Introduction
The spinal nerve ligation model (SNL) was first
proposed by Kim and Chung in 1992 (1). Since the manifestations of
neuropathological pain (NP), such as spontaneous pain, mechanical
allodynia and thermal hyperalgesia, can be quickly induced
postoperatively, and the pain maintenance time is long (1), SNL has become the most commonly used
model of NP. In recent years, animal experiments and clinical
research studies have shown that electroacupuncture (EA) treatment
has a clear analgesic effect towards NP (2,3).
Studies have shown that when NP occurs, the spinal levels of
purinergic receptor P2X, ligand-gated ion channel 4 (P2X4) and
microglial levels of brain-derived neurotrophic factor (BDNF) play
important roles in the generation and maintenance of NP (4,5);
however, it remains unclear whether EA affects the spinal
expression of P2X4 and BDNF in SNL model rats during analgesia.
In the present study, the up-down method was used to
measure the bipedal 50% mechanical paw withdrawal threshold (PWT),
electron microscopy was used to observe the ultrastructure of the
injured-side L5 nerve root (n=6) and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
performed to detect the mRNA levels of BDNF and P2X4 in the spinal
cord. The aim of the study was to investigate the relationship
between analgesia and repair, and thus provide a theoretical basis
for the mechanism of EA-induced analgesia.
Materials and methods
Experimental animals
A total of 60 male Sprague-Dawley (SD) rats,
weighing 220–250 g, were provided by the Animal Center of Shanghai
SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The animals
were then reared in separated cages in the Animal Center of the
Affiliated Municipal Hospital of Traditional Chinese Medicine
(TCM), Shanghai University of TCM (Shanghai, China). The room
conditions were maintained at 23±2°C and 50–60% humidity, with
day-night cycling lighting (12 h-12 h). All experimental steps were
performed with the purpose of minimizing the suffering of the
animals and operated in accordance with the relevant principles of
laboratory animal care. The experimental animals were reared in the
above environment for a week for adaption prior to the experiment.
This study was carried out in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (8th edition, 2011).
The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of Shanghai
Hospital of TCM, Shanghai University of TCM.
Preparation of the SNL model and animal
grouping
The animals were satisfactorily anesthetized with
10% chloral hydrate (300 mg/kg) and the fur was removed by shaving.
The rats were then fixed, disinfected and laid on a towel. The L5/6
spinous gap was positioned in the center, and an incision was made
~4 cm along the midline of the back. The skin and subcutaneous
tissues were cut and the paraspinal muscles were bluntly dissected
and fixed with a retractor. Following blunt removal of the left L5
lamina and zygopophysis to expose the left L5 nerve root and dorsal
root ganglia, a 5-0 Mersilk thread was used to ligate the L5 nerve
tightly. The wound was then washed with saline to fully stop the
bleeding, and a 4-0 Vicryl absorbable suture was used to suture the
muscle fascia, subcutaneous tissue and skin layer by layer.
Gentamicin was then administered by intramuscular injection to
prevent infection. The rats in the normal control group were not
subjected to surgery. All the animals were kept in individual
cages, with room temperature maintained at 23±2°C, natural lighting
and free access to water and food.
The 60 SD rats were randomly divided into three
groups, namely the spinal nerve ligation group (SNL group, n=20),
the normal control group (control group, n=20) and the
electroacupuncture group (SNL + EA group, n=20). The SNL group and
the control group were not given any treatment.
Determination of bipedal 50% mechanical
PWT
The behavioral test time was fixed at 8:00–12:00,
and the testers did not know the surgery grouping. The rat bipedal
pain thresholds were determined on preoperative day 1 and
postoperative days 1, 3, 5, 7, 10, 14 and 21. The rat was placed in
a transparent Plexiglass box, the bottom of which was a 5×5 mm wire
mesh and allowed to adapt for ~15 min. A series of standardized von
Frey fibers (Stoelting Co., Ltd., Wood Dale, IL, USA) was then used
to stimulate the center of the rat hindpaw, with a stimulating
force that caused the fiber to form a slight S-shape and continued
for 6–8 sec. When the rat had calmed down, the mechanical pain
threshold was measured according to the up and down method
described by Dixon (6). The
folding forces of the classical von Frey fibers were 0.4, 1.4, 2.0,
4.0, 6.0, 8.0 and 15.0 g. Started from 2.0 g, the von Frey fiber
was vertically pushed into the plantar skin of the rat hindpaw,
with sufficient force to cause the fiber to bend into an S-shape,
taking care to avoid the paw pad. Then, dependent upon the paw
withdrawal, the fiber was replaced with the next or previous fiber,
with the testing of each fiber lasting no longer than 8 sec, and
the interval between adjacent tests being 10 sec. The reactions of
the rats towards the different fibers in the series were recorded.
