Introduction
Fetal hydronephrosis, the incidence of which is
between 0.17 and 2.3%, is one of the most common fetal
abnormalities found in prenatal ultrasound examination (1). The majority of cases of fetal
hydronephrosis spontaneously regress prior to or following birth,
while only a few of them require further treatment postnatally
(2); however, parents of the
infants usually worry about the prognosis of the disease,
particularly about whether postnatal surgery or the long-term use
of antibiotics is required. It is, therefore, essential to
investigate how to use ultrasound to assess objectively the degree
of fetal hydronephrosis, and to predict the outcome, particularly
the likelihood of surgery.
The possibility of postnatal treatment for fetal
hydronephrosis is proportional to its severity (1–3).
Shapiro et al (4) proposed
a hydronephrosis index in 2008, which was used to quantify and
objectively reflect changes due to hydronephrosis (5). The dataset used to evaluate the
sample, however, was not large enough to explore the association
between the index and the prognosis of fetal hydronephrosis.
Three-dimensional ultrasound can accurately measure the volume of
different structures, particularly objects with irregular shapes.
Duin et al (6) reported in
2008 that six researchers measured the pelvis volumes of 15 fetuses
with pyelectasis using three-dimensional ultrasound, which was
suggested to be a reliable method due to its good reproducibility;
however, this research was only a methodological discussion. To the
best of our knowledge, there has been no reported clinical
application of three-dimensional ultrasound for the measurement of
pelvis volume or its application in assessing the degree and
prognosis of fetal hydronephrosis. In the present study, the
expanded renal pelvis volume and kidney volume were measured in 180
cases of fetal hydronephrosis using three-dimensional ultrasound,
and the degree of hydronephrosis was quantitatively evaluated using
renal parenchymal volume/kidney volume measurements.
Materials and methods
Patients
In the present study, 180 pregnant females with
fetal hydronephrosis were examined in the Women’s Hospital, School
of Medicine, Zhejiang University (Hangzhou, China) between January
2009 and October 2013. The enrolled patients were aged between 20
and 42 years with an average age of 28.56±4.47 years. The
pre-pregnancy weights of the females were between 40 and 72 kg with
an average weight of 56.33±7.37 kg, and the pre-pregnancy heights
were between 142 and 173 cm with an average height of 159.18±4.61
cm.
The inclusion criteria were as follows: i) ≥28 weeks
gestation; ii) pelvic anteroposterior diameter (APD) ≥10 mm; iii)
singleton pregnancy; and iv) no other obvious abnormalities found
by ultrasound examination during pregnancy with the exception of
hydronephrosis. Maternal pre-pregnancy weight and height and the
side of fetal hydronephrosis were recorded. Fetuses with
hydronephrosis were excluded if they had other system malformations
or chromosomal abnormalities. Written informed consent was obtained
from all patients and the study was approved by the Ethics
Committee of Zhejiang University.
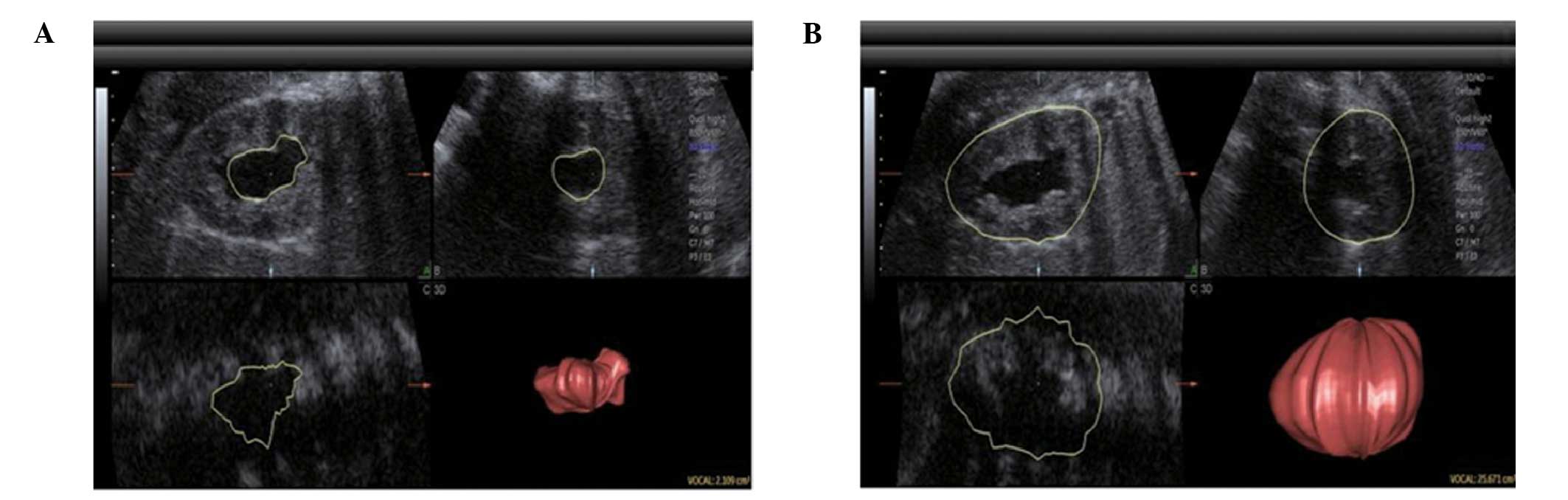
Three-dimensional ultrasound
measurements
The three-dimensional power Doppler mode was started
(Voluson™ 730 Expert and Voluson™ E8; GE
Healthcare, Vienna, Austria) when two-dimensional image quality was
adjusted to optimal and the kidney structure was clearly displayed.
