Introduction
Vascular smooth muscle cells (VSMCs) are a highly
specialized type of cell, whose primary functions in mature animals
include contraction (1) and the
production of matrix components of the blood vessel wall (2). The proliferation of VSMCs has been
demonstrated to be important in the development of atherosclerosis
(3). Fully differentiated or
mature smooth muscle cells (SMCs) express a unique repertoire of
contractile proteins, ion channels and signaling molecules that are
required for functionality.
VSMCs exhibit a variety of phenotypes at different
developmental stages, under different pathophysiological conditions
or normal conditions, depending on the anatomical location of the
vessels (4). Previous studies have
aimed to distinguish between the two phenotypes of VSMCs, the
spindle-shaped ‘contractile’ and epithelioid-shaped ‘synthetic’
phenotypes (5). Varicose veins
exhibit thickened vessel walls, as a result of the dysregulation of
the synthesis of extracellular matrix proteins in SMCs (6). The phenotypic modulation of SMCs can
alter extracellular matrix metabolism (7), and the etiology and physiopathology
of varicose disorders include venous wall remodeling associated
with abnormalities of SMCs and extracellular matrix (8).
NELIN is a novel F-actin-associated protein that has
been shown to have restricted expression in the heart, skeletal
muscle, arteries and veins (9).
The protein mediates cell motility and is important in the process
of cell migration and adhesion (10). SM22α is a calponin-related protein
(11) and one of the earliest
markers of differentiated SMCs (12). The 6.2-kilobase single copy gene is
composed of five exons, and is expressed in the smooth, cardiac and
skeletal muscle lineages during early embryogenesis, prior to
becoming restricted specifically to VSMCs during late fetal
development and adulthood (13).
The mechanisms and determinants underlying the
development of varicosities are not yet clearly defined. In the
present study, the transcriptional and translational expression
levels of NELIN and SM22α in varicose vein and normal vein tissues
were analyzed. The aims of the study were to verify the phenotypic
modulation of VSMCs in varicose vein tissues, and discuss the
possible mechanisms underlying the development of varicose
veins.
Materials and methods
Specimen collection
In total, 18 patients with lower limb varicose
veins, who had undergone treatment at the Qianfoshan Hospital
Affiliated to Shandong University (Jinan, China), were assigned to
the experimental group. The average age of the patients was
46.6±15.5 years, and 10 patients were male. In addition, 14
patients that had undergone great saphenous vein coronary artery
bypass surgery were assigned to the control group. These patients
had an average age of 46±13 years, and five patients were female.
The patients in the control group did not have a medical history of
varicose veins, which was verified by various preoperative
assessments. During surgery, tissue samples from the great
saphenous vein were obtained from the two groups, with two samples
collected for each case. One sample was quick-frozen and
immediately stored in a liquid nitrogen container, while the other
sample was soaked in 10% formaldehyde solution to be fixed for 24
h, then conventionally dehydrated, hyalinized, embedded in wax and
cut into 4-μm slices for preservation. Written informed consent was
provided by the patients, and sample and data collection were
approved by the Ethics Committee of Qianfoshan Hospital Affiliated
to Shandong University.
Main reagents
TransZol Up and two-step reverse transcription
polymerase chain reaction (RT-PCR) kits were purchased from Beijing
TransGen Biotech Co., Ltd. (Beijing, China). β-actin upstream and
downstream primers were synthesized by Takara Bio, Inc. (Shiga,
Japan). NELIN-specific, SM22α-specific and GAPDH upstream and
downstream PCR primers were synthesized by Beijing Dingguo
Changsheng Biotechnology Co., Ltd. (Beijing, China). A rabbit
anti-human anti-NELIN polyclonal antibody was purchased from
Sigma-Aldrich (St. Louis, MO, USA), while a concentrated rat
anti-human SM22α polyclonal antibody was purchased from Proteintech
Group, Inc. (Chicago, IL, USA). Two resistant kits for
immunohistochemical streptavidin-biotin complex (SABC) detection
were purchased from Wuhan Boster Biotechnology Co., Ltd. (Wuhan,
China). Diaminobenzidine (DAB) staining reagents were purchased
from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing,
China), and all other reagents were homebred and analytically
pure.