If the rat quickly flinched or licked the paw, the reaction was
recorded as positive, expressed as X; if there was no response,
then the reaction was considered as negative, expressed as O. A
series of Os or an O and X combination sequence was then be
obtained; an O anterior to X was set as the starting point, and six
consecutive stimuli, including the starting point, such as OXOXOO,
were used as the key sequence for the estimation of the 50%
mechanical PWT. The estimation formula was as follows: 50%
mechanical PWT (g) = (10[Xf+κδ])/10,000, where Xf is the
logarithmic value of the last von Frey fiber in the sequence; κ is
the value obtained by table search according to the measured X and
O sequence; and δ is the logarithmic average difference of each
fiber intensity, which in this study was ~0.224. The estimated 50%
PWT value by this formula may be >15.0 g or <0.4 g, while
15.0 and 0.4 g continued to be used as the maximum and minimum
mechanical pain thresholds. If the stimuli in the force range
2.0–0.4 g were all positive, the 50% PWT was recorded as 0.4 g; if
the stimuli in the force range 2.0–15.0 g were all negative, the
50% PWT was recorded as 15.0 g. Measurement of the mechanical pain
threshold of the uninjured side was conducted 15 min after that of
the injured side. If the rat appeared to have a preoperative right
paw 50% mechanical PWT ≤4.0 g, the rat was excluded from the study.
The rats were placed into the experimental observation box for 30
min/day one week prior to the experiment for adaption.
EA intervention
Seven days after modeling, the rats were
satisfactorily anesthetized, then fixed on a special board. The
acupoints of ‘Huantiao’ and ‘Zusanli’ in the injured side were
electrically stimulated with the BT701-1B EA device (Shanghai Huayi
Medical Instrument Co., Ltd., Shanghai, China) using the following
EA parameters: frequency, 2 Hz; current intensity, ≤1 mA (a slight
tremble in the left lower limb muscles was set as the limit).
Stimulation for 30 min/day for 7 days was a course of treatment and
two consecutive courses were conducted. The SNL + A group received
the EA treatment, while the rats in the other two groups were
anesthetized, but did not receive the EA treatment.
Sampling
All rats were intraperitoneally injected with 10%
chloral hydrate (300 mg/kg) for satisfactory deep anesthesia on
postoperative day 21. Twelve rats in each group were randomly
selected and sacrificed by decapitation. The L5-6 section of the
spinal cord was quickly removed, placed on ice, and put into a
200-μl RNAiso Plus EP tube, then frozen in liquid nitrogen for
RT-qPCR analysis. Two samples from each vertebral point of the
spinal cord were taken to form a PCR sample.
RT-qPCR
Total RNA was extracted from spinal cord samples
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA). RT was performed using an RT-for-PCR kit (Qiagen, Valencia,
CA, USA) following the manufacturer’s instructions. The primers
were designed and synthesized by Shanghai Biological Engineering
Technology & Services Co., Ltd. BDNF primer sequences:
5′-CAGTGGCTGGCTCTCATACC-3′ and 3′-CGGAAACAGAACGAACAGAA-5′; P2X4
primer sequences: 5′-CGTGGCGGACTATGTGATT-3′ and
3′-GGTGCTCTGTGTCTGGTTCA-5′; the internal reference was GAPDH.
RT-qPCR amplifications were performed using the SYBR Premix Ex Taq
kit (Takara Bio, Dalian, China) in an RG 3000 thermal cycler
(Corbett Research, Qiagen) with 4 μl sample per assay. The qPCR
conditions were as follows: holding stage, 95°C for 10 min; and
cycling stage, 95°C for 15 sec, 60°C for 1 min and 72°C for 35 sec,
followed by melt curve analysis to confirm the specificity of the
amplified products. The fold change was calculated by the
2−ΔΔCT method. PCR products were subjected to melting
curve analysis, while the data were quantified using RotorGene 6.0
analysis software (Qiagen). The experiments were repeated at least
three times.
Electron microscopy
The remaining six rats of each group were subjected
to perfusion-fixation, and then the ligated nerve root of the
injured-side L5, approximately the size of a grain of rice, was
immersed in a 2.5% glutaraldehyde-prepared EP tube (Sigma-Aldrich,
St. Louis, MO, USA) for observation under a CM120 electron
microscope (Philips, Zürich, Switzerland).
Statistical analysis
SPSS statistical software package, version 18.0
(SPSS, Inc., Chicago, IL, USA) was used for the statistical
analysis, The measurement data are expressed as the mean ± standard
deviation, the differences among the pain thresholds of the
repeated measurements in each group were subjected to analysis of
variance (ANOVA) with repeated measurement data, intergroup
comparisons at the same time point were conducted using ANOVA, with
P<0.05 considered to indicate a statistically significant
difference.
Results
50% mechanical PWT results
There was no significant difference among the
preoperative bipedal 50% PWT values in the different groups.