The three-dimensional volume was obtained in the absence of fetal
movement, when the patients were required to hold their breath and
avoid movement. The section showing the maximum fetal kidney was
set as the starting section to acquire the volume. The depth and
width were adjusted according to the size of the kidney so that it
occupied approximately three quarters of the screen. The scanning
angle varied according to gestational age. The volume included the
entire kidney, and the B plane (coronal section) of the volume was
checked subsequent to obtaining the three-dimensional volume. Two
or three valid three-dimensional kidney volumes were saved for
volume measurement selection for each fetus. The 4D
View® Vocal software (GE Kretztechnik, Zipf, Austria)
was used to measure the volumes, and the average was calculated.
The kidney volume and expanded renal pelvic volume of each kidney
with hydronephrosis were measured.
Main indicators for hydronephrosis
The following indicators for hydronephrosis were
included: i) Pelvic APD (the maximum pelvic APD of the kidney
cross-section); ii) the thickness of the renal parenchyma (the
distance from the outermost edge of the renal collecting system on
the long axis middle section to the outer edge of the kidney); iii)
Society for Fetal Urology (SFU) grades (grade 0, no hydronephrosis;
grade 1, mild renal pelvis dilatation; grade 2, mild calyx
expansion with one or several calyces showing expansion; grade 3,
expansion of all calyces; grade 4, calyx expansion with thinning of
the renal parenchyma) (7); iv)
hydronephrosis index (HI, %) [(total area of the kidney - area of
the expanded renal pelvis)/total area of the kidney ×100 (5), which was measured on the largest
hydronephrotic section on the long axis of the kidney]; and v)
renal parenchymal volume/kidney volume [(total volume of the kidney
- volume of the expanded renal pelvis)/total volume of the kidney]
(Fig. 1).
Postnatal follow-up of fetal
hydronephrosis
Hydronephrosis was examined by ultrasound one week
and one, three, six and 12 months after birth and reviewed every
six months thereafter, until spontaneous regression of the
hydronephrosis was observed. If hydronephrosis progressed rapidly,
the time interval between the follow-ups was shortened. If the
progression was rapid or the APD was ≥20 mm, radionuclide
renography or excretory urography and magnetic resonance imaging
examination were performed to determine the condition of the
impaired renal function and the cause of the obstruction, prior to
deciding whether surgery was required. Indications for surgery were
as follows: i) APD >30 mm; ii) APD >20 mm and associated with
calyx expansion; iii) split renal function <30%; iv) continued
decline in renal function; v) continual increase in hydronephrosis;
and vi) clear symptoms (3). The
patients with spontaneous regression were followed up until
hydronephrosis regression was observed. Patients who received
surgery were followed up for pathological conditions and
postoperative recovery.
Statistical analysis
Data were analyzed using SPSS software (version 13;
SPSS Inc., Chicago, IL, USA). A logistical regression method was
used to analyze the association between fetal hydronephrosis
outcome (whether to have surgery) and the side of hydronephrosis,
the pelvic APD, renal parenchymal thickness, SFU grade, HI and the
renal parenchymal volume/kidney volume value. Whether to have
surgery for the fetal hydronephrosis was used as an outcome
variable, the above-mentioned indicators were used as single
diagnostic indicators, and a combination of selected different
single indicators were used as comprehensive indicators to perform
receiver operating characteristic (ROC) curve analyses. The ROC
curves were plotted and the best prediction cutoff for each
indicator was calculated. A two-sample t-test was used to compare
the correlations between hydronephrosis side and regression time.
