published by the United States National Institutes of
Health (NIH Publication no. 85-23, revised 1996) and approved by
the ethics committee of Yantai University (Shandong, China).
Surgical procedures, stroke induction and animal sacrifice (at the
end of the observation period) were performed under general
anesthesia with intraperitoneal (i.p.) injection of chloral hydrate
(350 mg/kg).
The sodium salt of FA (i.e., sodium ferulate) was
obtained from Haikou Qili Pharmaceutical Co., Ltd. (Haikou, China)
and edaravone injection was provided by Nanjing Simcere Dongyuan
Pharmaceutical Co., Ltd. (Nanjing, China).
Rat cerebral ischemia study protocol
The middle cerebral artery occlusion (MCAO)
procedure was carried out according to a previously described
method with minor modifications (16). Briefly, rats were anesthetized with
10% chloral hydrate in 0.9% NaCl (350 mg/kg, i.p.) and placed in a
dorsal recumbent position. Under sterile conditions, a ventral neck
incision was made and the external carotid artery (ECA) and
internal carotid artery (ICA) were exposed and carefully isolated.
A nylon monofilament (40 mm in length and 0.25 mm in diameter), its
tip rounded by flame-heating, was inserted from the lumen of the
ECA to that of the right ICA to occlude the origin of the right
MCA. The filament was removed after 1 h. For the sham group, the
ECA and ICA underwent the same procedures without occlusion of the
MCA. The rats were kept under conditions of controlled temperature
(24–25°C) for the first 24 h after surgery.
The rats were randomly divided into six groups of 10
rats each. Three groups received different doses of FA (50, 100 and
200 mg/kg), and one group received edaravone (6 mg/kg) by
intravenous injection 30 min after ischemia, and once a day
thereafter for seven consecutive days. The rats in the sham and
vehicle-treated group were injected with saline.
Evaluation of neurological deficits
Neurological deficits were evaluated using a
modified six-point scoring method (17), by an investigator who was blinded
to each experimental group. The damage was graded on a scale of
0–5. The scale is: 0, no neurological deficits (normal); 1, failure
to extend left forepaw fully (mild); 2, circling to the left
(moderate); 3, falling to the left (severe); 4, no spontaneous
walking with a depressed level of consciousness (very severe); and
5, death.
Hematoxylin and eosin (H&E)
staining
Six rats from each group were selected for H&E
staining. The rats were deeply anesthetized with choral hydrate and
pericardially perfused with 0.9% saline and then with 4%
formaldehyde. The entire brain was embedded in paraffin. Nerve
injury in the hippocampus was determined by analysis under a
microscope (magnification, ×400; Olympus BX41; Olympus, Tokyo,
Japan). The damage was evaluated by counting the number of
surviving neurons per millimeter length of the hippocampus examined
under light microscopy.
Enzyme-linked immunosorbent assay
(ELISA)
EPO and G-CSF levels in the plasma were measured by
ELISA kits (EPO Quantikine ELISA Kit, catalogue no. MEP00B and
G-CSF Quantikine ELISA Kit, catalogue no. MCS00; R&D Systems,
Inc., Minneapolis, MN, USA). The rats in each group were sacrificed
24 h after the final administration of treatment and plasma was
collected. Monoclonal antibodies specific for EPO and G-CSF were
pre-coated onto microplates. Standards and samples were added to
the wells and were incubated for 30 min at 37°C. After washing away
the unbound substances, enzyme-linked polyclonal antibodies
specific for EPO and G-CSF were added to the wells and were
incubated for 60 min at 37°C. After removing any unbound
antibodies, substrates were added to the wells and were incubated
for 15 min at 37°C. The intensities of the colors developed were in
proportion to the amount of EPO and G-CSF bound to the wells. The
optical density of each well was measured with a scanning
multi-well spectrophotometer (SpectraMax M3, Molecular Devices,
Sunnyvale, CA, USA) at a wavelength of 450 nm.
Immunohistochemistry assays
After being deeply anesthetized, the rats were
transcardially perfused with saline solution, followed by 4%
paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) 24 h
after ischemia. Brains were removed and post-fixed in 4%
paraformaldehyde for 4 h, then transferred into 30% sucrose
solution until the brains sank to the bottom of the container.