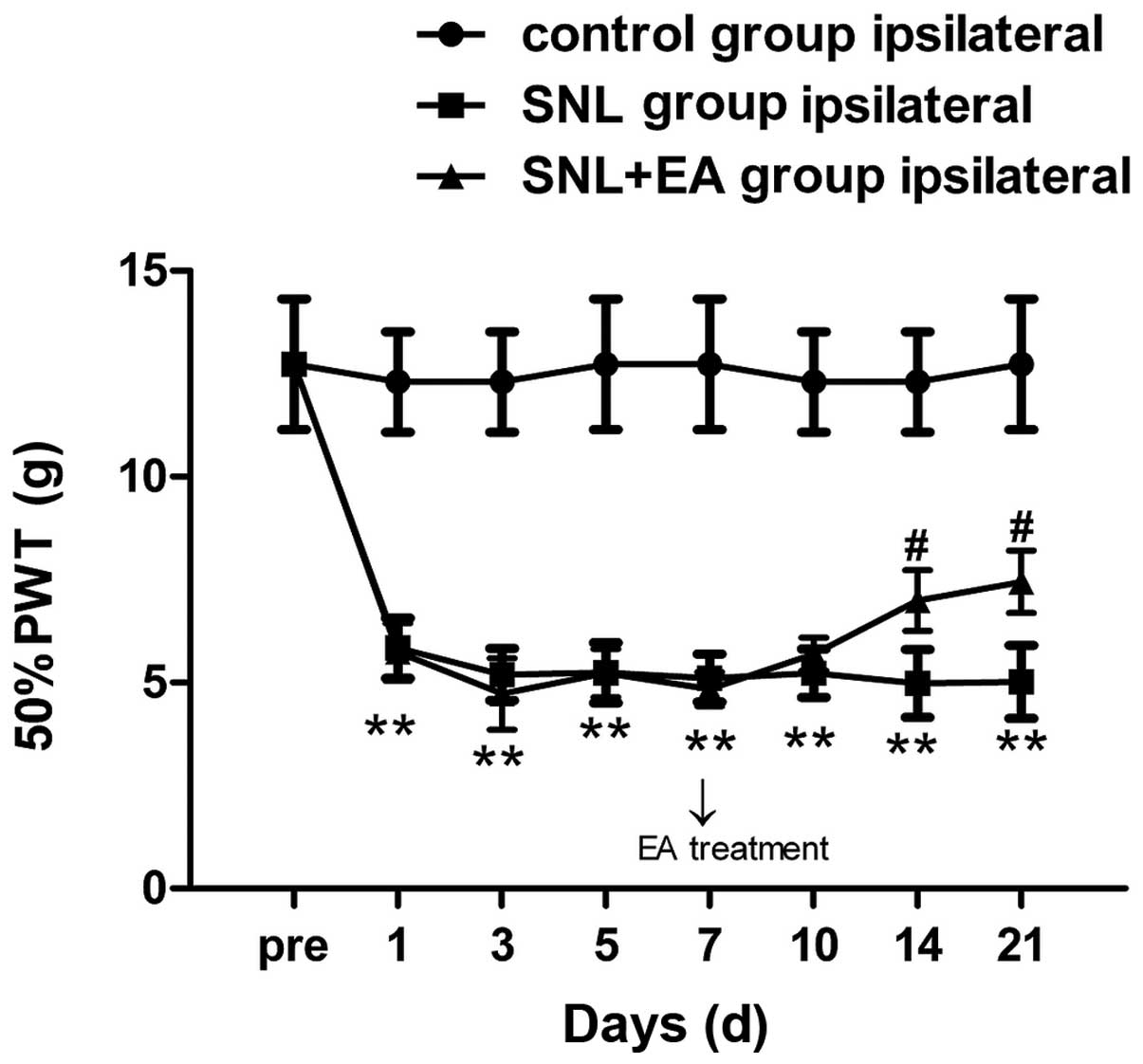
Compared with the preoperative baseline value, the injured-side 50%
PWT in the SNL group decreased significantly on postoperative day
1, and was maintained at a lower level from postoperative day 7 to
the end of the observation period and was significantly different
compared with that in the control group (P<0.01). Compared with
the control group, the mechanical pain threshold in the SNL + EA
group increased slightly (P>0.05). The increase in the
mechanical pain threshold in the SNL + EA group compared with that
in the SNL group was of statistical significance only on