Spearman rank correlation analysis was used to analyze the
correlation between the APD, the HI, the SFU grade, renal
parenchymal thickness, the renal parenchymal volume/kidney volume
value and the regression time.
Results
Pathological conditions of fetal
hydronephrosis
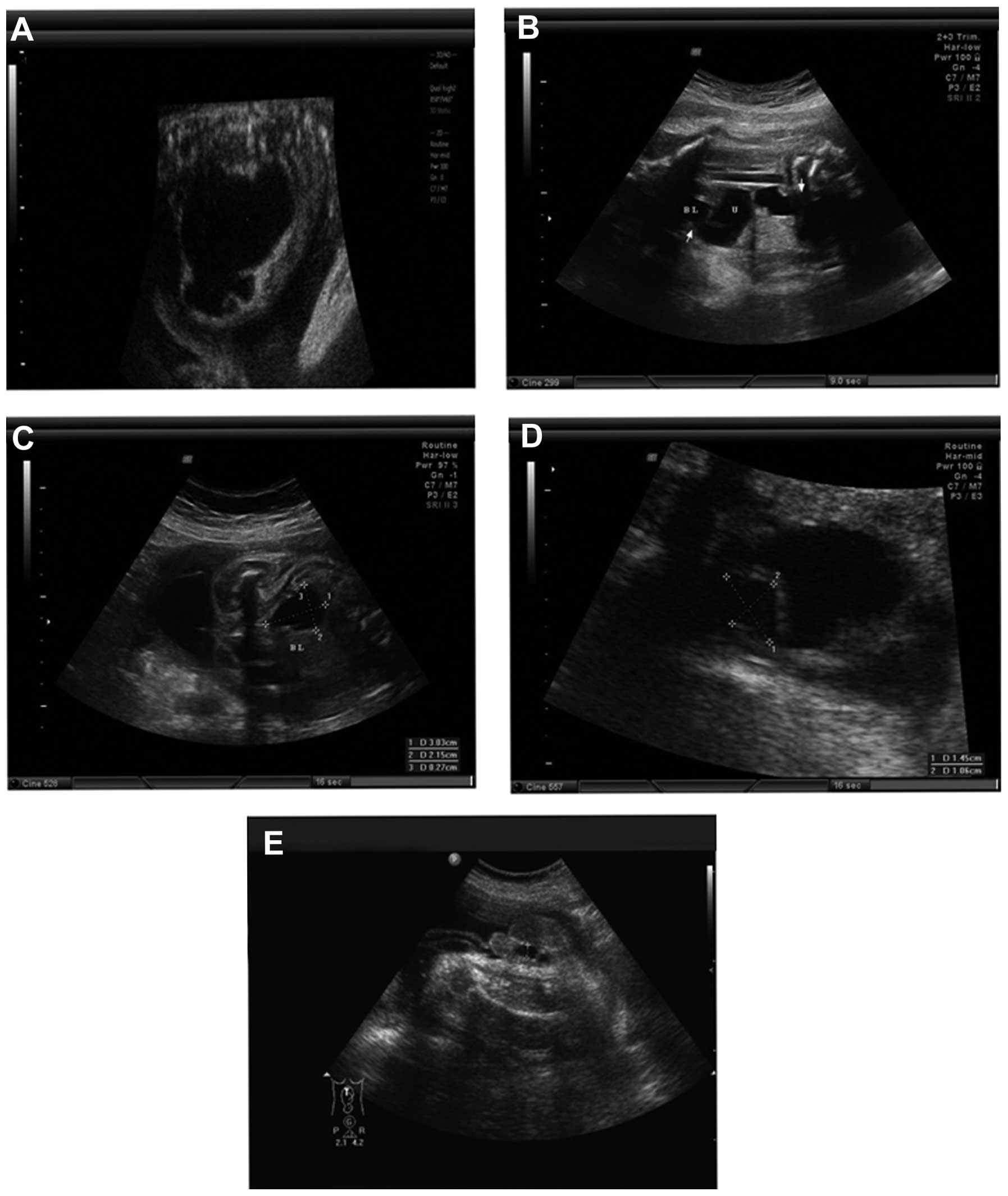
To examine the pathological condition of the fetal
hydronephrosis of the patients, two-dimensional ultrasound was
employed. In the 49 surgical cases, there were 35 cases of
pyelo-ureteral junction stricture (Fig. 2A), seven cases of vesicoureteral
reflux (Fig. 2B), three cases of
posterior urethral valve (Fig.