RT-PCR detection of NELIN and SM22α mRNA
expression in the vein tissue
Total RNA in the vein tissue was extracted with
TransZol Up, and the purity of the RNA samples was determined by an
ultraviolet (UV) spectrophotometer (EU-2800; Shanghai Onlab
Instruments Co., Ltd., Shanghai, China). The specific PCR primer
sequences of human β-actin and NELIN are shown in Table I, while the sequences of GAPDH and
SM22α are shown in Table II. The
synthesis of first-strand cDNA and PCR were conducted according to
the manufacturer’s instructions of the two-step RT-PCR kit. The
RT-PCR for NELIN included 2X EasyTaq PCR SuperMix (25 μl),
cDNA (10 μl), upstream and downstream primers of NELIN (1.0 μl
each), upstream and downstream primers of β-actin (1.0 μl each) and
double-distilled (dd) H20 (11 μl). The thermal cycle
format used in the RT-PCR was as follows: One cycle of preheating
at 94°C for 2 min; 31 amplified cycles with denaturation for 30 sec
at 94°C, annealing for 30 sec at 55°C and extension for 45 sec at
72°C; followed by a final incubation cycle at 72°C for 10 min. The
RT-PCR for SM22α PCR included cDNA (2 μl), upstream and downstream
primers of SM22α (0.5 μl each), upstream and downstream primers of
GAPDH (0.5 μl each), 2X TransTaq™ High Fidelity PCR SuperMix
II (25 μl) and ddH20 (21 μl). The thermal cycle format
used in the RT-PCR was as follows: 33 cycles of denaturation for 30
sec at 94°C, annealing for 30 sec at 56°C and extension for 30 sec
at 72°C; followed by a final incubation cycle at 72°C for 10 min.
Products of 10 μl (each) were analyzed by electrophoresis in 1.5%
agarose gels and visualized under UV illumination. The products
were photographed using an independent softwae package controlled
by a gel imaging system (GelDol2000; Bio-Rad, Hercules, CA, USA)
and semi-quantified with density scanning. The objective stripe and
internal control products were compared. The ratio of the density
scanning values of NELIN against the GAPDH control was used to
evaluate the expression level of NELIN mRNA. To analyze the
differences between the experimental and control groups, the mRNA
expression level of SM22α was evaluated by comparing the ratio of
the density scanning values of SM22α against the GAPDH control.
 | Table IPrimer sequences used for reverse
transcription polymerase chain reaction analysis of NELIN. |
Table I
Primer sequences used for reverse
transcription polymerase chain reaction analysis of NELIN.
| Gene | Primer | Amplified fragment
length (bp) |
|---|
| NELIN |
| Positive-strand |
5′-AGGAGTGGCTCTATTCAA-3′ | 611 |
| Negative-strand |
5′-GGTAAGTAAAGGCAGTAAG-3′ | |
| β-actin |
| Positive-strand |
5′-TTCTGTGGCATCCACGAAACT-3′ | 312 |
| Negative-strand |
5′-TTCTGTGGCATCCACGAAACT-3′ | |
 | Table IIPrimer sequences used for reverse
transcription polymerase chain reaction analysis of SM22α. |
Table II
Primer sequences used for reverse
transcription polymerase chain reaction analysis of SM22α.