postoperative days 14 and 21 (P<0.05), while no statistical
significance was identified at the other time points (P>0.05;
Fig. 1). The 50% PWT of the
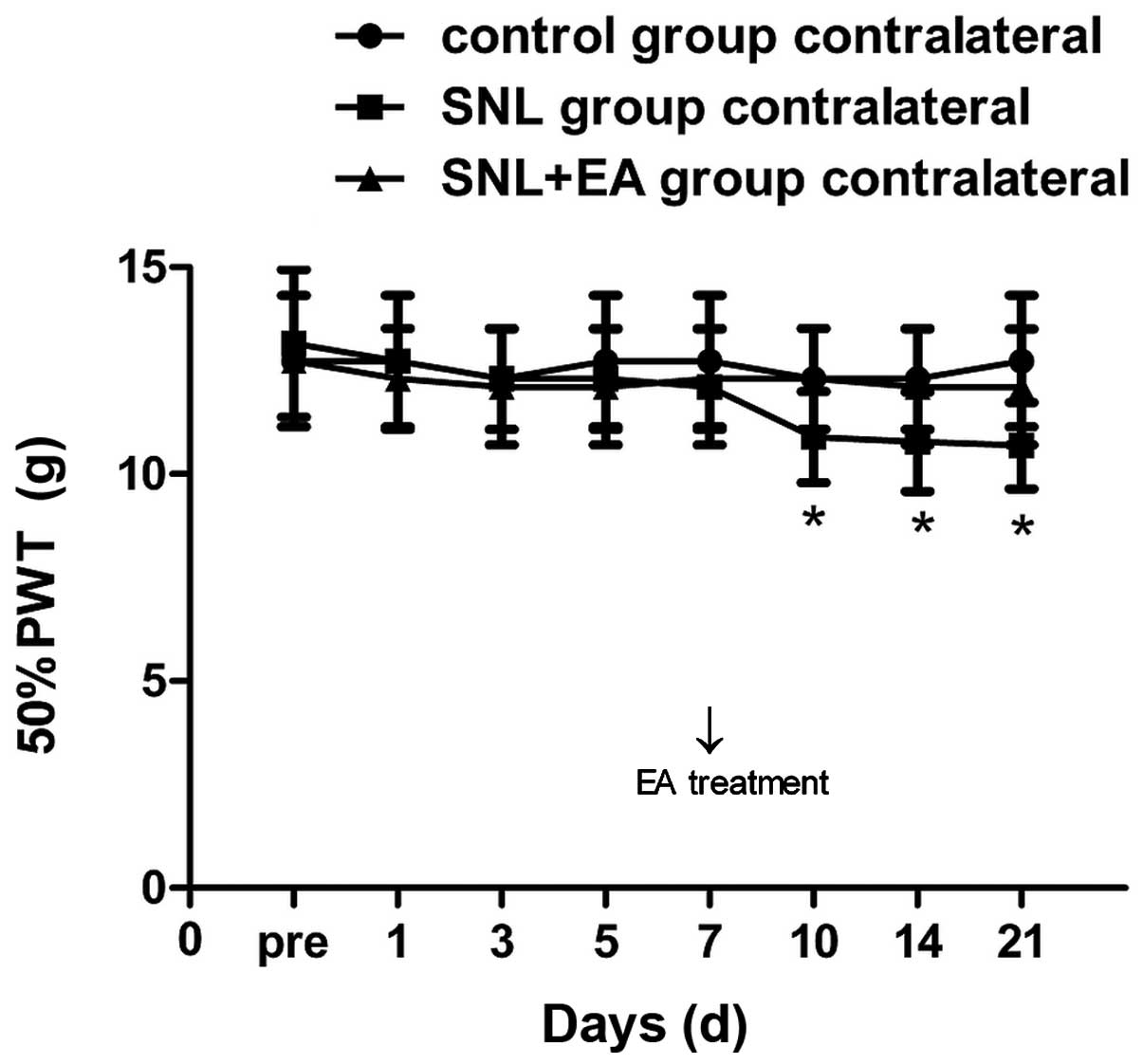
uninjured-side hindpaw in the SNL group was decreased compared with
that of the control group from postoperative day 10, exhibiting a
significant difference on postoperative days 10, 14 and 21
(P<0.05); no significant differences were identified at the
other time points. The 50% PWT of the uninjured-side hindpaw in the
SNL + EA group was significantly different compared with that in
the SNL group on days 10, 14 and 21 (P<0.05; Fig. 2).