2C), three cases of ureterocele (Fig. 2D) and one case of anterior urethral
valve (Fig. 2E). With the
exception of one case that had left kidney resection 10 months
after birth due to severe left hydronephrosis, the post-operative
follow-ups of the remaining surgical patients were good. In the 131
non-surgical patients, there were seven cases of vesicoureteral
reflux, nine cases of extrarenal pelvis and 115 cases with no
obvious pathological condition. A total of 110 cases exhibited
spontaneous regression and 21 cases showed no significant changes
in the degree of hydronephrosis during the follow-ups.
Three-dimensional ultrasound can be used
to predict whether fetal hydronephrosis requires surgery
To investigate the correlation between ultrasonic
indicators and the outcome of fetal hydronephrosis, multivariate
logistic regression analysis was performed using
unilateral/bilateral hydronephrosis (unilateral, 0; bilateral, 1),
the APD, the renal parenchymal thickness of the hydronephrotic
side, the SFU grade, the HI and the renal parenchymal volume/kidney
volume value as independent variables, and the hydronephrosis
outcome (no surgery, 0; surgery, 1) as a dependent variable. Upon
examination, unilateral/bilateral hydronephrosis, the APD and the
renal parenchymal volume/kidney volume values were introduced into
the model. Bilateral hydronephrosis, a larger APD or a lower renal
parenchymal volume/kidney volume value suggested a greater risk of
surgery (Tables I and II); therefore, a logistic regression
model was established as follows: Logit (P) = 3.79 + 0.82 ×
unilateral/bilateral hydronephrosis + 1.05 × APD − 8.96 × renal
parenchymal volume/kidney volume.
 | Table IMultivariate logistic regression
analysis of each indicator and hydronephrosis outcome. |
Table I
Multivariate logistic regression
analysis of each indicator and hydronephrosis outcome.
| Variables | β | SE | Wald | v | P-value | OR (95% CI) |
|---|
| Unilateral/bilateral
hydronephrosis | 0.91 | 0.43 | 4.60 | 1.00 | 0.03 | 2.50 (1.08–5.76) |
| HI | 0.51 | 2.68 | 0.04 | 1.00 | 0.85 | 1.67
(0.01–318.09) |
| SFU grade | 0.12 | 0.43 | 0.08 | 1.00 | 0.78 | 1.13 (0.49–2.59) |
| APD | 1.06 | 0.54 | 3.91 | 1.00 | 0.05 | 2.89 (1.01–8.28) |
| Thickness of the
renal parenchyma | 0.37 | 1.63 | 0.05 | 1.00 | 0.82 | 1.44
(0.06–34.98) |
| Renal parenchymal
volume/kidney volume | −9.25 | 2.88 | 10.29 | 1.00 | <0.01 | 0.00 (0.00–0.03) |
| Constant | 2.20 | 3.44 | 0.41 | 1.00 | 0.52 | |
 | Table IILogistic regression analysis of three
indicators and hydronephrosis outcome. |
Table II
Logistic regression analysis of three
indicators and hydronephrosis outcome.
| Variables | β | SE | Wald | v | P-value | OR (95% CI) |
|---|
| Unilateral/bilateral
hydronephrosis | 0.82 | 0.41 | 4.09 | 1.00 | 0.04 | 2.27 (1.03–5.04) |
| APD | 1.05 | 0.49 | 4.53 | 1.00 | 0.03 | 2.85 (1.09–7.48) |
| Renal parenchymal
volume/kidney volume | −8.96 | 2.37 | 14.32 | 1.00 | <0.01 | 0.00 (0.00–0.01) |
| Constant | 3.79 | 2.31 | 1.64 | 1.00 | 0.20 | |
In addition, ROC curve analysis was performed using
the requirement for surgery for the fetal hydronephrosis as the
outcome variable (surgery, 1; no surgery, 0), and
unilateral/bilateral hydronephrosis, the APD, the thickness of the
renal parenchyma, the SFU grade, the HI and the renal parenchymal
volume/kidney volume value as single diagnostic indicators
(Table III). The results showed
that the APD, the thickness of the renal parenchyma, the SFU grade,
the HI and the renal parenchymal volume/kidney volume value, but
not unilateral/bilateral hydronephrosis, had predictive
significance for the requirement for postnatal surgery. The renal
parenchymal volume/kidney volume value had the highest forecast
accuracy, and the APD had the second highest (Table IV and Fig. 3A).
