Introduction
Implantation is a critical step during normal
pregnancy. The implantation process occurs after 4–6 days of
pregnancy in rats (plug presented day = gestation day 1) (1), and a similar phenomenon also occurs
in humans between days luteinizing hormone (LH)+6 and LH+8
(2). Successful implantation
requires a receptive endometrium, a normal embryo at the blastocyst
developmental stage and a synchronized dialogue between maternal
and embryonic tissues (3).
A prerequisite for implantation is increased
endometrial angiogenesis and vascular remodeling at the
implantation site. Leukemia-inhibitory factor (LIF) is a
well-characterized cellular factor that is a promising candidate as
an endometrial receptivity biomarker in mice (4) and humans (5,6). LIF
knock-out female mice are infertile, and implantation of the embryo
does not occur (7). LIF has a role
in the adhesive and invasive phases of implantation (8). Angiopoietin-2 (Ang-2) destabilizes
the quiescent endothelium and primes it to respond to exogenous
stimuli, thereby modulating angiogenic cytokine activity (9,10).
Ang-2 is expressed in the peri-implantation endometrium in a
spatiotemporal manner and participates in angiogenesis and the
vascular remodeling process (11).
Although LIF and Ang-2 are essential for normal blastocyst
implantation, it is not yet clear whether there is an association
between LIF and Ang-2.
The controlled ovarian hyperstimulation (COH) with
gonadotropin-releasing hormone agonist (GnRHa) long protocol is an
important approach in IVF. COH results in high-quality embryos;
however, even with ongoing advances, implantation rates are still
relatively low (12). A number of
studies have demonstrated that COH may directly change endometrial
characteristics compared with those of the natural cycle (13–15),
and these differences may alter endometrial receptivity (16). Furthermore, high serum estradiol
levels or other hormonal alterations that result from COH may
indirectly adversely affect implantation (17,18).
Although the current understanding of implantation
has increased, therapeutic options remain limited (19). Further study is required to
investigate clinical treatment options for infertility patients
with implantation failure. In China, traditional Chinese medicine
(TCM) harmonizes the endocrine environment to assist with assisted
reproductive technology (20–22),
and integrating the principles and knowledge from TCM may be useful
in clinical practice to provide better strategies for treating
infertility (23). Therefore, in
the present study, Bu Shen Huo Xue Decoction (BSHXD) was
established to aid in preparing the endometrium for implantation
(Table I).
 | Table IBu Shen Huo Xue Decoction (BSHXD)
composition. |
Table I
Bu Shen Huo Xue Decoction (BSHXD)
composition.
| Component | Ratio |
|---|
| (1) | Sheng Di
[Rehmannia glutinosa (Gaertn.) Libosch., root] | 15 |
| (2) | Dan Shen (Salviae
miltiorrhizae Bge., root) | 10 |
| (3) | Dang Gui
[Angelica sinensis (Oliv.) Diels., root] | 12 |
| (4) | Chuan Duan
(Dipsacus asperoides C. Y. Cheng et T .M. Ai., root) | 15 |
| (5) | Du Zhong
(Eucommia ulmoides Oliv., cortex) | 12 |
| (6) | Shan Yao
(Dioscorea opposita Thunb., rhizome) | 15 |
| (7) | Mei Gui-hua
(Rosa rugosa Thunb., flower) | 6 |
| (8) | Chuan Xiong
(Ligusticum chuanxiong Hort., rhizome) | 6 |
| (9) | Yi Yi-ren [Coix
lacryma-jobi L. var. ma-yuen (Roman.) Stapf., seed] | 12 |
In the present study, endometrial LIF and Ang-2
protein and mRNA expression was investigated, and the number of
implantation sites and live births in a COH rat model were
determined to elucidate the side-effects of COH on fertility. It
was hypothesized that BSHXD can ameliorate these side-effects and
improve endometrial receptivity and pregnancy outcome. The results
may therefore provide evidence to support the use of BSHXD in
assisted reproduction.
Materials and methods
Ethics statement
All the experimental protocols were approved by the
Ethics Committee of the Beijing University of Chinese Medicine
Animal Care and Use Committee (no. 2012-087-R; Beijing, China).
BSHXD preparation
The drugs present in BSHXD were obtained from the
Pharmacy Department of Dongfang Hospital of Beijing University of
Chinese Medicine (Beijing, China). The quality of the raw herbs was
controlled according to the requirements of the Pharmacopoeia of
the People’s Republic of China. An aqueous extract of BSHXD was
prepared in accordance with the following procedure. In brief, the
components (as shown in Table I)
were mixed in proportion and were macerated for 1 h with 8 volumes
of distilled water and then decocted for 2 h. The cooled extract
was filtered. The extraction procedure was repeated twice. The
extracts were then combined and concentrated by boiling to a final
volume of 100 ml (4.12 g/ml). This dilution was used in the
following preliminary experiments in a range of concentrations
(between 1.03 and 4.12 g/ml).