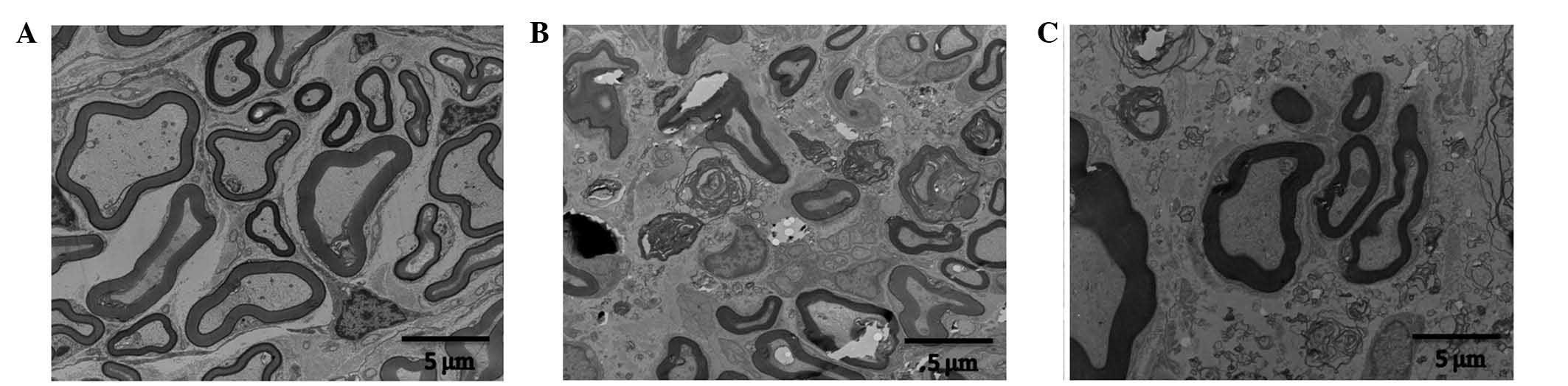
Electron microscopy results
In the control group, the myelin structure of the
nerve root was integrated, the axonal structure was normal and
arranged regularly, and the Schwann cells were normal. In the SNL
group, the majority of the myelin structure of the nerve root had
disappeared, axons were bare and arranged irregularly, while the
axonal structure was integrated, Schwann cells proliferated and
myelin debris was visible in the cytoplasm, with largely
proliferated Schwann cell nuclei. In the SNL + EA group, the axons
of the nerve root were partially demyelinated, the majority of the
axonal myelin was complete but thinner, the axonal structure was
integrated although arranged irregularly, the proliferation of
Schwann cells was not evident and vascular proliferation was
observable within the field of vision (Fig. 3).
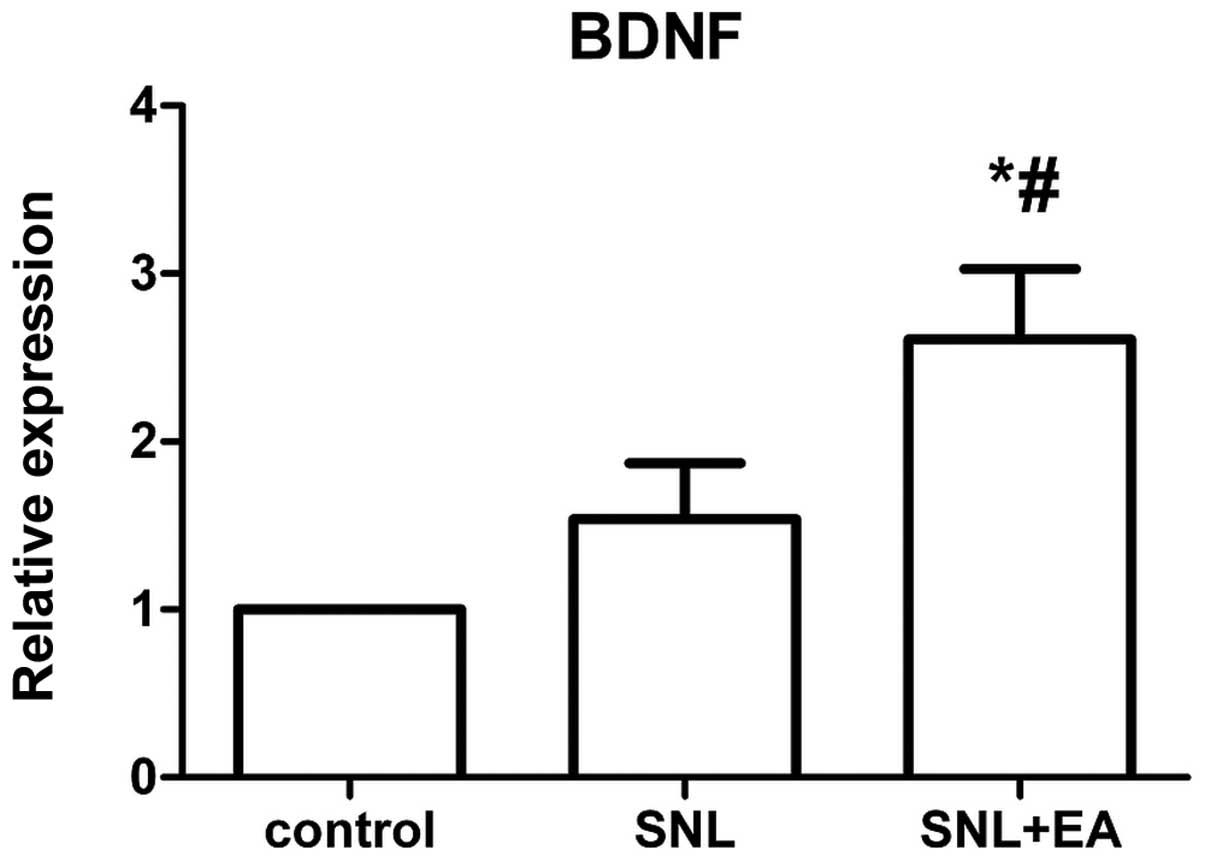
Expression levels of BDNF mRNA
The expression level of BDNF mRNA in the SNL + EA
group was significantly higher compared with that in the SNL group
(P<0.05), and the expression of BDNF mRNA in the SNL group was
not significantly different compared with that in the control group
(P>0.05; Fig. 4).