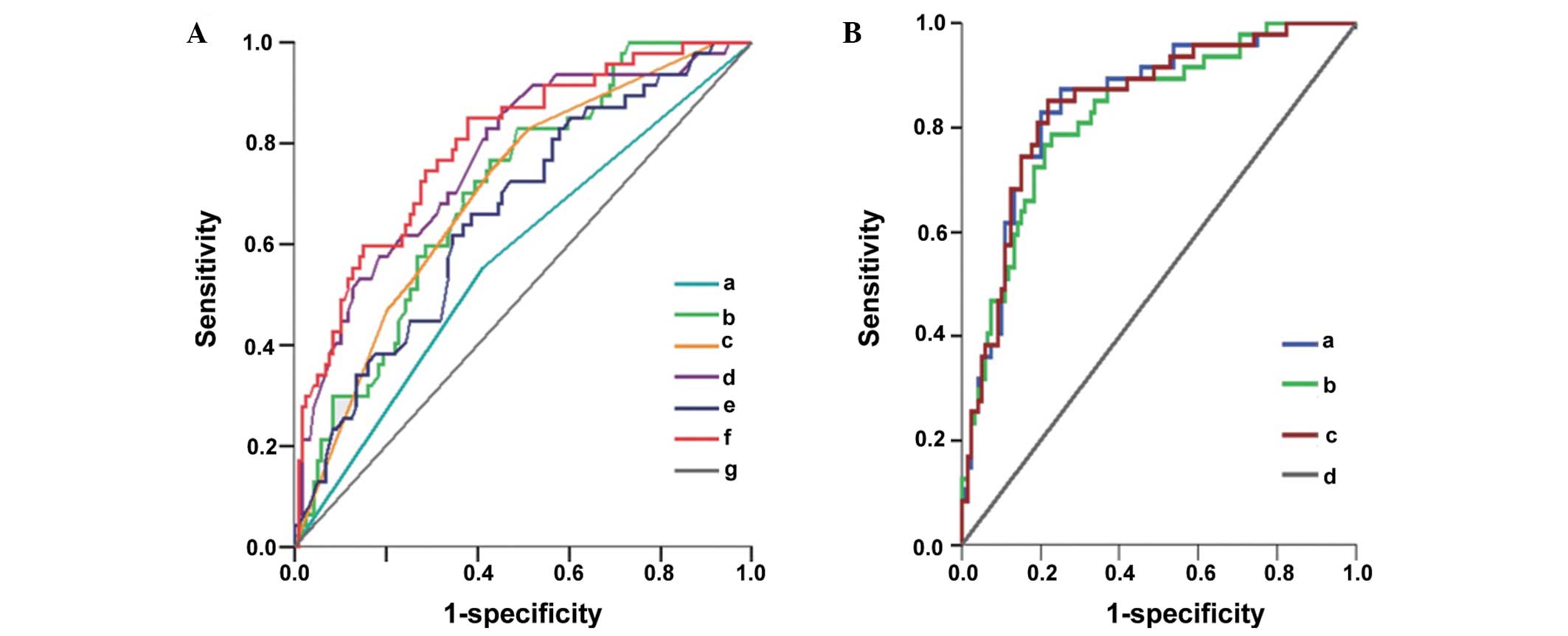 | Figure 3Receiver operating characteristic
curves for the prediction of fetal hydronephrosis outcome. (A)
Single indicator curve showing all the indices with the exception
of lateral. a, Unilateral/bilateral hydronephrosis; b,
hydronephrosis index; c, Society of Fetal Urology grade; d, pelvic
APD; e, renal parenchymal thickness; f, renal parenchymal/kidney
volume value; g, reference line. (B) Individual indicator
combination curve showing that the efficiency of the combination of
three indices is similar to that of the combination of six indices.
a, Six indicators; b, pelvic APD + renal parenchymal volume/kidney
volume value; c, unilateral/bilateral hydronephrosis + pelvic APD +
renal parenchymal/kidney volume value; d, reference line. APD,
anteroposterior diameter. |
 | Table IIIReceiver operating characteristic
curve analysis of each indicator and fetal hydronephrosis
outcome. |
Table III
Receiver operating characteristic
curve analysis of each indicator and fetal hydronephrosis
outcome.
