Introduction
Autism and autism spectrum disorder (ASD) are
frequent and severe developmental disorders of the central nervous
system, characterized by dysfunctional social interactions and
communication skills, along with repetitive and stereotypical
verbal and nonverbal behaviors (1). The etiology of ASD remains unclear;
however, the condition most likely results from a complex
combination of genetic, environmental and immunological factors
(2,3). Although prescription drugs and
education may reduce some symptoms of autism (4), there is currently no cure available
for ASD. Stem cell transplantation via subarachnoid cavity
injection is reported to be a novel, promising treatment for
specific types of autistic children (5). Although lumbar puncture is a safe and
relatively short procedure, the patients are required to remain
motionless during the process. Autistic children with cognitive
handicaps are unable to cooperate with this request and present
unique challenges to the medical team; therefore, a rapid and
effective induction of anesthesia is indispensable for autistic
patients during the lumbar puncture procedure.
Sevoflurane, a novel type of inhalation anesthetic
with rapid induction and fine control ability, is non-pungent and
non-irritating to the respiratory tract (6). Sevoflurane can be inhaled using a
face mask, thus making it suitable for short procedures and for use
in patients with venipuncture difficulties. Alternatively, as
short-acting, intravenous anesthetics, propofol and etomidate have
been found to have a rapid onset time and short duration (7,8).
These agents are typically used for the induction of general
anesthesia and for sedation during short procedures, such as
gastroenterological endoscopy, cervix examination or tracheal
intubation (9–12). Several studies have revealed that,
when compared with propofol, etomidate did not decrease or only
slightly decreased arterial blood pressure and cardiac output
during the induction of anesthesia (9,13),
indicating a lower risk of cardiovascular depression.
In the present study, we hypothesized that etomidate
administration would achieve a greater hemodynamic stability and
less respiratory depression when used as an anesthetic in autistic
children. The purpose of this clinical, prospective, randomized,
double-blind study was to comparatively study the efficacy and
adverse effects of two anesthetics, propofol and etomidate,
administered by injection, in order to determine which, if any,
would be suitable for the induction of short-term anesthesia in
autistic children during stem cell transplantation, when used in
combination with sevoflurane inhalation.
Materials and methods
General approach and patient cohort
The study protocol was reviewed and approved by the
Ethics Committee of the Second Artillery General Hospital of PLA
(Beijing, China), and written informed consent was obtained from
the subjects, and parents of minors. A total of 60 autistic
children with American Society of Anesthesiologists physical status
I, aged 2–12 years and scheduled for stem cell transplantation via
lumbar puncture during January 2011 to October 2012, were recruited
in the study. The enrolled patients (51 males and nine females)
were randomly allocated equally to the propofol group (group P) and
the etomidate group (group E). Exclusion criteria consisted of a
history of epilepsy, necessary use of central nervous system
medications prior to or following the surgery, an allergy to the
anesthetics used in this study and evidence of corticoadrenal
insufficiency.
Propofol and etomidate are both opaque white
liquids, which allowed the study to be conducted under double-blind
conditions. The recommended dose of etomidate is between 0.15 and
0.3 mg/kg and that of propofol is between 1.5 and 2.5 mg/kg
(7,8). In the present study, the patients
received either 0.2 mg/kg intravenous etomidate or 2 mg/kg
intravenous propofol according to their group assignment.
Anesthesia procedure
Patients were fasted for 6 h and water-deprived for
4 h prior to the surgery, and no premedication was administered.
The general anesthesia procedure was as follows: First, the
patients received an inhalation induction with 8% sevoflurane
(Jiangsu Hengrui Medicine Co., Ltd., Shanghai, China) at an oxygen
flow of 3 l/min. Once the patients had fallen asleep, the face mask
was rapidly removed and standard anesthesia monitoring and
peripheral venous access were quickly established. Each child was
intravenously injected with 2.0 mg dexamethasone and 0.01 mg/kg
scopolamine; then 0.2 mg/kg etomidate (Jiangsu Nhwa Pharmaceutical
Ltd. by Share Ltd., Xuzhou, China) was intravenously administered
to group E patients and 2 mg/kg propofol (AstraZeneca, London, UK)
to group P. Supplemental doses of 0.1 mg/kg etomidate in group E
and 1 mg/kg propofol in group P were repeatedly given if sedation
was not adequate, as defined by a Ramsay sedation score (RSS)
(14) of <4. Anesthetic drugs
were administered slowly, ≥30 sec.