| Gene | Primer | Amplified fragment
length (bp) |
|---|
| SM22α |
| Positive-strand |
5′-TGGTGAACAGCCTGTACCCT-3′ | 235 |
| Negative-strand |
5′-CACGGTAGTGCCCATCATTC-3′ | |
| β-actin |
| Positive-strand |
5′-ACCACAGTCCATGCCATCAC-3′ | 472 |
| Negative-strand |
5′-TCCACCACCCTGTTGCTGTA-3′ | |
SABC immunohistochemistry staining
Tissue samples were cut into slices, dewaxed and
immersed in water. Endogenous peroxide was blocked by incubating
the tissues with 3% H2O2, after which antigen
retrieval (hot repair with microwave) was conducted. Tissues were
blocked with 5% bovine serum albumin, and incubated with primary
antibodies against NELIN (rabbit anti-human, 1:150) and SM22α
(polyclonal, 1:100) for 60 min at room temperature. Subsequently,
the samples were incubated with a secondary antibody for 20 min,
which was followed by the addition of the avidin-biotin peroxidase
complex. Staining was visualized with DAB and tissues were
counterstained with hematoxylin solution, then for transparent and
sealed in piece with neutral gum. At every stage of the dyeing
process, phosphate-buffered saline was used to replace the primary
antibodies as the negative control.
Under a light microscope (BX-51; Olympus
Corporation, Center Valley, PA, USA), positive staining (yellow or
brown-yellow) was observed in the cytoplasm of the VSMCs. For image
analysis, five evenly dyed and completely non-overlapping outlines
were selected from each slice to be photographed under 10×40
magnification; all the images were exactly the same size. Integral
optical density (IOD) values of each image were determined using
Image-Pro Plus 5.0 image analysis software (Media Cybernetics,
Rockville, MD, USA) and the average value was calculated. A
positive correlation was observed between the IOD value and the
protein expression of positive cells; this value was regarded as
the semi-quantitative value of the sample.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Mean values were compared by analysis of variance with
the Student-Newman Keuls test, using SPSS 10.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
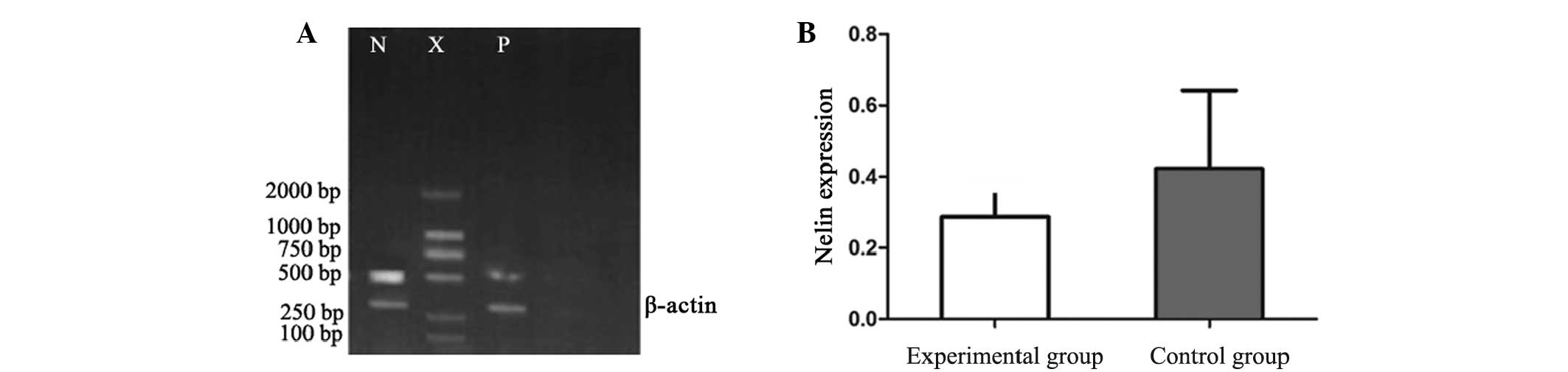
RT-PCR
RT-PCR analysis revealed that the mRNA expression
levels of NELIN in the VSMCs were significantly decreased in the
experimental group (0.2867±0.1025) when compared with the control
group (0.4221±0.220; P<0.01; Fig.