| Variables | Area under the curve
(95% CI) | P-value |
|---|
| Unilateral/bilateral
hydronephrosis | 0.57 (0.47–0.67) | 0.156 |
| HI | 0.71 (0.62–0.79) | <0.001 |
| SFU grade | 0.70 (0.62–0.79) | <0.001 |
| APD | 0.77 (0.69–0.85) | <0.001 |
| Thickness of renal
parenchyma | 0.66 (0.57–0.75) | 0.001 |
| Renal parenchymal
volume/kidney volume | 0.80 (0.72–0.87) | <0.001 |
 | Table IVBest predictive cutoff values of each
single indicator. |
Table IV
Best predictive cutoff values of each
single indicator.
| Indicators | Best predictive
cutoff value | Sensitivity (%) | Specificity (%) | Youden index |
|---|
| HI | 0.69 | 82.98 | 51.26 | 0.34 |
| SFU grade | 2.00 | 69.52 | 57.05 | 0.27 |
| APD | 1.35 | 82.98 | 57.98 | 0.41 |
| Thickness of renal
parenchyma | 0.50 | 72.34 | 52.94 | 0.25 |
| Renal parenchymal
volume/kidney volume | 0.81 | 85.11 | 62.18 | 0.47 |
In addition, ROC curve analysis was performed using
the combination of three indicators (unilateral/bilateral
hydronephrosis, the APD and the renal parenchymal volume/kidney
volume) or the combination of two indicators (the APD and renal
parenchymal volume/kidney volume). The test results showed that the
predictive accuracy of the combined three indicators was higher
than that of the single indicators (Table V and Fig. 3B). When using 0.255 as the
predictive possibility cutoff, the Youden index was the highest
(0.64), with the sensitivity being 85.71% and the specificity being
78.46%.
 | Table VReceiver operating characteristic
curve analysis of the combinations of indicators and hydronephrosis
outcome. |
Table V
Receiver operating characteristic
curve analysis of the combinations of indicators and hydronephrosis
outcome.
| Variables | Area under the
curve (95% CI) | P-value |
|---|
| Combination of six
indicators | 0.85
(0.78–0.91) | <0.001 |
|
Unilateral/bilateral hydronephrosis + APD
+ renal parenchymal/kidney volume | 0.84
(0.78–0.91) | <0.001 |
| APD + renal
parenchymal/kidney volume | 0.82
(0.76–0.89) | <0.001 |
Using unilateral/bilateral hydronephrosis, the APD,
renal parenchymal volume/kidney volume and hydronephrosis outcome,
the logistic regression model was established to give the following
formula: P = e3.786 + 0.822 × unilateral/bilateral
hydronephrosis + 1.048 × APD − 8.955 × renal parenchymal
volume/kidney volume/1 + e3.786 + 0.822 ×
unilateral/bilateral hydronephrosis + 1.048 × APD − 8.955 × renal
parenchymal volume/kidney volume.
According to this formula, the probability of fetal
hydronephrosis outcome (surgery) could be calculated. A P-value
≥0.255 was judged as positive (surgery was required), while a
P-value <0.255 was judged as negative (no surgery was required).
Using this model to retrospectively evaluate the discrimination
effect on the samples, the accuracy was 78.77%, the sensitivity was
81.63% and the specificity was 77.69%. These data demonstrated that
ultrasound, particularly three-dimensional ultrasound, can be used
to predict whether surgery is required for the treatment of fetal
hydronephrosis.
Three-dimensional ultrasound can be
employed to predict the regression time of non-surgical cases
subsequent to birth
To determine the correlation between fetal
hydronephrosis indicators and the postnatal regression time of
non-surgical cases, the association between any of the
aforementioned six ultrasound indicators and the regression time of
hydronephrosis was individually analyzed. The results showed that
bilateral hydronephrosis had a longer regression time than
unilateral hydronephrosis (t=−2.82, P=0.01). A larger APD, a higher
SFU grade and a lower renal parenchymal volume/kidney volume value
suggested a longer regression time (r=0.191, P=0.030; r=0.189,
P=0.031; r=−0.293, P=0.001, respectively). The HI and the thickness
of the renal parenchyma had no significant correlation with the
regression time of hydronephrosis (r=−0.082, P=0.358; r=−0.144,
P=0.155). These data indicated that three-dimensional ultrasound
could predict the regression time of non-surgical cases following
birth.
Discussion
Studies by Ismaili et al (8) and Thornburg et al (9) indicated that ultrasound in late
pregnancy could better predict whether to perform surgery for fetal
hydronephrosis compared with that in mid-term pregnancy. Ismaili
et al (8) reported that the
positive predictive value of a late pregnancy APD of >0.7 cm was
69%, while that of a mid-term pregnancy APD of >0.4 cm was only
49%, since the majority (80%) of mid-term pregnancy cases of fetal
hydronephrosis, particularly mild hydronephrosis, would regress in
late pregnancy or following birth (10,11).