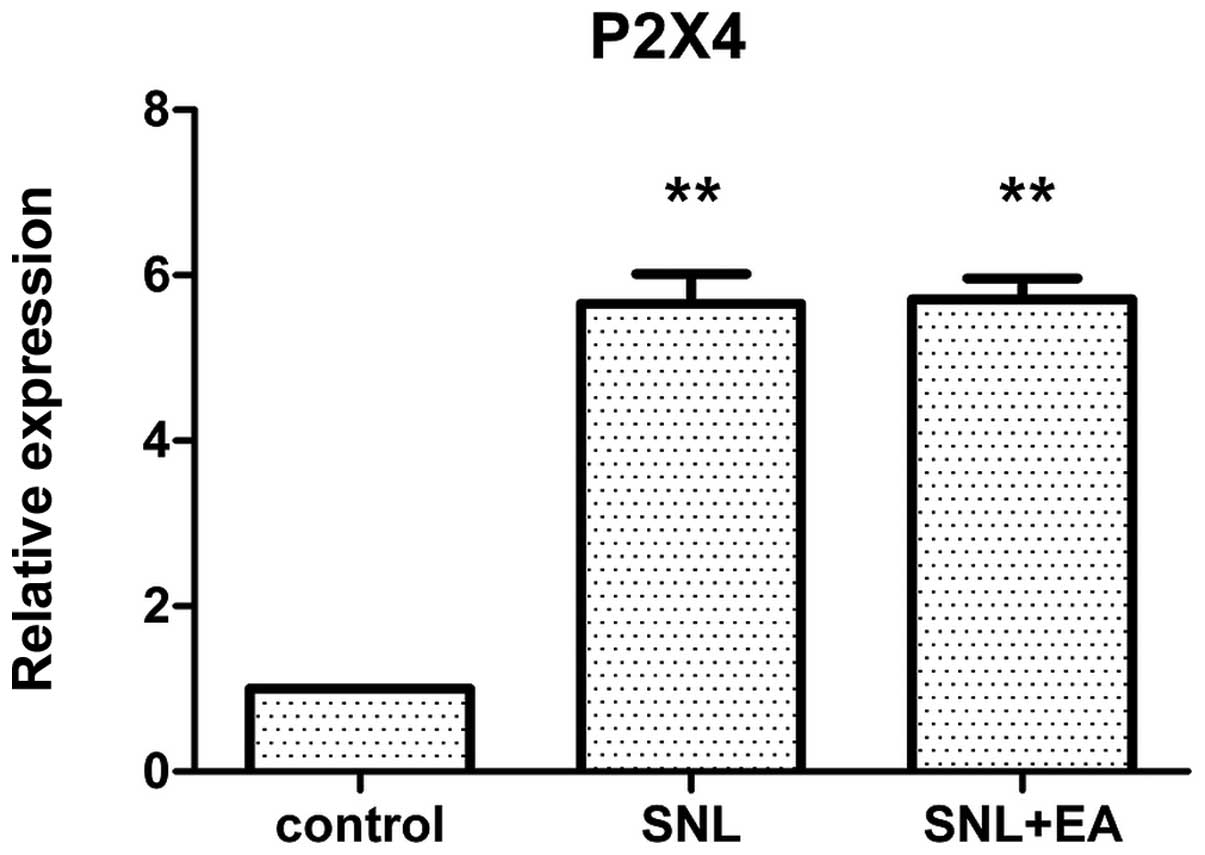
Relative expression of P2X4 mRNA
No significant difference was identified in the
expression levels of P2X4 mRNA between the SNL + EA and the SNL
groups (P>0.05). The P2X4 mRNA expression levels in the SNL and
SNL +EA groups were significantly higher than that in the control
group (P<0.01; Fig. 5).
Discussion
The results of this study demonstrated that compared
with the preoperative baseline value, the postoperative 50% PWT in
the SNL group decreased progressively, and the hyperalgesia was
significant, indicating that the SNL modeling was successfully
established. In addition, the mechanical hyperalgesia was present
on postoperative day 1 in the SNL group, and was significantly
decreased on postoperative day 3, reaching its lowest level on
postoperative day 7 and maintaining a low level until the end of
the observation period; the results were consistent with those of
previous studies (1). Compared
with the preoperative state, hyperalgesia of the uninjured-side
hindpaw was statistically significant on postoperative days 10–21
(P<0.05), the so-called ‘mirror pain’ phenomenon, which is
consistent with the results of Arguis et al (7). Mirror pain refers to the concept that
when a peripheral nerve is injured, pain can be perceived not only
from the injured area, but also from sites a certain distance
outside the injured area (8).