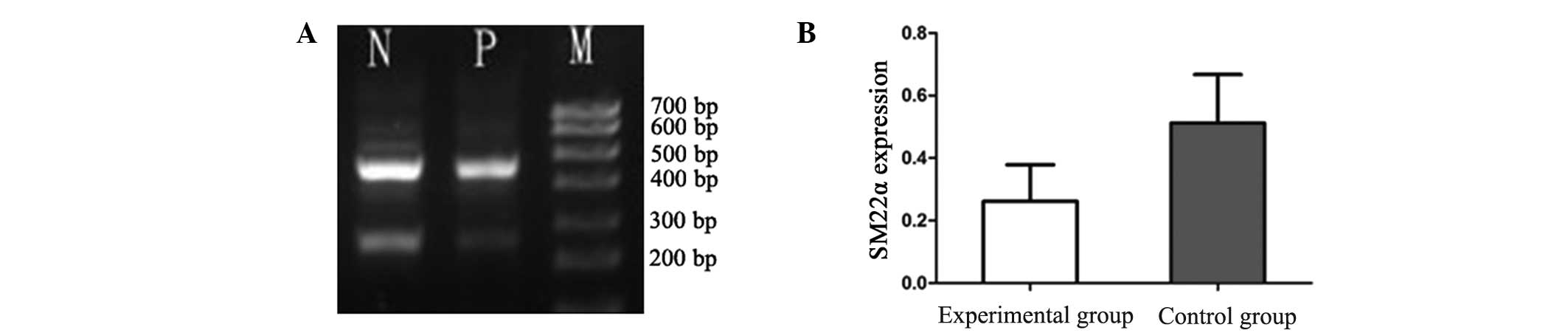
1). Similarly, the mRNA expression levels of SM22α were
significantly reduced in the experimental group (0.2614±0.1168)
when compared with the control group (0.5114±0.1554; P<0.01;
Fig. 2).
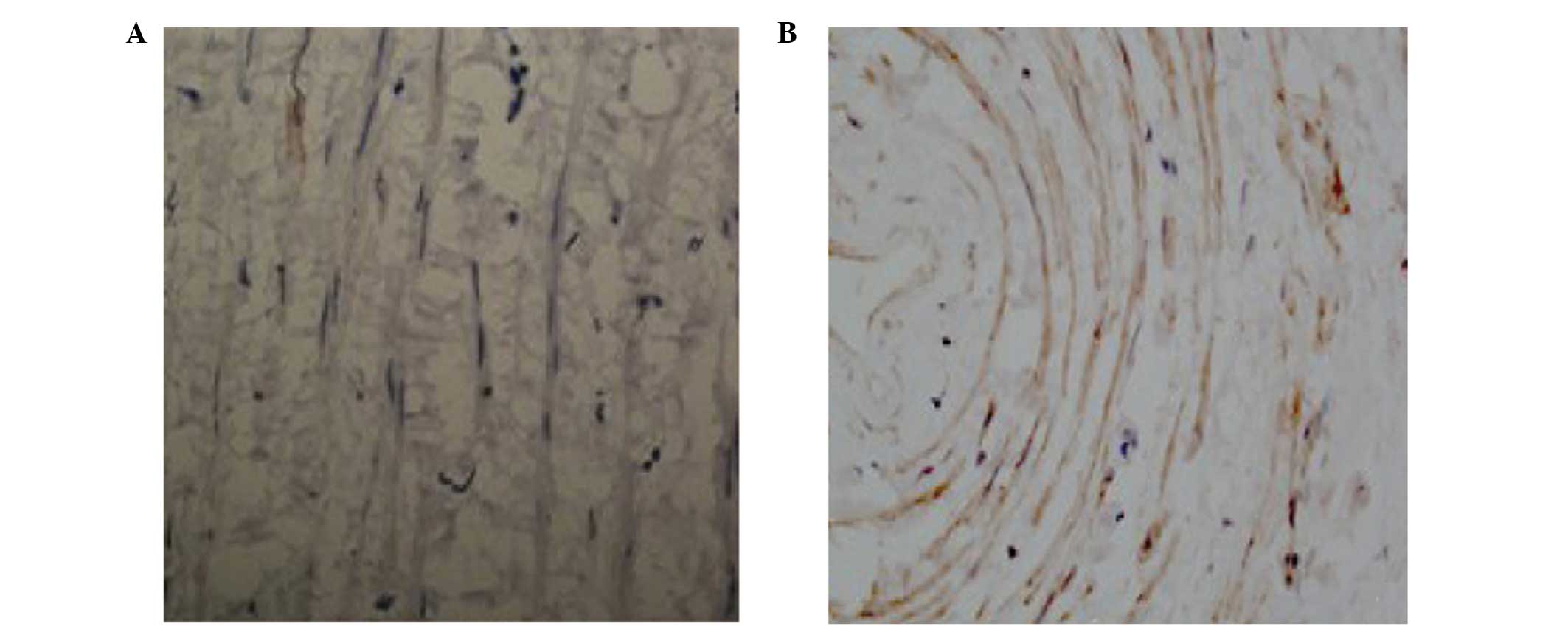
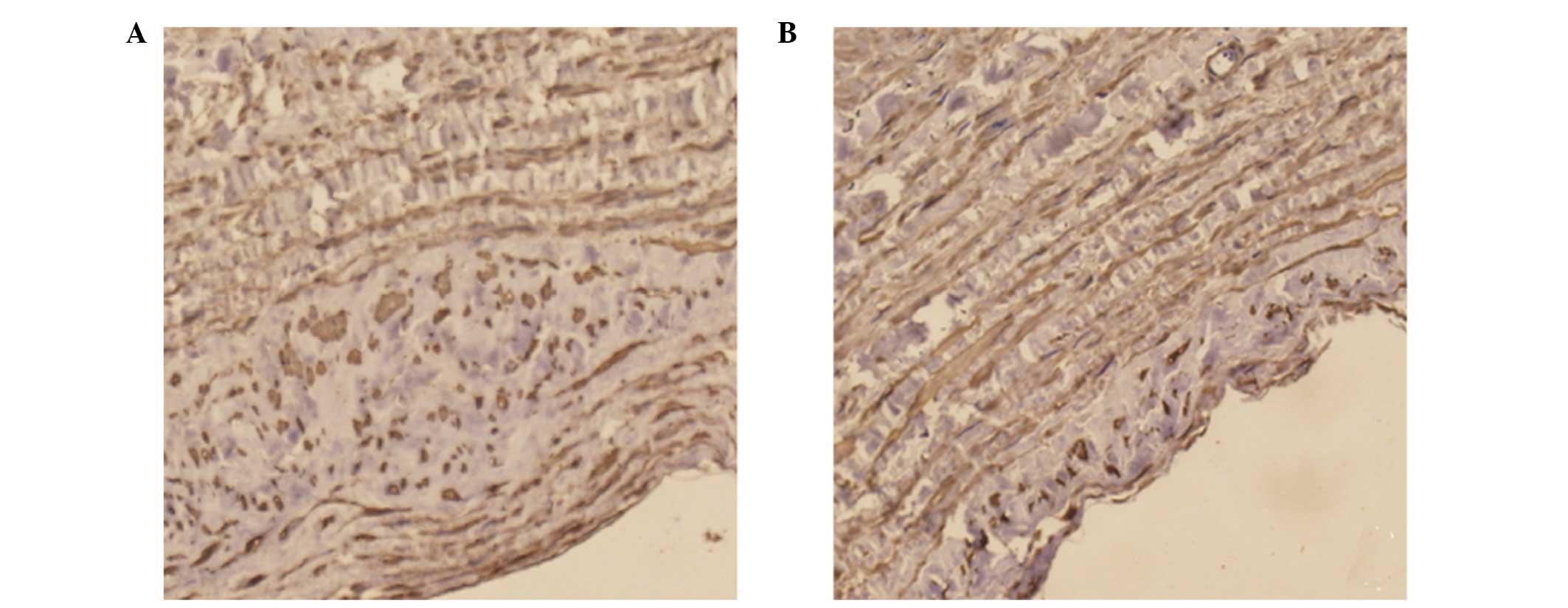
SABC immunohistochemistry
Positive staining of NELIN (Fig. 3) and SM22α (Fig. 4) was observed in the cytoplasm of
the cells. Immunohistochemical staining revealed a significant
decrease in the expression levels of NELIN and SM22α in the
experimental group when compared with the control (P<0.05).