Stem cell transplantation
Stem cell transplantation was performed with the
patients oriented in a lateral decubitus position to maximize the
spinal interspaces for lumbar punctures. The puncture areas were
infiltrated with 1 ml 0.5% lidocaine. Routine lumbar punctures were
performed by the same physician and cerebrospinal fluids were
collected in aseptic test tubes for testing. Stem cells were then
injected into the subarachnoid space. During the procedure, the
patients were given oxygen at a flow rate of 3 l/min using a face
mask.
Observation indices
Data from clinical parameters, such as heart rate
(HR), mean arterial pressure (MAP), pulse oxygen saturation
(SpO2), respiratory rate (RR), RSS and recovery time
(time to reach an RSS of 2), were continuously monitored throughout
the procedure. Adverse effects, such as respiratory depression (RR
<8 breaths/min), hypotension (decrease in MAP >30% from the
baseline), hypertension (increase in MAP >30% from the
baseline), arrhythmia, bradycardia (HR <60 bpm) and hypoxemia
(SpO2 <95%) were recorded. Oxygen was supplied for
each patient throughout the surgery, and their physician
satisfaction was evaluated using an objective four-point scale
(very good, good, fair or poor). Discrete time-points included
study onset (T0), the administration of the propofol or etomidate
(T1), and 5 min after the administration of the propofol or
etomidate (T5min).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 13; SPSS Inc., Chicago, IL, USA). Continuous and
discrete variables are expressed as the mean ± standard deviation
and the numbers of patients, respectively. Data were analyzed using
the independent samples t-test, repeated-measures analysis of
variance or least significant difference t-test when appropriate.
Numeration data were analyzed using a χ2 test. P<0.05
and P<0.01 were considered to indicate statistically significant
and highly statistically significant differences, respectively.
Results
Physical characteristics and
cardiovascular indices
The physical characteristics of the patients were