Sometimes the pain occurs in the contralateral side, and the
contralateral pain is similar in nature to the ipsilateral pain;
while not significant, the mirror pain phenomenon of the animal
model is often ignored in studies (9). Certain patients with complex regional
pain syndrome (CRPS) or post-herpetic neuralgia also appear to
experience mirror pain (10,11);
however, the mechanism remains unclear, with current hypotheses
focusing on neural network and immune activation theories (12). The immune activation theory
considers that when the peripheral nerves are injured, a large
number of metabolites are produced at the injured site, and a large
number of immune cells infiltrate; the damage-induced metabolites
and the immune response may be associated with the activation of
spinal glial cells and the release of inflammatory mediators
(13,14). However, the immune theory cannot
explain the presence of allodynia in the bilateral corresponding
regions. As the nervous system has anatomical symmetry, peripheral
and central neural pathways are likely to involve in the process of
mirror pain. In addition, the study of anatomy has determined that
symmetric neural connections occur at the level of the spinal cord
(15). The exact reaction site
characteristics on the opposite side could only be completed
through a neural pathway. Therefore, in the current study, it is
hypothesized that the only immune factor is a pain signaling
molecule, and that immune factors, associated with the specific
neural pathways, perform the functions together. A recent study
(16) reported that in the chronic
constriction injury model, enhanced MRI revealed that when a
unilateral nerve was injured, activities were exhibited by nerves
bilaterally. Therefore, studies of mirror pain are required to not
only investigate mirror pain itself, but also to determine the
nature of the pain, which may improve the understanding of how the
two sides of the body are connected together.
The RT-qPCR results demonstrated that the BDNF mRNA
expression level in the SNL + EA group was higher compared with
that in the SNL group, with statistical significance (P<0.05),
while the 50% PWT in the SNL + EA group was also higher than that
in the SNL group. The combination of these two results shows that
EA induces the body to secrete BDNF, and thus exhibits analgesic
effects. The increase in the pain threshold was positively
correlated with the EA treatment time. The level of BDNF mRNA
expression in the SNL group was increased slightly compared with
that in the control group, without statistical significance, while
the pain threshold was significantly lower than that in the control
group. A previous study (17)
found that in the EA-non-intervention SNL model, quantitative ELISA
analysis confirmed that BDNF appears with a secretion peak in the
early stage of injury (within 24–48 h), and returns to the
preoperative control level in 28 days. In the present study, it was
found that following EA intervention, the BDNF content in the
spinal dorsal horn significantly increased on postoperative day 21,
indicating that repeated EA treatment continued to promote BDNF
secretion. Other studies (18,19)
have shown that when a nerve is injured, ATP release from the
central process endings of spinal dorsal horn neurons is increased,
which activates the P2X4 receptor on the microglia of the spinal
dorsal horn, leading to the opening of nerve cell membrane
Ca2+ channels, and thereby promoting the release of
BDNF. The released BDNF then binds to its receptor trkB, regulating
the phosphorylation and decreasing the activity of the
K+-Cl– co-transporter 2 (KCC2), causing
dysfunction of inhibitory neurons, and thus generating pain; that
is, pain is induced by the P2X4R-BDNF-trkB-KCC2 pathway in
microglial cells. The present study observed that in the SNL group
the P2X4 content of the spinal dorsal horn increased significantly
compared with that in the control group, with statistical
significance (P<0.05), while compared with the SNL + EA group,
the P2X4 content showed no significant differences. Therefore, it
may be presumed that the analgesic mechanism of EA was not
associated with changes in the expression of P2X4.
Electron microscopy results revealed that after
modeling, the axons of the ligated nerve root were irregular,
Schwann cells proliferated, and myelin debris was visible in the
cytoplasm. A large number of proliferated Schwann cell nuclei were
observed and the number of endoplasmic reticulums increased. In
addition, the axons were irregularly arranged, and medullary
droplets were present, all of which are consistent with the
characteristics of Wallerian degeneration following injury of the
peripheral nerve. However, following EA treatment, axonal
demyelination in the SNL + EA group was reduced compared with that
in the SNL group, Schwann cell proliferation was not evident, and
vascular proliferation was visible in the field of vision.
Therefore, it may be concluded that EA reduced the mechanical
damage-induced demyelination of nerve roots, promoted the vascular
proliferation of the nerve root, improved blood supply to the nerve
root and promoted neurological recovery. This result is consistent
with a previous study (20);
however, the exact mechanism remains unclear. The effect of EA may
be associated with the formation of a stable electric field that
may promote injured nerve regeneration (21), or with the rhythmic contractions of
stimuli-involved muscles, which may promote local blood circulation
(22).