Coronal sections (10 μm) were made using a Leica CM1950S cryostat
(Leica Microsystems GmbH, Wetzlar, Germany). Sections were blocked
with 3% normal goat serum (diluted in PBS containing 0.3% Triton
X-100) for 1 h and incubated with rabbit anti-rat polyclonal
primary antibodies [anti-EPO (BA0843) and anti-G-CSF (BA0746),
1:200, Wuhan Boster Biological Engineering Co., Ltd., Wuhan, China]
overnight at 4°C. After rinsing with PBS, sections were incubated
with horseradish peroxidase-conjugated goat anti-rabbit polyclonal
IgG (ZDR-5306; Beijing Zhongshan Jinqiao Biological technology Co.
Ltd., Beijing, China) as secondary antibodies (1:200) for 2 h at
room temperature. The images from five fields of each ischemic
region from six rats in each group were examined using the same
brightness and exposure settings. Image-Pro Plus software (Media
Cybernetics, Silver Spring, MD, USA) was used to analyze positive
expression of EPO or G-CSF in each photograph. The artificial unit
of mean optical density (MOD) × total per area (TPA) was employed
for measurement in the stereological analysis, which indicated the
integrated optical density (IOD) of the positive signal in the
stained tissue.
Statistical analysis
Neurological deficit scores between groups were
analyzed using a non-parametric test. Quantitative data from the
experiments were expressed as mean ± standard deviation (SD), and
significance was determined by one-way analysis of variance (ANOVA)
followed by Tukey’s test. In all cases, differences were considered
significant if P<0.05.
Results
Effects of FA on neurological deficit
scores in rats following MCAO
On the seventh day after the surgery, the results
showed that FA induced a significant reduction in the neurological
deficit score compared with that of the rats treated with vehicle.