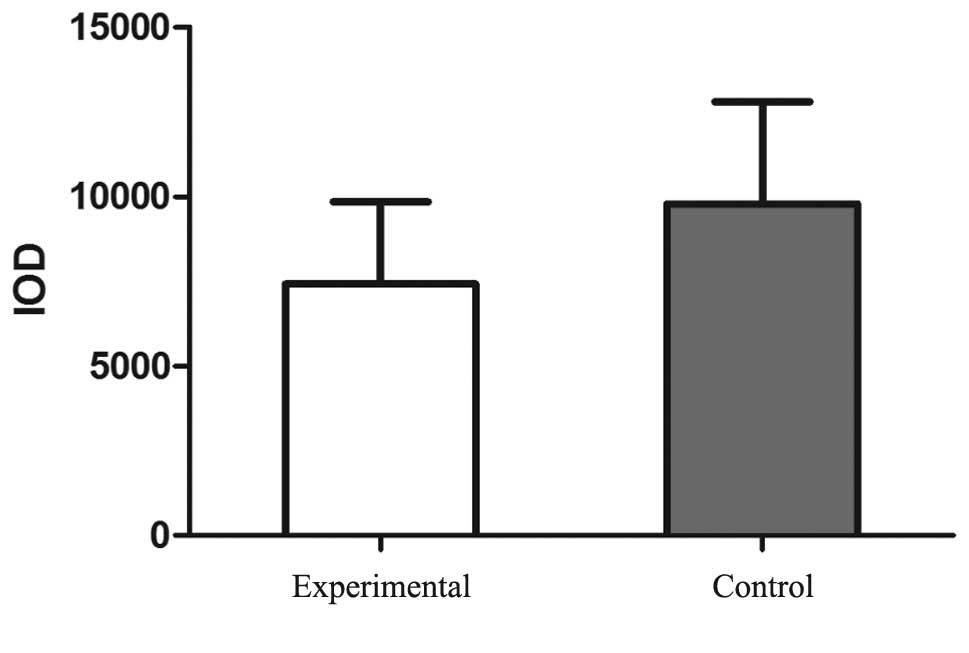
According to the statistical analysis, the IOD value of the NELIN
protein staining was significantly reduced in the experimental
group (7422.56±2423) when compared with the control group
(9785.85±3005.85; P<0.05; Fig.
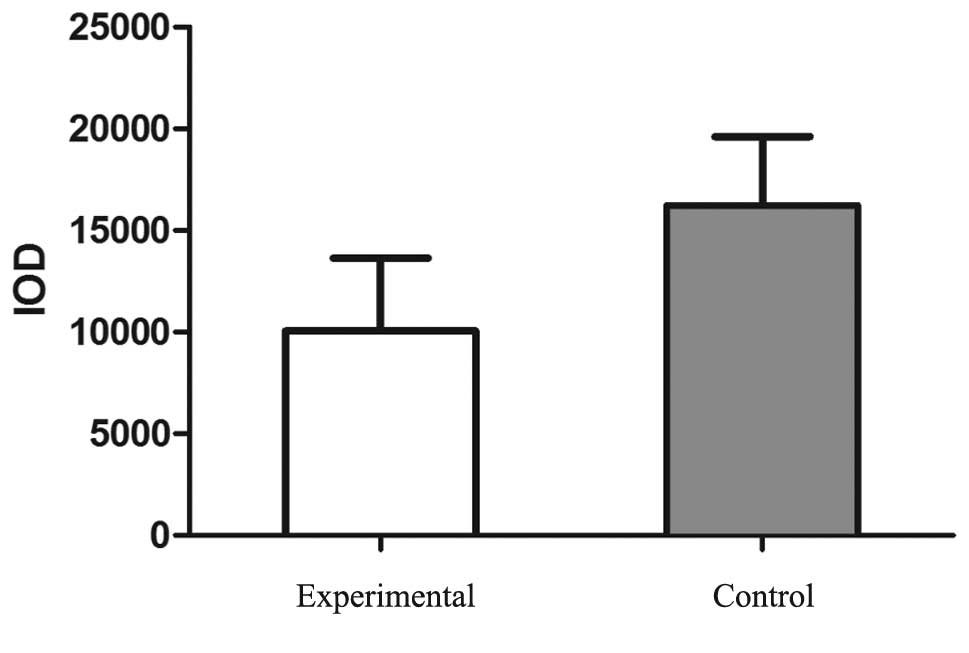
5). Similarly, the IOD value of SM22α staining was
significantly reduced in the experimental group (10054.79±3584.07)
when compared with the control group (16226.05±3378.16; P<0.05;
Fig. 6).