comparable in the two groups, and no statistically significant
differences were found in age, gender, weight or physical status
(P>0.05, Table I). Following
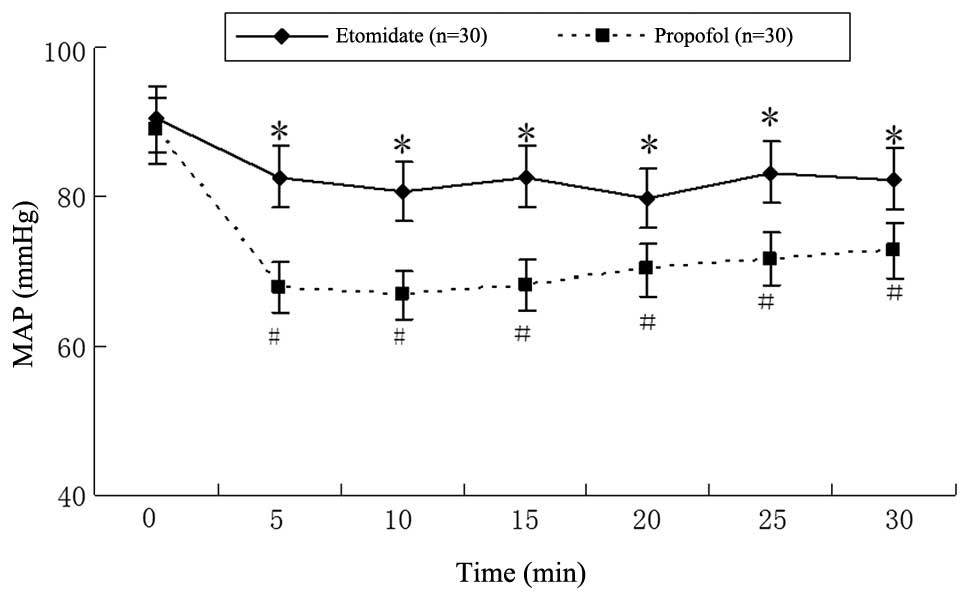
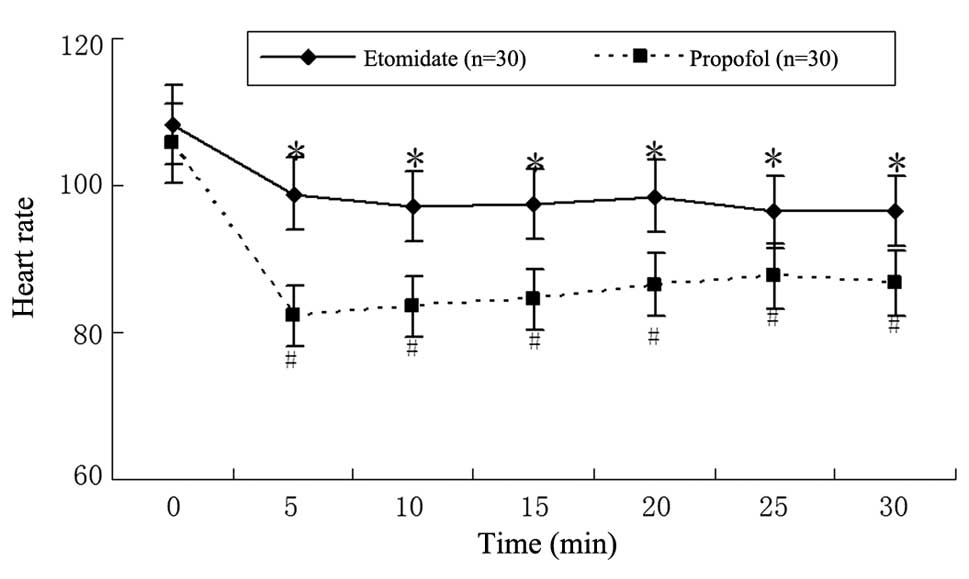
anesthesia, blood pressure and HR were decreased in groups E and P
compared with the baseline values (Figs. 1 and 2), but the levels were significantly
lower in group P than those in group E (P<0.05). The MAP and HR
of patients in group P significantly decreased during the first
5-min interval (88.9±5.7 mmHg and 105.7±3.7 bpm at T1 to 67.9±3.9
mmHg and 82.3±8.7 bpm at T5min, respectively;
P<0.01). The same tendency existed in group E patients, where
MAP and HR were respectively decreased from 90.4±4.2 mmHg and
108.3±4.8 bpm at T1 to 82.6±3.6 mmHg and 98.8±7.8 bpm at
T5min following anesthesia; however, this difference did
not reach statistical significance (P>0.05).
 | Table IGeneral comparison between group E and
group P patients. |
Table I
General comparison between group E and
group P patients.
| Group E, n=30 | Group P, n=30 |
|---|
| Demographic data |
| Age (years)a | 4.66±2.28 (2–12) | 4.49±2.88 (2–12) |
| Body weight
(kg)a | 19.58±8.97
(15–48) | 20.11±9.08
(16–45) |
| Gender
(male/female) | 25/5 | 26/4 |
| ASA | I | I |
| Physician
satisfaction scores |
| Very good | 20 | 22 |
| Good | 9 | 8 |
| Fair | 1 | 0 |
| Poor | 0 | 0 |
| Clinical data |
| Etomidate total dose
(mg/kg)b | 0.34±0.11 | 0 |
| Propofol total dose
(mg/kg)b | 0 | 2.20±0.48 |
| Anesthesia duration
(min)b,c | 1.50±0.35 | 1.60±0.28 |
| Surgery duration
(min)b | 9.66±2.56 | 9.42±2.54 |
| Recovery time
(min)b | 6.80±2.38 | 7.20±2.05 |
During the procedure a decrease in SpO2
was observed within each group. The SpO2 in group E was
reduced from 98.7±0.7% at T1 to 96.8±0.8% at T5min
(P<0.05), while propofol produced a larger decrease in
SpO2 from 98.6±0.8% at T1 to 95.8±0.9% at
T5min (P<0.05). Due to the oxygen supply during the
entire procedure, however, no significant difference in
SpO2 was observed between the two groups.
