Introduction
Chronic myelogenous leukemia (CML) is the most
common myeloproliferative disease, and the majority of cases of CML
have a (9;22) cytogenetic disorder, resulting in Breakpoint Cluster
Region/ Abelson murine leukaemia (BCR/ABL) fusion which activates
tyrosine kinase and leads to the uncontrolled proliferation of
myeloid cells (1–3). The clinical course of CML is
characterized by three phases: the chronic phase, the accelerated
phase and blast crisis phase. The blast crisis phase is accompanied
by an increasing percentage of blast cells in the peripheral blood
and bone marrow, while certain patients with CML develop
extramedullary blast crisis (EBC) caused by the extramedullary
infiltration of blast cells (4–6).
CML-EBC is rare with a poor prognosis and is may be difficult to
distinguish from the co-existence of two hematological
neoplasms.
Few cases concerning extramedullary T-lymphoblastic
blast crisis of CML have been reported in the literature, and the
outcome of EBC, even with allogeneic stem cell transplantation, is
poor (5–11). In this study, we report the case of
a patient with extramedullary T-lymphoblastic blast crisis of CML
treated with human leukocyte antigen (HLA)-mismatched stem cell
transplantation who remained in complete remission for 51 months.
The study was approved by the Ethics Committee of The Third
Military Medical University (Chongqing, China).
Case report
The patient was a 44-year-old male who presented in
March 2009 with palpitations, dyspnea and lymphadenectasis in the
neck. The patient was treated with antibiotics for one week, during
which time the symptoms were not alleviated. A subsequent
ultrasonic examination showed several low echo-level masses in the
bilateral axillary, submaxillary regions, neck and supraclavicular