A pelvic APD <1 cm usually means there is no disease (2,12–17).
Woodward et al (2) reported
that, in cases with an APD of <1 cm, only 3% had a deformity. In
the study by Woodward et al, fetuses aged ≥28 weeks with
hydronephrosis and an APD of ≥1 cm were studied. Using six
indicators, namely unilateral/bilateral hydronephrosis, the APD,
the thickness of the renal parenchyma, the SFU grade, the HI and
the renal parenchymal volume/kidney volume value, the association
between each indicator and the requirement for postnatal surgery
was analyzed. The results showed that all six indicators could
predict whether to perform postnatal surgery for the fetal
hydronephrosis. Bilateral hydronephrosis, a larger APD, a thinner
renal parenchyma, a higher SFU grade, a lower HI and a lower renal
parenchymal volume/kidney volume value suggested a greater
possibility of surgery. The cutoff for each indicator used to
predict the possibility of surgery was obtained using the ROC
curve. If the APD was >1.35 cm, the thickness of the renal
parenchyma was <0.5 cm, the SFU grade was >2, the HI was
<0.69 and the renal parenchymal volume/kidney volume value was
<0.81, then a significant possibility of surgery was suggested.
In the present study, a novel three-dimensional indicator, the
renal parenchymal volume/kidney volume value, was introduced for
the first time; this indicator had the best predictive performance,
with the largest area under the ROC curve (0.8) and the highest
Youden index (0.47). This could be associated with the high
accuracy of three-dimensional ultrasound in measuring the expanded
renal pelvis volume and kidney volume, which led to objective
evaluation of the degree of hydronephrosis.
Using combined indicators to predict fetal
hydronephrosis outcome usually has a higher accuracy than using
single indicators. Zhan et al (18) used 0–3 points to score the APD, the
thickness of the renal parenchyma and the renal pelvis shape
according to the degree of hydronephrosis, and found that, when the
combined score of the three indicators equaled ≤3, ≤4, ≤5, ≤6, ≤7
and ≥8, the proportions of pathological hydronephrosis were 0,
11.11, 28.57, 50.00, 80.00 and 100%, respectively. The best cutoff
value to diagnose pathological fetal hydronephrosis was six, with
the sensitivity and specificity being 88.46 and 94.49%,
respectively. The present study indicated that combined indicators
were more accurate than single indicators in predicting whether to
perform surgery, and the area under the ROC curve for the
combination of the six indicators was 0.85. Multivariate regression
analysis showed that the accuracy of the combination of three
indicators, unilateral/bilateral hydronephrosis, the APD and the
renal parenchymal volume/kidney volume value, was close to that of
the combination of six indicators, sharing similar 95% confidence
intervals (0.78–0.91). The surgery possibility calculated using the
logistic regression model constructed by the three indicators had
an accuracy of 78.77%, a sensitivity of 81.63% and a specificity of
77.69%, using a P-value of 0.255 as the cutoff. The results
suggested that the combination of the three indicators had a higher
accuracy in predicting the possibility of postnatal surgery for
fetal hydronephrosis.
To the best of our knowledge, only a few studies
have reported the correlation between the degree of fetal
hydronephrosis and postnatal regression time; these studies found
that the lower the APD, the shorter the spontaneous regression time
(19–21). The present study comprehensively
analyzed the correlation between whether hydronephrosis could
regress and each indicator, and the correlation between regression
time and each indicator. The results showed that bilateral
hydronephrosis, a larger APD, a higher SFU grade or a smaller renal
parenchymal volume/kidney volume value indicated a longer
spontaneous regression time, suggesting that more severe
hydronephrosis was associated with a longer regression time. Among
the indicators, the renal parenchymal volume/kidney volume value
was the most relevant, suggesting a high application value for
predicting whether spontaneous regression of fetal hydronephrosis
would occur.
Acknowledgements
The present study was supported by grants from the
Department of Health of Zhejiang Province (no. 2011KYA099) and the
Department of Science and Technology of Zhejiang Province (no.
2012C23103).