Adverse effects
The adverse effects caused by anesthesia are
summarized in Table II. The
results showed that the episodes of respiratory depression,
bradycardia, hypotension and pain on injection of propofol were
significantly higher than the number of adverse episodes identified
in the etomidate-treated group (P<0.05). By contrast, myoclonus
was estimated to occur in 26.7% of the children anesthetized by
etomidate, while no equivalent symptom was identified in group P
patients (P<0.01). For the analysis of physician satisfaction,
no significant difference was identified between the two groups
(Table I).
 | Table IIAdverse effects of propofol or
etomidate injection following sevoflurane inhalation. |
Table II
Adverse effects of propofol or
etomidate injection following sevoflurane inhalation.
| Group E, n=30 | Group P, n=30 | P-value |
|---|
| Respiratory
depression | 5 | 15 | 0.006 |
| Nausea and
vomiting | 0 | 0 | |
| Bradycardia | 0 | 5 | 0.019 |
| Hypotension | 0 | 12 | 0.0001 |
| Myoclonus | 8 | 0 | 0.002 |
| Pain at injection
site | 0 | 5 | 0.019 |
Surgery parameters
No statistically significant differences were found
between groups E and P with respect to the length of anesthesia
induction (the time from the start of sevoflurane inhalation to the
onset of sleep), surgery duration and recovery time (P>0.05,
Table I). Similarly, no
significant difference was observed with regard to the RSSs between
the two groups, indicating that propofol and etomidate may achieve
similar levels of sedation within 20 min of the injection of the
anesthetics (Table III).
 | Table IIIComparison of Ramsay sedation scores
between patients in groups E and P. |
Table III
Comparison of Ramsay sedation scores
between patients in groups E and P.
| Time (min) | Group E, n=30 | Group P, n=30 |
|---|
| 0 | 1.00±0.00 | 1.00±0.00 |
| 2 | 5.03±0.88 | 5.11±0.38 |
| 4 | 5.53±0.68 | 5.67±0.44 |
| 6 | 5.36±0.67 | 5.48±0.42 |
| 8 | 5.33±0.75 | 5.42±0.33 |
| 10 | 5.07±0.86 | 5.12±0.77 |
| 12 | 3.50±1.14 | 3.60±1.25 |
| 14 | 2.53±0.73 | 2.55±1.01 |
| 16 | 1.83±0.59 | 1.82±0.89 |
| 18 | 1.43±0.63 | 1.49±0.65 |
| 20 | 1.27±0.45 | 1.25±0.66 |
Discussion
Autism is a complex developmental disability that is
typically observed during childhood and that may result from a
neurological disorder that affects the functioning of the human
brain. Children diagnosed with ASD are often characterized by
significant deficits in reciprocal social interaction, verbal and
nonverbal communication skills and imaginative activity. As a
result of their highly unusual clinical presentation, autistic
children present a unique perioperative challenge to the pediatric
surgical team (15); therefore,
stem cell transplantation for children with autism must be
performed under general anesthesia.
The main goals of anesthesia are to establish a
rapid recovery, with reduced postoperative pain, few complications,
and minimal respiration and circulatory system changes during the
perioperative period. As short-acting intravenous anesthetics,
propofol and etomidate have been used for the induction and
maintenance of anesthesia in a rapid and safe manner (16–18).
Thus, in the present study, the efficacy and adverse effects of
these two anesthetics were comparatively studied in 60 autistic
children during stem cell transplantation, to find which anesthetic
would be suitable for induction of short-term anesthesia in
autistic children.
The results presented showed that, unlike propofol,
which markedly suppressed the circulatory system, etomidate
administration only slightly reduced the blood pressure and HR of
the pediatric patients. This was unsurprising since etomidate has
been reported to neither selectively decrease sympathetic nervous
system activity nor inhibit cardiovascular function (7,8).