fossa. The largest mass was 3.0×2.2 cm in size and the blood supply
was abundant. An enlarged spleen and liver were also detected. A
complete blood count (CBC) test obtained the following results:
hemoglobin 10.2 g/dl, leukocyte count 169.87×109
cells/l, (neutrophils, 84.93×109 cells/l; lymphocytes,
1.70×109 cells/l; monocytes, 5.07×109
cells/l; eosinophils, 0.84×109 cells/l; basophils,
2.55×109 cells/l) and platelet count 129×109
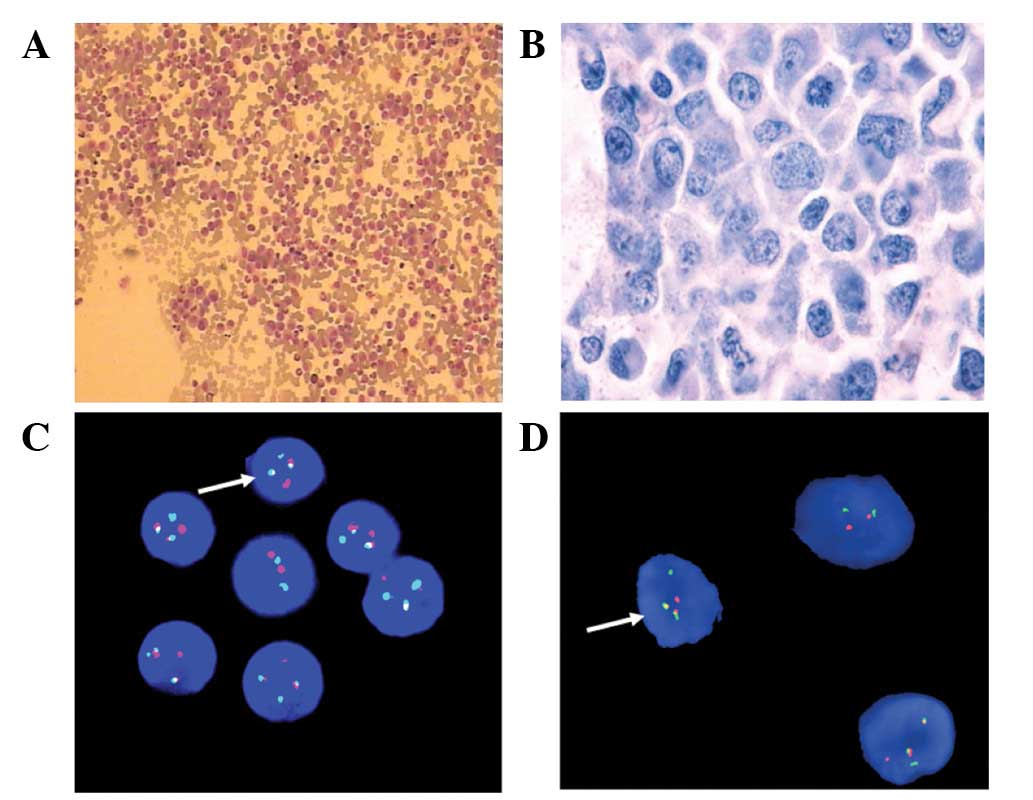
cells/l. Bone marrow analysis revealed a myeloproliferative
disorder with hyperactivity in the medullary system (Fig. 1A and B). The fluorescence in
situ hybridization (FISH) test for BCR/ABL was positive
(Fig. 1C). The patient was
diagnosed with CML in the chronic phase, and hydroxyurea was then
administered from March 5, 2009. Informed consent was obtained from
the patient prior to this study.
When the ultrasonic examination was performed, the
patient underwent a left cervical lymph node biopsy.
Immunohistochemical staining showed that the lymphoblastic cells
expressed CD7, CD3, PAX5, Bcl-2, terminal deoxynucleotidyl
transferase (TdT) and, to a lesser extent, CD20. By contrast,
staining for CD10 and CD21 was negative. The Ki-67/MIB-1 labeling
index was 70% (Fig. 2). A
preliminary diagnosis of a precursor T-lymphoblastic lymphoma
(T-LBL) was made. A week later, the FISH test for BCR/ABL was
positive in lymph node section (Fig.
1D), and the final diagnosis of EBC of CML was made.
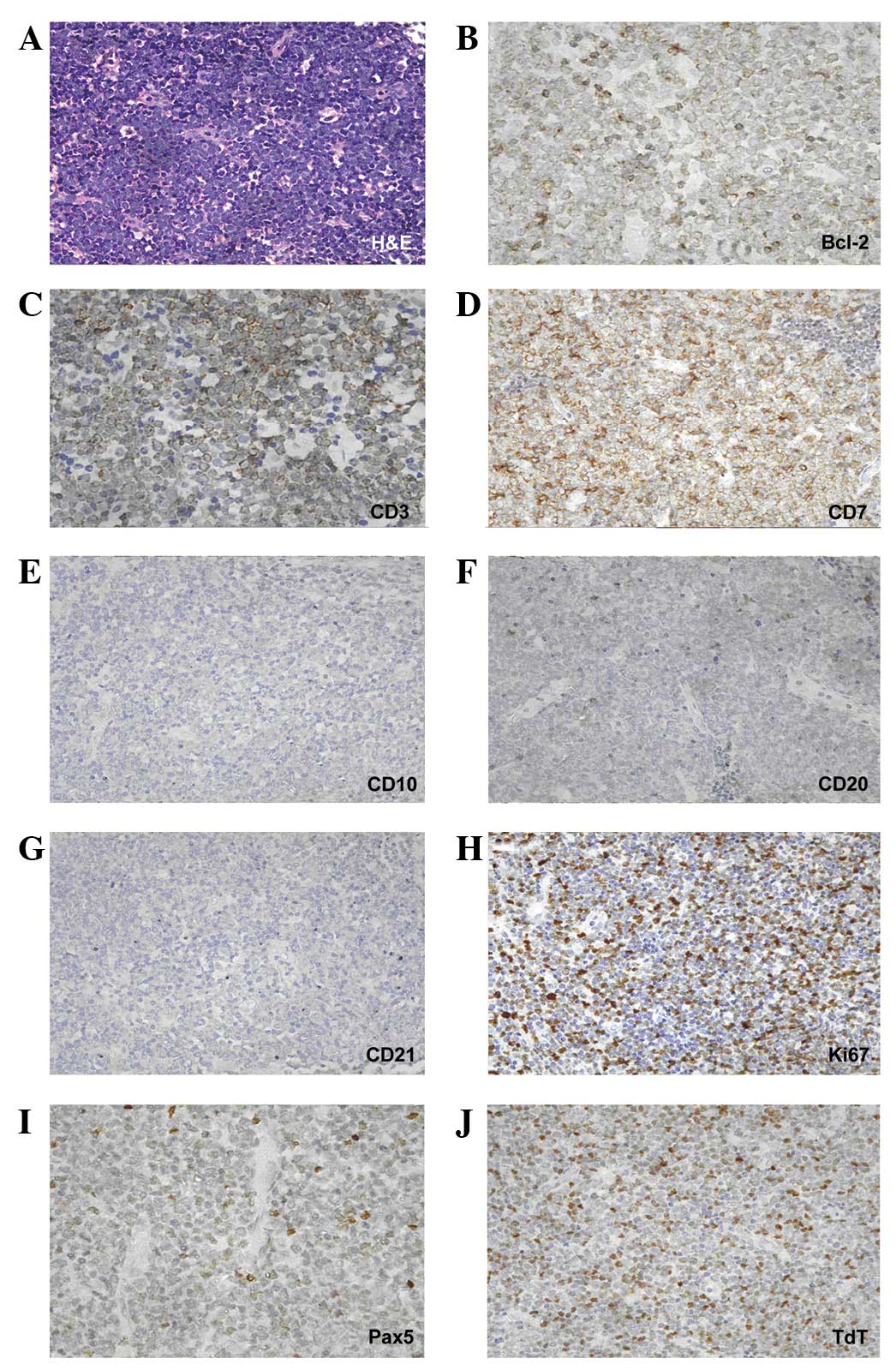 | Figure 2(A) High magnification showed that
lymph node cells were replaced by blast cells (hematoxylin and
eosin; magnification, ×400). Immunostaining in blasts was positive
for (B) Bcl-2 antigen (magnification, ×630), (C) CD3 antigen