Treatment with FA at doses of 100 and 200 mg/kg but not 50 mg/kg
significantly reduced the neurological deficit score in a
dose-dependent manner in the MCAO model rats. Edaravone treatment,
as a positive control, also significantly decreased the
neurological deficit score (Table
I).
 | Table IEffects of FA on neurological deficit
scores in rats following MCAO (n=10). |
Table I
Effects of FA on neurological deficit
scores in rats following MCAO (n=10).
| Group | Neurological deficit
scores (median/range) |
|---|
| Sham | - |
| Vehicle | 4/2 |
| Edaravone | 2/4b |
| FA (50 mg/kg) | 4/4 |
| FA (100 mg/kg) | 3/4a |
| FA (200 mg/kg) | 2/4a |
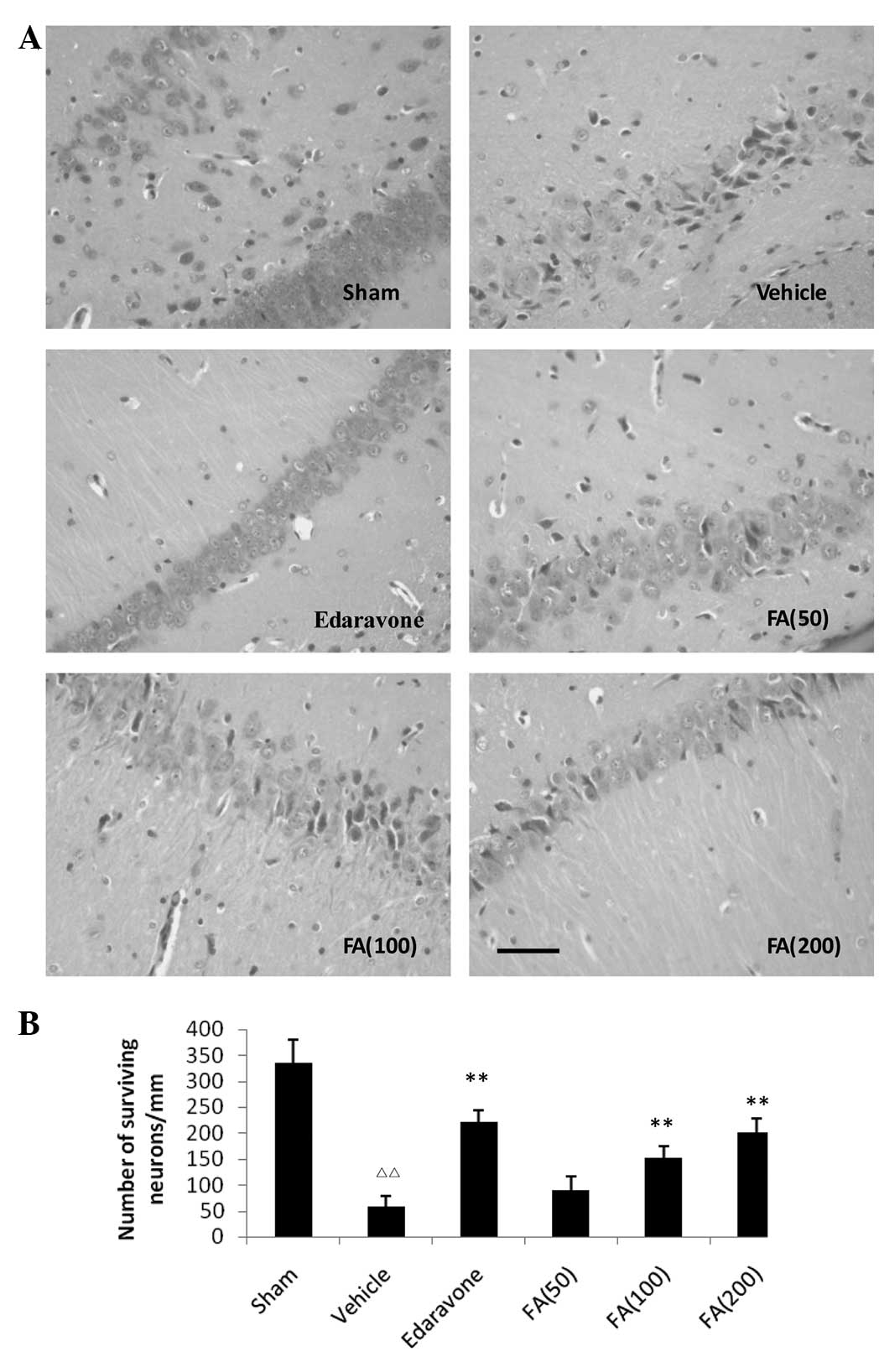
Effects of FA on neuronal damage in the
hippocampus of rats following MCAO
Extensively damaged neurons in the hippocampus were
observed and the number of surviving neurons was significantly
reduced in the vehicle-treated ischemic rats compared with the
sham-treated rats. Neuronal shrinkage and chromatin condensation of
nuclei were also observed in the ischemic rats. However, the number
of surviving neurons in the FA-treated rats was higher compared
with that in the vehicle-treated ischemic rats and numerous
surviving neurons were observed in the hippocampus (Fig. 1).
Effects of FA on EPO and G-CSF levels in
the peripheral blood of rats following MCAO
FA induced significant enhancements in the EPO level
in peripheral blood compared with that in the vehicle-treated rats
(P<0.05). With regard to G-CSF levels, the rats treated with FA
showed no significant difference compared with the vehicle-treated
rats (Table II).
 | Table IIEffects of FA on EPO and G-CSF levels
in the peripheral blood of rats following MCAO (mean ± standard
deviation; n=6). |
Table II
Effects of FA on EPO and G-CSF levels
in the peripheral blood of rats following MCAO (mean ± standard
deviation; n=6).