References
|
1
|
Kim SH and Chung JM: An experimental model
for peripheral neuropathy produced by segmental spinal nerve
ligation in the rat. Pain. 50:355–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan LP, Wu XT, Yin ZY and Ma C: Effect of
electroacupuncture on the levels of amino acid neurotransmitters in
the spinal cord in rats with chronic constrictive injury. Zhen Ci
Yan Jiu. 36:353–356. 3792011.(In Chinese).
|
|
3
|
Tu WZ, Cheng RD, Cheng B, et al: Analgesic
effect of electroacupuncture on chronic neuropathic pain mediated
by P2X3 receptors in rat dorsal root ganglion neurons. Neurochem
Int. 60:379–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beggs S, Trang T and Salter MW:
P2X4R+ microglia drive neuropathic pain. Nat Neurosci.
15:1068–1073. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsuda M, Masuda T, Tozaki-Saitoh H and
Inoue K: P2X4 receptors and neuropathic pain. Front Cell Neurosci.
7:1912013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dixon WJ and Mood AM: A method for
obtaining and analyzing sensitivity data. J Am Stat Assoc.
43:109–126. 1948. View Article : Google Scholar
|
|
7
|
Arguis MJ, Perez J, Martinez G, Ubre M and
Gomar C: Contralateral neuropathic pain following a surgical model
of unilateral nerve injury in rats. Reg Anesth Pain Med.
33:211–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fitzgibbon BM, Enticott PG, Bradshaw JL,
et al: Motor cortical excitability and inhibition in acquired
mirror pain. Neurosci Lett. 530:161–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dani M, Guindon J, Lambert C and Beaulieu
P: The local antinociceptive effects of paracetamol in neuropathic
pain are mediated by cannabinoid receptors. Eur J Pharmacol.
573:214–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Acerra NE and Moseley GL: Dysynchiria:
watching the mirror image of the unaffected limb elicits pain on
the affected side. Neurology. 65:751–753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shenker NG, Haigh RC, Mapp PI, Harris N
and Blake DR: Contralateral hyperalgesia and allodynia following
intradermal capsaicin injection in man. Rheumatology (Oxford).
47:1417–1421. 2008. View Article : Google Scholar
|
|
12
|
Huang D and Yu B: The mirror-image pain:
an unclered phenomenon and its possible mechanism. Neurosci
Biobehav Rev. 34:528–532. 2010. View Article : Google Scholar
|
|
13
|
Schreiber KL, Beitz AJ and Wilcox GL:
Activation of spinal microglia in a murine model of peripheral
inflammation-induced long-lasting contralateral allodynia. Neurosci
Lett. 440:63–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obata H, Sakurazawa S, Kimura M and Saito
S: Activation of astrocytes in the spinal cord contributes to the
development of bilateral allodynia after peripheral nerve injury in
rats. Brain Res. 1363:72–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dubový P, Klusáková I, Svízenská I and
Brázda V: Spatio-temporal changes of SDF1 and its CXCR4 receptor in
the dorsal root ganglia following unilateral sciatic nerve injury
as a model of neuropathic pain. Histochem Cell Biol. 133:323–337.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Behera D, Behera S, Jacobs KE and Biswal
S: Bilateral peripheral neural activity observed in vivo following
unilateral nerve injury. Am J Nucl Med Mol Imaging. 3:282–290.
2013.PubMed/NCBI
|
|
17
|
Sasaki M, Radtke C, Tan AM, et al:
BDNF-hypersecreting human mesenchymal stem cells promote functional
recovery, axonal sprouting and protection of corticospinal neurons
after spinal cord injury. J Neurosci. 29:14932–14941. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Janssen SP, Gerard S, Raijmakers ME, et
al: Decreased intracellular GABA levels contribute to spinal cord
stimulation-induced analgesia in rats suffering from painful
peripheral neuropathy: the role of KCC2 and GABA (A)
receptor-mediated inhibition. Neurochem Int. 60:21–30. 2012.
View Article : Google Scholar
|
|
19
|
Geng SJ, Liao FF, Dang WH, et al:
Contribution of the spinal cord BDNF to the development of
neuropathic pain by activation of the NR2B-containing NMDA
receptors in rats with spinal nerve ligation. Exp Neurol.
222:256–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawasaki Y, Xu ZZ, Wang X, et al: Distinct
roles of matrix metalloproteases in the early- and late-phase
development of neuropathic pain. Nat Med. 14:331–336. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blesch A, Lu P, Tsukada S, et al:
Conditioning lesions before or after spinal cord injury recruit
broad genetic mechanisms that sustain axonal regeneration:
superiority to cAMP-mediated effects. Exp Neurol. 35:162–173.
2011.
|
|
22
|
Rupp A, Dornseifer U, Fischer A, et al:
Electrophysiologic assessment of sciatic nerve regeneration in the
rat: surrounding limb muscles feature strongly in recordings from
the gastrocnemius muscle. J Neurosci Methods. 166:266–277. 2007.
View Article : Google Scholar : PubMed/NCBI
|