(magnification, ×630) and (D) CD7 antigen (magnification, ×630),
(E) negative for CD10 antigen (magnification, ×400), (F) low level
positive for CD20 antigen (magnification, ×400), (G) negative for
CD21 antigen (magnification, ×400), (H) positive for Ki-67 antigen
(magnification, ×400) with a labeling index of 70%, (I) positive
for PAX5 antigen (magnification, ×630) and (J) positive for
terminal deoxynucleotidyl transferase (TdT; magnification,
×400). |
The initial chemotherapy regimen CHOP was
administered from March 21, 2009 and, after the first course of
chemotherapy, the patient’s lymphadenectasis disappeared. Two
further courses of the chemotherapy regimen followed between April
and May 2009. Hematopoietic stem cell transplantation (HSCT) from a
matched relative donor had been considered, but no donor was
identified. While the patient’s sister was revealed to be
HLA-mismatched donor, only HLA-DQ was mismatched. The allo-HSCT was
administered from May 27, 2009 with the conditioning regimen:
Bu/CY/Ara-c/CCNU (BU, busulfan; CY, cyclophosphamide; Ara-c,
aracytidine; CCNU, lomustine). On June 3, 2009 peripheral blood
stem cells were collected from the donor and infused into the
patient. The following day, bone marrow was collected from the
donor and administered to the patient by infusion. During the
transplantation period, anti-graft-versus-host disease (anti-GVHD)
medications, including CsA, MTX, mycophenolate mofetil and
anti-thymocyte globulin (ATG) were administered to the patient.
Four weeks later, hematopoietic reconstitution was definite.
Accompanied by the chemotherapy, the patient received lumbar
puncture and intrathecal chemotherapy eight times, and the
cerebrospinal fluid was continuously normal.