Treatment
Female Sprague Dawley rat virgins aged 7–8 weeks old
(weighing 210–220 g) were maintained in the laboratory on a 12 h
light, 12 h dark regimen with free access to water and a standard
diet. The estrous stage was identified by vaginal smear. Only
female rats with regular cycles were used. The rats were randomly
allocated into four groups: control, COH, BSHXD and COH+BSHXD
groups (n=30 in each group). A total of 18 rats from each group
were used for the western blot and quantitative polymerase chain
reaction (qPCR) analyses, 6 rats were used to assess the
implantation site number and 6 rats were used to assess pregnancy
outcomes.
Rats in the COH group were administered 1 ml/100 g
body weight/day distilled water for 12 days and treated with the
GnRHa long protocol. In brief, a GnRH agonist (Diphereline; Ipsen
Pharma Biotech, Signes, France) was injected intraperitoneally at
1.5 μg/100 g body weight/day between the third and ninth day of
estrous. Pregnant mare’s serum gonadotropin (Chifeng Bo’En
Pharmaceutical Co. Ltd., Chifeng, China) was injected
intraperitoneally at 5 IU/100 g body weight between the third and
ninth day of estrous followed by 10 IU/100 g human chorionic
gonadotropin (hCG; Yantai North China Pharmaceutical Co., Ltd.,
Yantai, China) after 28 h. In the BSHXD group, the animals were
administered 1 ml BSHXD/100 g body weight/day for 12 days followed
by saline injections at the same time and volume as the COH group.
Animals in the COH+BSHXD group were administered 1 ml BSHXD/100 g
body weight/day for 12 days and were then subjected to the same
GnRHa long protocol as the COH group. In the control group, the
rats were administered 1 ml distilled water/100 g body weight/day
for 12 days, followed by saline injections at the same time and
volume as the COH group. The female rats were housed overnight with
males (1:1) following hCG or saline administration. Successful
mating was assessed daily by the presence of a vaginal plug. The
day that the plug was first observed was designated as day 0 of
gestation (D0).
On each of D3, D4 and D5, 6 rats were sacrificed
from each group. The uteri were removed without excess fat and
connective tissue and the whole sample was stored at −80°C until
protein and mRNA extraction.
Western blot analysis
The uterus was sectioned, and slices were incubated
and lysed in RIPA lysis buffer (C1053; Applygen Technologies,
Beijing, China) supplemented with protease inhibitor (P1265;
Applygen Technologies). The protein concentration was quantified
with bicinchoninic acid (P1511; Applygen Technologies). Sodium
dodecyl sulfate-polyacrylamide gel electrophoresis was performed
using a 10% polyacrylamide gel, and the samples were transferred to
nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Membranes
were blotted with anti-LIF (sc-1336; Santa Cruz Biotechnology,
Heidelberg, Germany) or anti-Ang-2 (AP23297PU-N; Acris Antibodies,
Herford, Germany) primary antibodies at a 1:1,000 dilution and
incubated overnight at 4°C. Following incubation, the membranes
were washed 3 times with Tris-buffered saline and Tween 20 buffer
and then incubated with the secondary antibodies (P1308, P1309;
Applygen Technologies) at a 1:10,000 dilution at room temperature
for 1 h. The blots were visualized using the Super ECL Plus
detection reagent (P1010; Applygen Technologies). The enhanced
chemiluminescence signals were detected using Quantity One software
(Bio-Rad). GAPDH (blotted with ab8245; Abcam, Cambridge, UK) was
used as an internal control to validate the quantity of protein
loaded onto the gel.
qPCR analysis
LIF and Ang-2 gene expression was measured using
qPCR. Total RNA was extracted from the uteri of the control, COH,
BSHXD and COH+BSHXD rats using TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA), in accordance with the
manufacturer’s instructions. The RNA was thawed on ice and
quantified spectrophotometrically; the quality was assessed using
sodium dodecyl sulfate-agarose gel electrophoresis. Reverse
transcription was performed with 8 μl total RNA per 20 μl reaction
using a standard cDNA synthesis kit (Takara Bio, Otsu, Japan).
Target gene primer sequences are listed in Table II.
 | Table IIQuantitative polymerase chain
reaction primer sequences. |
Table II
Quantitative polymerase chain
reaction primer sequences.
| Gene | Primer sequence
5′→3′ | Length | Amplicon |
|---|
| LIF | F:
CCCTTCCCATCACCCCTGTA | 20 | 102 bp |
| R:
TGCCGTTGAGTTGAGCCAGT | 20 | |
| Ang-2 | F:
CGGACTCTGTCACAAGCAAGAA | 22 | 237 bp |
| R:
AGCACAAGACGGAACAACGAA | 21 | |
| GAPDH | F:
TGCTGAGTATGTCGTGGAG | 19 | 288 bp |
| R:
GTCTTCTGAGTGGCAGTGAT | 20 | |
For each qPCR assay, the thermal cycling conditions
included an initial activation step at 95°C for 5 min, 40
amplification cycles and a final melting curve (65–95°C). PCR
reactions were performed on an ABI Prism 7700 Sequence Detection
System (Applied Biosystems, Foster City, CA, USA). Target mRNA
levels were normalized against those of GAPDH. Target mRNA
expression was analyzed using the 2−ΔΔCt algorithm.