Previous studies have also suggested that etomidate has minimal
effects on healthy patients or those with cardiac diseases, making
it more suitable for patients with hypotension, hypovolemia or
cardiovascular disorders (8,19).
By contrast, propofol has been reported to clearly inhibit
sympathetic nerve activity and baroreceptor reflexes (20), thereby making it more likely to be
associated with bradycardia and hypotension (19). In the current study, bradycardia
occurred in five children in group P during their recovery, while
no bradycardia occurred in group E children, which corroborated the
results of previous studies.
Another serious problem associated with the use of
propofol is reputed to be the high incidence of pain at the
injection site (25–50%) (8) In the
present study, a total of five children receiving a propofol
infusion complained about pain on injection, while no one developed
this complication following etomidate administration. In addition,
etomidate has previously resulted in less apnea or respiratory
depression when compared with propofol (21). Due to the continuous oxygen supply,
however, no significant difference in SpO2 was detected
between the two groups; therefore, the same conclusion could not be
drawn from the present study.
The most prominent adverse effect reported with
etomidate was myoclonus, which has been suggested to be
dose-dependent and to occur in 20–45% of patients (22). The findings of the current study
showed a 26.7% incidence of myoclonus in group E children while
under anesthesia; the practice of slow etomidate administration may
explain this lower incidence found.
A major concern associated with etomidate infusion
is the possibility of adrenal suppression (23,24).
Etomidate has been previously found to inhibit 11-β-hydroxylase, an
enzyme that promotes the conversion of 11-deoxycortisol to cortisol
(25), and long-term, high-dosage
use of etomidate may cause adrenal suppression. While it was
conceivable that the short-term use of etomidate was not likely to
lead to adrenocortical suppression (8), in the present study, although
adrenocortical function was not directly measured, certain outward
signs of adrenal suppression, including hypotension and arrhythmia,
were monitored. The results showed that there was not any outward
sign of adrenal suppression identified following etomidate
infusion.
In addition to myoclonus and adrenal suppression,
postoperative nausea and vomiting are frequent side effects of
etomidate. A previous study reported a post-operative nausea and
vomiting incidence of 25–30% following etomidate induction, higher
than that when propofol was used (9); however, in the current study, no
children developed nausea or vomiting following etomidate
administration. This was perhaps due to dexamethasone infusion
prior to the procedure, which may have inhibited the development of
nausea and vomiting (26).
No statistically significant difference was found
between group E and P patients with respect to the length of
anesthesia induction, surgery duration and recovery time
(P>0.05). The RSS, a tool for measuring the quality of sedation
in patients, was also evaluated in this study, with no significant
difference observed between the two groups. This indicated that
propofol and etomidate may achieve similar levels of sedation
within 20 min after intravenous injection.
In conclusion, the results of the present study
suggested that a sevoflurane-etomidate combination achieved a more
stable hemodynamic response and resulted in fewer adverse effects
compared with a sevoflurane-propofol combination. The
sevoflurane-etomidate combination would therefore be more suitable
for the induction of short-term anesthesia in autistic children
during stem cell transplantation.
Acknowledgements
This study was supported by The Capital Health
Research and Development of Special Funding (2011-5011-01).
References
|
1
|
Mefford HC, Batshaw ML and Hoffman EP:
Genomics, intellectual disability, and autism. N Engl J Med.
366:733–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dantzer R and Kelley KW: Autistic
children: a neuroimmune perspective. Brain Behav Immun. 22:804–805.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hughes JR: Update on autism: a review of
1300 reports published in 2008. Epilepsy Behav. 16:569–589. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dove D, Warren Z, McPheeters ML, Taylor
JL, Sathe NA and Veenstra-VanderWeele J: Medications for
adolescents and young adults with autism spectrum disorders: a
systematic review. Pediatrics. 130:717–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ichim TE, Solano F, Glenn E, et al: Stem
cell therapy for autism. J Transl Med. 5:302007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nathan N and Odin I: Induction of
anaesthesia: a guide to drug choice. Drugs. 67:701–723. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forman SA: Clinical and molecular
pharmacology of etomidate. Anesthesiology. 114:695–707. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pandit JJ: Intravenous anaesthetic agents.