Two months after transplantation, the patient
received a repeated ultrasonic examination and the results showed
that lymphadenectasis had disappeared and the sizes of spleen and
liver were normal. FISH detection demonstrated changes in the
patient’s sex chromosomes from XY to XX and the test for BCR/ABL
was negative. Until now (September 2013)the patient obtained
continuous remission for 51 months and recent ultrasonic
examination, FISH detection, bone marrow smear and biopsy results
were normal.
Discussion
Blast crisis phase is the terminal stage of CML and
it constitutes a different form of CML. EBC is a special form of
blast crisis and has been observed in <10% of patients with CML
(12–14). EBC commonly occurs in bone, skin,
lymph nodes and certain other soft tissues, even though the bone
marrow of CML patients is still in the chronic stage (7,11).
The majority of Blast Crisis (BC) cells of CML are of myeloid
lineage; therefore, when the biopsy of the lymph node or other
extramedullary tissue reveals the evidence of myeloid origin and
the BCR/ABL fusion gene is positive, the diagnosis is
straightforward. When the EBC cells are of lymphoid lineage and the
lymphadenopathy is the only symptom prior to final diagnosis, it
may be misdiagnosed as lymphoma. In the current case, the biopsy of
the lymph node showed that the lymphoblastic cells expressed CD7,
CD3, PAX5, Bcl-2 and TdT, whereas the tests for CD10 and CD21 were
negative. The Ki-67/MIB-1 labeling index was 70%. Initially, we
made a misdiagnosis of precursor T-LBL accompanied by CML.
Subsequently, we obtained a positive result for BCR/ABL in the
lymph node by FISH test and the final diagnosis of EBC of CML was
made. Ichinohasama et al reported two cases of Ph-negative
non-Hodgkin’s lymphoma (NHL) occurring in CML and reviewed the
literature concerning Ph+ and Ph− lymphoma
(15); the FISH of BCR/ABL was
considered to be the essential test for blast crisis of CML while
the PCR of BCR/ABL was not. The majority of cases of so-called
‘Ph+ lymphoma’ occurred in patients without a diagnosis
of CML or acute lymphocytic leukemia (ALL) and it was difficult to
state whether the NHL carried the BCR/ABL gene translocation
without FISH.
The majority of cases of EBC occur several months or
years after the diagnosis of CML and the relevant therapy,
including hydroxycarbamide and imatinib, has been regarded as the
cause of EBC (5). Kim et al
reported two cases who received imatinib therapy after being
diagnosed with CML in the chronic phase (5). After 3–5 months, the two patients
presented with lymphadenectasis and the enlarged lymph nodes were
subsequently excised and revealed T-cell-type acute lymphatic
leukemia (T-ALL). However, in the present study the EBC was
diagnosed together with CML in chronic phase, so the exact
pathogenesis of EBC awaits further clarification.
The prognosis of EBC is poor, as the majority of
patients succumb within 6–8 months of the final diagnosis (7–10).
Hydroxycarbamide is effective for the treatment of leucocytosis and
lymphadenectasis, but is capable of changing the pathogenesis of
CML. Imatinib is able to target tyrosine kinase and decrease the
synthesis of BCR/ABL fusion gene, Naito et al reported their
experience of treating a patient with CML accompanied by EBC with
imatinib and obtained favorable results (16). Another study has shown that CML
patients in the chronic phase without EBC may eventually acquire
EBC during imatinib therapy (5).
Based on the evidence above, the effects of imatinib on EBC remain
uncertain. In the present case, hydroxycarbamide and combined
sequential chemotherapy were administered first and followed by a
successful HLA-mismatched HSCT. The patient obtained genetic
remission from CML and no lymphadenectasis was observed following
hematopoietic reconstitution. The continuous remission status was
maintained for 51 months till now (September 2013).
In conclusion, extramedullary T-lymphoblastic blast
crisis of CML as a first diagnosis is rare and may be misdiagnosed
as lymphoma without BCR/ABL detection by FISH. Despite the view
that allografts may be the cause of an extramedullary relapse or
blast crisis for CML (10),
allo-HSCT therapy may be an effective therapy for EBC of CML.