| Group | EPO (μg/ml) | G-CSF (μg/ml) |
|---|
| Sham | 8.32±2.85 | 0.39±0.09 |
| Vehicle | 8.63±0.97 | 0.78±0.24c |
| Edaravone | 8.62±2.51 | 0.75±0.19 |
| FA (50 mg/kg) | 12.68±3.84a | 0.62±0.11 |
| FA (100 mg/kg) | 13.28±3.32a | 0.72±0.12 |
| FA (200 mg/kg) | 12.38±1.91b | 0.60±0.12 |
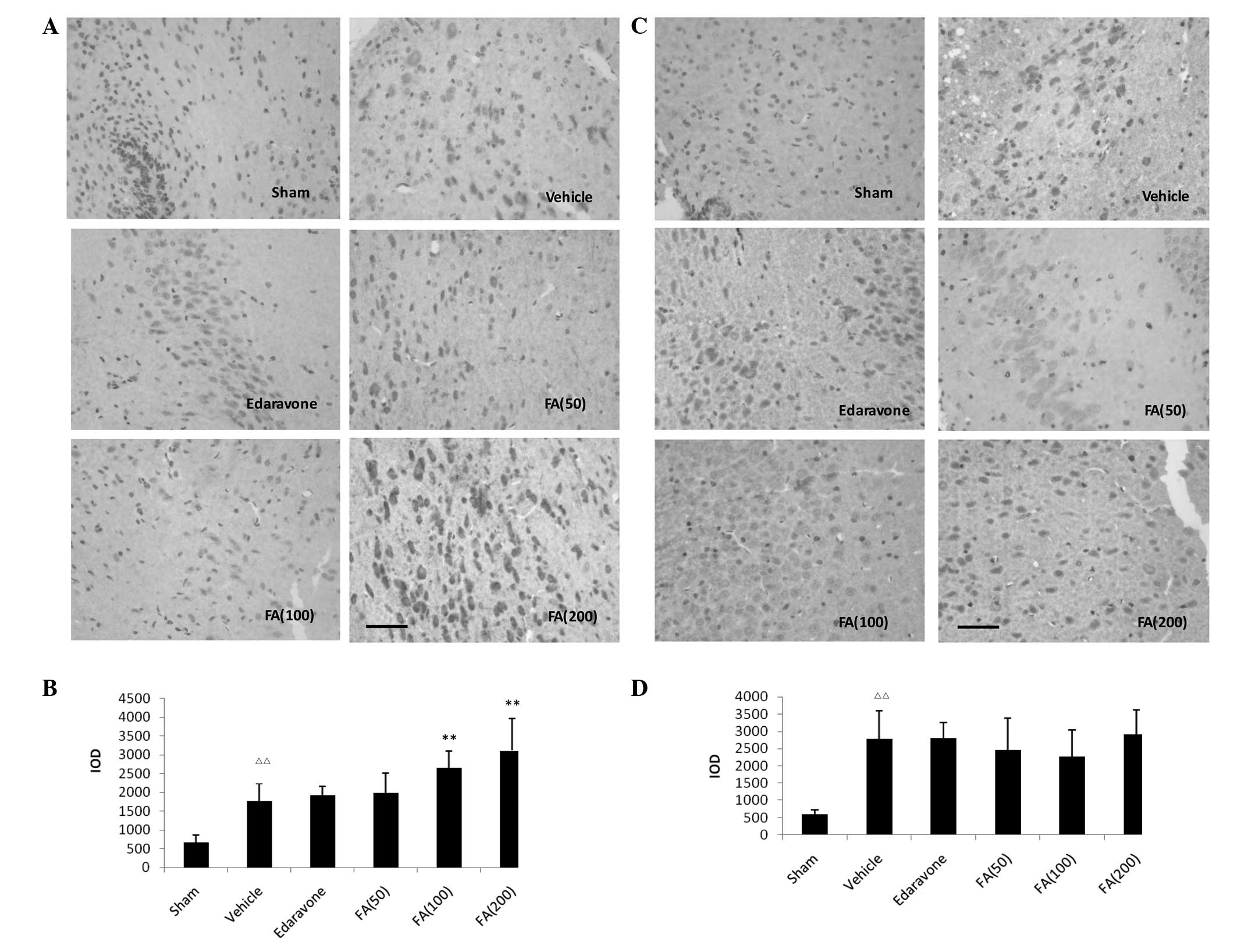
Effects of FA on EPO and G-CSF expression
in the hippocampus of rats following MCAO
The results showed that EPO expression within the
infarct region in the vehicle-treated group was significantly
increased compared with that of the sham-operated group.
Administration of FA induced a significant enhancement in EPO
expression levels in a dose-dependent manner (P<0.01). With
regard to G-CSF, cerebral ischemia induced a significant increase
in its expression, whereas treatment with FA was not observed to
induce a significant difference in the expression of the protein in
the infarct region compared with that in the vehicle-treated rats
(Fig. 2).