Discussion
As shown in a previous study, VSMCs cultivated in
vitro change from a contractile to a synthetic phenotype
following four subcultures, with the symbol of phenotypic
transition being a clear reduction in SM22α expression in the VSMCs
(14). Therefore, in the present
study, the expression of SM22α was analyzed to assess the
occurrence of VSMC phenotypic transition in the varicose vein
tissues. The results revealed that the VSMCs in the control group
were primarily of a contractile phenotype (15), while in the control group, a number
of VSMCs had changed from a contractile to a synthetic phenotype
(16). This result was consistent
with previous study of VSMC in vitro culturing. The increase
in synthetic phenotype VSMCs may result in an increase in
extracellular matrix immaturity, resulting in a decrease in the
normal extracellular matrix maintenance of cell stability and wall
integrity. Subsequently, structural changes may occur in the normal
vein tissues, which can be verified by the morphological
differences of the two groups of specimens observed under
immunohistochemical light and electron microscopy. Based on the
phenotypic transition, the vein wall structure changes and causes
venous dilation (17). An increase
in the vein wall tension augments the expression/activity of matrix
metalloproteinases, which induces the degradation of the
extracellular matrix proteins and affects the structural integrity
of the vein wall, ultimately leading to chronic and progressive
venous insufficiency and varicose vein formation (18).
NELIN expression was observed in the VSMCs of the
vein wall; however, the mRNA and protein expression levels were
markedly reduced in the lower limb varicose vein tissue. These
results indicated that the downregulation of NELIN expression may
be a branch sign of VSMC phenotypic transition. A previous study
reported that NELIN was a novel F-actin-binding protein that was
colocalized with F-actin and filamin in the cytoplasm of cells
(19). Therefore, NELIN was
hypothesized to be associated with the skeleton organization of
VSMCs, regulating a variety of biological behaviors, including the
phenotype, form and systolic function of the cytoskeleton, directly
or indirectly.
Sequence analysis has previously indicated that
amino acids 154–161 of SM22α may be an actin-binding site (20). In addition, amino acids 175–195
have been shown to exhibit a high homology with tandem repeat
domains in the COOH-terminal of calponin h1 and h2; in calponin,
these tandem repeat sequences are important for full actin affinity
(21). NELIN and SM22α were
hypothesized to combine with actin simultaneously, resulting in a
change of form and function of the cytoskeleton. A cell phenotypic
transition may subsequently occur, promoting the occurrence of
varicose veins.
In conclusion, the present study demonstrated that
VSMCs in varicose vein tissue transformed from a contractile to a
synthetic phenotype with varicosity. Furthermore, NELIN and SM22α
are important for the phenotypic transition. Since NELIN and SM22α
are actin-binding proteins, a synergistic effect may exist between
them. However, the specific regulatory and molecular mechanisms
underlying the mutual coordination remain unclear and require
further study.
References
|
1
|
Miller DC, Thapa A, Haberstroh KM and
Webster TJ: Endothelial and vascular smooth muscle cell function on
poly (lactic-co-glycolic acid) with nano-structured surface
features. Biomaterials. 25:53–61. 2004. View Article : Google Scholar
|
|
2
|
Galis ZS and Khatri JJ: Matrix
metalloproteinases in vascular remodeling and atherogenesis the
good, the bad, and the ugly. Circ Res. 90:251–262. 2002.PubMed/NCBI
|
|
3
|
Arita Y, Kihara S, Ouchi N, et al:
Adipocyte-derived plasma protein adiponectin acts as a
platelet-derived growth factor-BB-binding protein and regulates
growth factor-induced common postreceptor signal in vascular smooth
muscle cell. Circulation. 105:2893–2898. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshida T and Owens GK: Molecular
determinants of vascular smooth muscle cell diversity. Circ Res.