References
|
1
|
Hao Z and Cowell JK: CML: a model disease
with a defined oncogenic driver. Clin Adv Hematol Oncol. 9:247–248.
2011.PubMed/NCBI
|
|
2
|
Leitner AA, Hochhaus A and Müller MC:
Current treatment concepts of CML. Curr Cancer Drug Targets.
11:31–43. 2011. View Article : Google Scholar
|
|
3
|
Maziarz RT: Transplantation for CML: 2010.
Blood. 115:1860–1861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ross DM: Extramedullary relapse of chronic
myeloid leukemia in blast crisis: more questions than answers. Leuk
Lymphoma. 50:517–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim AS, Goldstein SC, Luger S, Van Deerlin
VM and Bagg A: Sudden extramedullary T-lymphoblastic blast crisis
in chronic myelogenous leukemia: a nonrandom event associated with
imatinib? Am J Clin Pathol. 129:639–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ganessan K, Goel R, Kumar K and Bakhshi S:
Biphenotypic extramedullary blast crisis as a presenting
manifestation of Philadelphia chromosome-positive CML in a child.
Pediatr Hematol Oncol. 24:195–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tondon R, Singh PA, Misra V, Singh M and
Mohan S: Extramedullary blast crisis in chronic myeloid leukemia -
a case report. Indian J Pathol Microbiol. 48:253–254. 2005.
|
|
8
|
Matsuda M, Morita Y, Shimada T, et al:
Extramedullary blast crisis derived from 2 different clones in the
central nervous system and neck during complete cytogenetic
remission of chronic myelogenous leukemia treated with imatinib
mesylate. Int J Hematol. 81:307–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Breccia M, Nanni M, Mancini F, Russo E,
Mecarocci S and Alimena G: Extramedullary blast crisis occurring in
a Philadelphia-positive chronic myeloid leukemia patient with major
cytogenetic response to imatinib. Haematologica.
89:ECR112004.PubMed/NCBI
|
|
10
|
Kroschinsky F, Friedrich K, Hanel M, et
al: Extramedullary blast crisis of chronic myeloid leukemia after
allogeneic hematopoietic stem cell transplantation mimicking
aggressive, translocation t(14; 18)-positive B-cell lymphoma. Ann
Hematol. 82:47–52. 2003.PubMed/NCBI
|
|
11
|
Beedassy A, Topolsky D, Styler M and
Crilley P: Extramedullary blast crisis in a patient with chronic
myelogenous leukemia in complete cytogenetic and molecular
remission on interferon-alpha therapy. Leuk Res. 24:733–735. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Specchia G, Palumbo G, Pastore D, Mininni
D, Mestice A and Liso V: Extramedullary blast crisis in chronic
myeloid leukemia. Leuk Res. 20:905–908. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kini H, Adhikari P, Shenoy UD and Kini UA:
Extramedullary blast crisis of chronic myeloid leukaemia in a lymph
node aspiration. J Indian Med Assoc. 94:3231996.PubMed/NCBI
|
|
14
|
Van Dorpe J, Van Damme S, Jacobs V, et al:
T-lymphoid extramedullary (lymphadenopathic) blast crisis in CML.
Acta Clin Belg. 50:121–125. 1995.PubMed/NCBI
|
|
15
|
Ichinohasama R, Miura I, Takahashi N, et
al: Ph-negative non-Hodgkin’s lymphoma occurring in chronic phase
of Ph-positive chronic myelogenous leukemia is defined as a
genetically different neoplasm from extramedullary localized blast
crisis: report of two cases and review of the literature. Leukemia.
14:169–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naito K, Mori T, Miyazaki K, Tsukada Y,
Ikeda Y and Okamoto S: Successful treatment of extramedullary blast
crisis of chronic myelogenous leukemia with imatinib mesylate
(STI571). Intern Med. 42:740–742. 2003. View Article : Google Scholar : PubMed/NCBI
|