Discussion
Nerve injury resulting from focal or global cerebral
ischemia is a major cause of mortality and disability in the adult
population. Neuronal cell death has been observed to occur several
days after ischemic insult and predominantly affects sensitive
areas of the brain, such as the hippocampus and the cortex
(18,19).
The results of the present study demonstrated that
neuronal cell death occurred in the ischemic brain primarily in the
hippocampal area seven days after ischemia. Treatment with FA
significantly reduced the neurological deficit score and
hippocampal neuron damage in a dose-dependent manner in the rats
following MCAO. The results indicated that treatment of the rats
with FA for seven days following MCAO ameliorated the neuronal
damage.
EPO stimulates erythroid cell production, which
supports the survival, proliferation and differentiation of
erythroid progenitor cells. The sites of expression of the EPO
receptor (EPO-R) and the EPO response are in hematopoietic cells
and other cell types, including endothelial and neural cells
(20). The targeted deletion of
EPO or the EPO-R causes mice to lack definitive erythropoiesis and
mature erythrocytes (21). In such
mice, increased levels of apoptosis in the brain are also observed,
which suggests that EPO, in addition to being necessary for the
production of mature red blood cells, may also contribute to normal
brain development (22). EPO is
produced in fetal liver, adult kidney and also in the brain, in
astrocytes and neurons; EPO production is induced by hypoxia, and
may persist for 24 h or longer (23).
In the present study, it was observed that FA
induced a significant enhancement of the level of EPO expression in
the ischemic brain. The results also demonstrated that FA increased
the EPO level in peripheral blood; EPO nay be transferred from the
blood into the brain through the blood-brain barrier (BBB)
(24). Therefore, the increasing
levels of EPO in the brain and blood may contribute to the
neuroprotective effect of FA.
The hematopoietic factor G-CSF was recently
discovered to act as a protective and neurotrophic factor in the
brain. Several studies have described the infarct-reducing and
recovery-enhancing effects of G-CSF following ischemic stroke
(25–27). The main actions of G-CSF are
mediated via binding to the G-CSF receptors present on neuronal
cells. In the present study, however, treatment with FA for seven
days demonstrated no significant effect on the levels of G-CSF in
the ischemic brain and peripheral blood. Although the traditional
Chinese medicine Angelicae sinensis is commonly used to
promote erythropoiesis for the treatment of anemia, and FA has
previously been demonstrated to increase the levels of EPO and
G-CSF (8,9), the results of the present study
suggest that the neuroprotective effect of FA is not associated
with G-CSF.
The findings indicate that FA has certain protective
effects against the nerve injury induced by cerebral ischemia, and
suggest that the promotion of EPO expression in the ischemic brain
and peripheral blood may be one of the neuroprotective mechanisms
of FA.
Acknowledgements
This study was supported by the Project of Shandong
Province Higher Educational Science and Technology Program (No.
10LF76), the Foundation for Outstanding Middle-age and Young
Scientists (No. BS2011YY061) and Taishan Scholar Project. The
authors are grateful to Professor Tongshen Liu for providing
technical assistance in the pathological observations.
References
|
1
|
Gorelick PB: Stroke prevention therapy
beyond antithrombotics: unifying mechanisms in ischemic stroke
pathogenesis and implications for therapy: an invited review.
Stroke. 33:862–875. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fisher M: Advances in stroke 2007:
introduction. Stroke. 39:250–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honkaniemi J, Massa SM, Breckinridge M and
Sharp FR: Global ischemia induces apoptosis-associated genes in
hippocampus. Mol Brain Res. 42:79–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruan YW, Ling GY, Zhang JL and Xu ZC:
Apoptosis in the rat striatum after transient forebrain ischemia
and the effects of ischemic severity. Brain Res. 982:228–240. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu C, Li J, Meng FY, et al:
Polysaccharides from the root of Angelica sinensis promotes
hematopoiesis and thrombopoiesis through the PI3K/AKT pathway. BMC
Complement Altern Med. 10:792010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Y, Zhao HP, Zhang J, et al: Effect of
ferulic acid on learning and memory impairments of vascular
dementia rats and its mechanism of action. Yao Xue Xue Bao.
47:256–260. 2012.(In Chinese). PubMed/NCBI
|
|
7
|
Yi L, Liang Y, Wu H and Yuan D: The
analysis of Radix Angelicae Sinensis (Danggui). J Chromatogr A.