96:280–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao H, Gabbiani G and Bochaton-Piallat ML:
Arterial smooth muscle cell heterogeneity implications for
atherosclerosis and restenosis development. Arterioscler Thromb
Vasc Biol. 23:1510–1520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sansilvestri-Morel P, Rupin A,
Badier-Commander C, et al: Imbalance in the synthesis of collagen
type I and collagen type III in smooth muscle cells derived from
human varicose veins. J Vasc Res. 38:560–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Badier-Commander C, Couvelard A, Henin D,
Verbeuren T, Michel JB and Jacob MP: Smooth muscle cell modulation
and cytokine overproduction in varicose veins. An in situ study. J
Pathol. 193:398–407. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sansilvestri-Morel P, Rupin A, Jaisson S,
Fabiani JN, Verbeuren TJ and Vanhoutte PM: Synthesis of collagen is
dysregulated in cultured fibroblasts derived from skin of subjects
with varicose veins as it is in venous smooth muscle cells.
Circulation. 106:479–483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, Wei YJ, Cao HQ and Ding JF:
Molecular cloning of NELIN, a putative human cytoskeleton
regulation gene. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao
(Shanghai). 33:19–24. 2001.
|
|
10
|
Wang W, Zhang W, Han Y, et al: NELIN, a
new F-actin associated protein, stimulates HeLa cell migration and
adhesion. Biochem Biophys Res Commun. 330:1127–1131. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Miano JM, Cserjesi P and Olson EN:
SM22 alpha, a marker of adult smooth muscle, is expressed in
multiple myogenic lineages during embryogenesis. Circ Res.
78:188–195. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Solway J, Seltzer J, Samaha FF, et al:
Structure and expression of a smooth muscle cell-specific gene,
SM22 alpha. J Biol Chem. 270:13460–13469. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Liu Z, Mercer B, Overbeek P and
Olson EN: Evidence for serum response factor-mediated regulatory
networks governing SM22alpha transcription in smooth, skeletal, and
cardiac muscle cells. Dev Biol. 187:311–321. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han Y, Wu G, Deng J, et al: Cellular
repressor of E1A-stimulated genes inhibits human vascular smooth
muscle cell apoptosis via blocking P38/JNK MAP kinase activation. J
Mol Cell Cardiol. 48:1225–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steitz SA, Speer MY, Curinga G, et al:
Smooth muscle cell phenotypic transition associated with
calcification: upregulation of Cbfa1 and downregulation of smooth
muscle lineage markers. Circ Res. 89:1147–1154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chamley-Campbell JH, Campbell GR and Ross
R: Phenotype-dependent response of cultured aortic smooth muscle to
serum mitogens. J Cell Biol. 89:379–383. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elsharawy MA, Naim MM, Abdelmaguid EM and
Al-Mulhim AA: Role of saphenous vein wall in the pathogenesis of
primary varicose veins. Interact Cardiovasc Thorac Surg. 6:219–224.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raffetto JD and Khalil RA: Mechanisms of
varicose vein formation: valve dysfunction and wall dilation.
Phlebology. 23:85–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Meng X, Cao H, et al:
Characteristics of the binding features of NELIN with F-actin and
screening NELIN interactive proteins. Chinese Science Bulletin.
49:2487–2490. 2004.
|
|
20
|
Zeidan A, Swärd K, Nordström I, et al:
Ablation of SM22alpha decreases contractility and actin contents of
mouse vascular smooth muscle. FEBS Lett. 562:141–146. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu Y, Liu HW, Forsythe SM, et al:
Mutagenesis analysis of human SM22: characterization of actin
binding. J Appl Physiol (1985). 89:1985–1990. 2000.
|