References
|
1
|
Wollenberg A, Neuhaus TJ, Willi UV and
Wisser J: Outcome of fetal renal pelvic dilatation diagnosed during
the third trimester. Ultrasound Obstet Gynecol. 25:483–488. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woodward M and Frank D: Postnatal
management of antenatal hydronephrosis. BJU Int. 89:149–156. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wickstrom E, Maizels M, Sabbagha RE,
Tamura RK, Cohen LC and Pergament E: Isolated fetal pyelectasis:
assessment of risk for postnatal uropathy and Down syndrome.
Ultrasound Obstet Gynecol. 8:236–240. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shapiro SR, Wahl EF, Silberstein MJ and
Steinhardt G: Hydronephrosis index: a new method to track patients
with hydronephrosis quantitatively. Urology. 72:536–539. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Venkatesan K, Green J, Shapiro SR and
Steinhardt GF: Correlation of hydronephrosis index to society of
fetal urology hydronephrosis scale. Adv Urol. 9604902009.PubMed/NCBI
|
|
6
|
Duin LK, Willekes C, Vossen M, Beckers M,
Offermans J and Nijhuis JG: Reproducibility of fetal renal pelvis
volume measurement using three-dimensional ultrasound. Ultrasound
Obstet Gynecol. 31:657–661. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grignon A, Filion R, Filiatrault D,
Robitaille P, Homsy Y, Boutin H and Leblond R: Urinary tract
dilatation in utero: classification and clinical applications.
Radiology. 160:645–647. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ismaili K, Hall M, Donner C, Thomas D,
Vermeylen D and Avni FE; Brussels Free University Perinatal
Nephrology study group. Results of systematic screening for minor
degrees of fetal renal pelvis dilation in an unselected population.
Am J Obstet Gynecol. 188:242–246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thornburg LL, Pressman EK, Chelamkuri S,
Hulbert W, Rabinowitz R and Mevorach R: Third trimester ultrasound
of fetal pyelectasis: predictor for postnatal surgery. J Pediatr
Urol. 4:51–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toiviainen-Salo S, Garel L, Grignon A, et
al: Fetal hydronephrosis: is there hope for consensus? Pediatr
Radiol. 34:519–529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pates JA and Dashe JS: Prenatal diagnosis
and management of hydronephrosis. Early Hum Dev. 82:3–8. 2006.
View Article : Google Scholar
|
|
12
|
Filly RA and Feldstein VA: The fetal
genitourinary tract. Ultrasonography in Obststrics and Gynecology.
Callen PW: 4th edition. WB Saunders; Philadelphia, PA: pp. 515–550.
2000
|
|
13
|
Langer B: Fetal pyelectasis. Ultrasound
Obstet Gynecol. 16:1–5. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sairam S, Al-Habib A, Sasson S and
Thilaganathan B: Natural history of fetal hydronephrosis diagnosed
on mid-trimester ultrasound. Ultrasound Obstet Gynecol. 17:191–196.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siemens DR, Prouse KA, MacNeily AE and
Sauerbrei EE: Antenatal hydronephrosis: thresholds of renal pelvic
diameter to predict insignificant postnatal pelviectasis. Tech
Urol. 4:198–201. 1998.
|
|
16
|
Plevani C, Locatelli A, Paterlini G,
Ghidini A, Tagliabue P, Pezzullo JC and Vergani P: Fetal
hydronephrosis: natural history and risk factors for postnatal
surgery. J Perinat Med. 42:385–391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
St Aubin M, Willihnganz-Lawson K, Varda
BK, et al: Society for fetal urology recommendations for postnatal
evaluation of prenatal hydronephrosis - will fewer voiding
cystourethrograms lead to more urinary tract infections? J Urol.
190(4 Suppl): 1456–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan X, Tao G, Cheng L, Fu Q, Li H, Liu F
and Liu S: Ultrasound scoring in predicting the prognosis of fetal
hydronephrosis. Zhongguo Chaosheng Yixue Zazhi. 25:1176–1179.
2009.(In Chinese).
|
|
19
|
Dhillon H: Prenatally diagnosed
hydronephrosis: the Great Ormond Street experience. Br J Urol.
81(Suppl 2): 39–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coplen DE, Austin PF, Yan Y, Blanco VM and
Dicke JM: The magnitude of fetal renal pelvic dilatation can
identify obstructive postnatal hydronephrosis, and direct postnatal
evaluation and management. J Urol. 176:724–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coelho GM, Bouzada MC, Pereira AK,
Figueiredo BF, Leite MR, Oliveira DS and Oliveira EA: Outcome of
isolated antenatal hydronephrosis: a prospective cohort study.
Pediatr Nephrol. 22:1727–1734. 2007. View Article : Google Scholar : PubMed/NCBI
|