1216:1991–2001. 2009. View Article : Google Scholar
|
|
8
|
Ma ZC, Hong Q, Wang YG, et al: Effects of
ferulic acid on hematopoietic cell recovery in whole-body gamma
irradiated mice. Int J Radiat Biol. 87:499–505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan S, Xie Y, Zhu B, Han Y and Guo W:
Effect comparison of different formulation of Dang-Gui-Bu-Xu-Tang
on myelosuppression mouse. Asian Pac J Trop Med. 4:556–559. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng CY, Su SY, Tang NY, et al: Ferulic
acid provides neuroprotection against oxidative stress-related
apoptosis after cerebral ischemia/reperfusion injury by inhibiting
ICAM-1 mRNA expression in rats. Brain Res. 1209:136–150. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van der Kooij MA, Groenendaal F, Kavelaars
A, Heijnen CJ and van Bel F: Neuroprotective properties and
mechanisms of erythropoietin in in vitro and in vivo experimental
models for hypoxia/ischemia. Brain Res Rev. 59:22–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
England TJ, Gibson CL and Bath PM:
Granulocyte-colony stimulating factor in experimental stroke and
its effects on infarct size and functional outcome: A systematic
review. Brain Res Rev. 62:71–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamada M, Burke C, Colditz P, Johnson DW
and Gobe GC: Erythropoietin protects against apoptosis and
increases expression of non-neuronal cell markers in the
hypoxia-injured developing brain. J Pathol. 224:101–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prass K, Scharff A, Ruscher K, et al:
Hypoxia-induced stroke tolerance in the mouse is mediated by
erythropoietin. Stroke. 34:1981–1986. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sevimli S, Diederich K, Strecker JK, et
al: Endogenous brain protection by granulocyte-colony stimulating
factor after ischemic stroke. Exp Neurol. 217:328–335. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang W, Fu F, Tian J, Zhu H and Hou J:
Curculigoside A attenuates experimental cerebral ischemia injury in
vitro and vivo. Neuroscience. 192:572–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minematsu, Li L, Sotak CH, Davis MA and
Fisher M: Reversible focal ischemic injury demonstrated by
diffusion-weighted magnetic resonance imaging in rats. Stroke.
23:1304–1310. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pulsinelli WA, Brierley JB and Plum F:
Temporal profile of neuronal damage in a model of transient
forebrain ischemia. Ann Neurol. 11:491–498. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noguchi CT, Asavaritikrai P, Teng R and
Jia Y: Role of erythropoietin in the brain. Crit Rev Oncol Hematol.
64:159–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu H, Liu X, Jaenisch R and Lodish HF:
Generation of committed erythroid BFU-E and CFU-E progenitors does
not require erythropoietin or the erythropoietin receptor. Cell.
83:59–67. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai PT, Ohab JJ, Kertesz N, et al: A
critical role of erythropoietin receptor in neurogenesis and
post-stroke recovery. J Neurosci. 26:1269–1274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chikuma M, Masuda S, Kobayashi T, Nagao M
and Sasaki R: Tissue-specific regulation of erythropoietin
production in the murine kidney, brain, and uterus. Am J Physiol
Endocrinol Metab. 279:E1242–E1248. 2000.PubMed/NCBI
|
|
24
|
Yang Y and Rosenberg GA: Blood-brain
barrier breakdown in acute and chronic cerebrovascular disease.
Stroke. 42:3323–3328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugiyama Y, Yagita Y, Oyama N, et al:
Granulocyte colony-stimulating factor enhances arteriogenesis and
ameliorates cerebral damage in a mouse model of ischemic stroke.
Stroke. 42:770–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Floel A, Warnecke T, Duning T, et al:
Granulocyte-colony stimulating factor (G-CSF) in stroke patients
with concomitant vascular disease--a randomized controlled trial.
PLoS One. 6:e197672011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gibson CL, Bath PM and Murphy SP: G-CSF
administration is neuroprotective following transient cerebral
ischemia even in the absence of a functional NOS-2 gene. J Cereb
Blood Flow Metab. 30:739–743. 2010. View Article : Google Scholar : PubMed/NCBI
|