Anaesth Intens Care Med. 12:144–150. 2011. View Article : Google Scholar
|
|
9
|
Toklu S, Iyilikci L, Gonen C, et al:
Comparison of etomidate-remifentanil and propofol-remifentanil
sedation in patients scheduled for colonoscopy. Eur J Anaesthesiol.
26:370–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vinson DR and Bradbury DR: Etomidate for
procedural sedation in emergency medicine. Ann Emerg Med.
39:592–598. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu ZY, Wang X, Si YY, et al: Intravenous
remifentanil and propofol for gastroscopy. J Clin Anesth.
20:352–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryu JH, Kim JH, Park KS and Do SH:
Remifentanil-propofol versus fentanyl-propofol for monitored
anesthesia care during hysteroscopy. J Clin Anesth. 20:328–332.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sams L, Braun C, Allman D and Hofmeister
E: A comparison of the effects of propofol and etomidate on the
induction of anesthesia and on cardiopulmonary parameters in dogs.
Vet Anaesth Analg. 35:488–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adesanya AO, Rosero E, Wyrick C, Wall MH
and Joshi GP: Assessing the predictive value of the bispectral
index vs patient state index on clinical assessment of sedation in
postoperative cardiac surgery patients. J Crit Care. 24:322–328.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seid M, Sherman M and Seid AB:
Perioperative psychosocial interventions for autistic children
undergoing ENT surgery. Int J Pediatr Otorhinolaryngol. 40:107–113.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ho WM, Yen CM, Lan CH, et al: Comparison
between the recovery time of alfentanil and fentanyl in balanced
propofol sedation for gastrointestinal and colonoscopy: a
prospective, randomized study. BMC Gastroenterol. 12:1642012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miner JR, Danahy M, Moch A and Biros M:
Randomized clinical trial of etomidate versus propofol for
procedural sedation in the emergency department. Ann Emerg Med.
49:15–22. 2007. View Article : Google Scholar
|
|
18
|
Sokolove PE, Price DD and Okada P: The
safety of etomidate for emergency rapid sequence intubation of
pediatric patients. Pediatr Emerg Care. 16:18–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bendel S, Ruokonen E, Pölönen P and Uusaro
A: Propofol causes more hypotension than etomidate in patients with
severe aortic stenosis: a double-blind, randomized study comparing
propofol and etomidate. Acta Anaesthesiol Scand. 51:284–289. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sellgren J, Ejnell H, Elam M, Pontén J and
Wallin BG: Sympathetic muscle nerve activity, peripheral blood
flows, and baroreceptor reflexes in humans during propofol
anesthesia and surgery. Anesthesiology. 80:534–544. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schaeuble J, Heidegger T, Gerig HJ, Ulrich
B and Schnider TW: Comparison of etomidate and propofol for
fibreoptic intubation as part of an airway management algorithm: a
prospective, randomized, double-blind study. Eur J Anaesthesiol.
22:762–767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Falk J and Zed PJ: Etomidate for
procedural sedation in the emergency department. Ann Pharmacother.
38:1272–1277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hildreth AN, Mejia VA, Maxwell RA, Smith
PW, Dart BW and Barker DE: Adrenal suppression following a single
dose of etomidate for rapid sequence induction: a prospective
randomized study. J Trauma. 65:573–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Albert SG, Ariyan S and Rather A: The
effect of etomidate on adrenal function in critical illness: a
systematic review. Intensive Care Med. 37:901–910. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dörr H, Kuhnle U, Holthausen H,
Bidlingmaier F and Knorr D: Etomidate: a selective adrenocortical
11 beta-hydroxylase inhibitor. Klin Wochenschr. 62:1011–1013. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Henzi I, Walder B and Tramér MR:
Dexamethasone for the prevention of postoperative nausea and
vomiting: a quantitative systematic review. Anesth Analg.
90:186–194. 2000. View Article : Google Scholar : PubMed/NCBI
|