Implantation sites and live births
On D10, 6 rats from each group were sacrificed. The
uteri were removed without excess fat and connective tissue, and
the conceptuses were removed from the uteri. The number of
implantation sites in the uterine horn was recorded. The average
number of implantation sites was calculated as the total number of
implantation sites/number of rats. Following conception and birth,
the number of newborn rats from each group was recorded. The
average number of live births was calculated as the total number of
newborn rats/number of rats.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. One-way analysis of variance and least significant
difference tests were used. P<0.05 was considered to indicate a
statistically significant difference. Graphs of the data were
produced using Microsoft Excel software.
Results
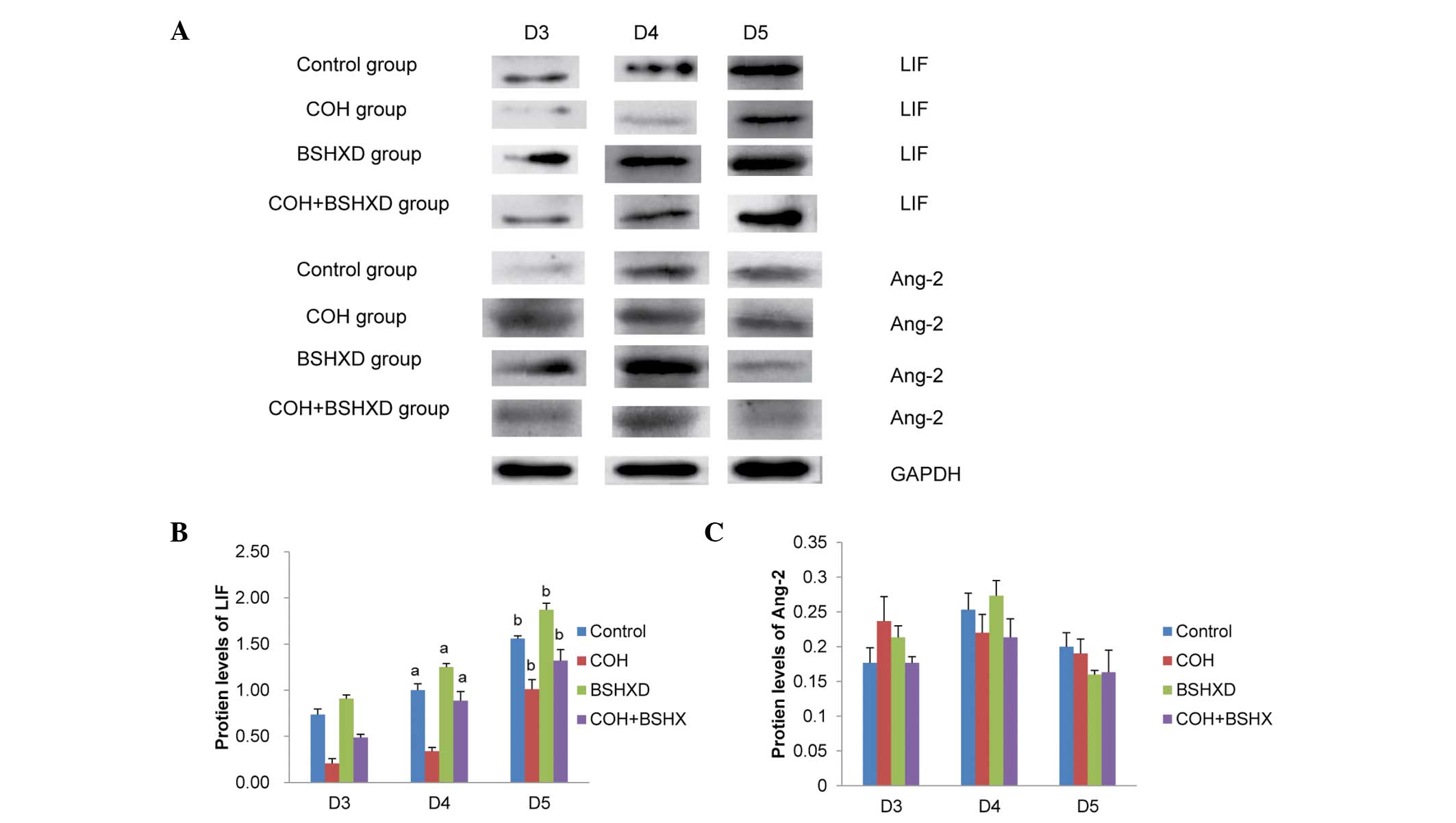
Western blot analysis
Endometrial LIF and Ang-2 protein expression levels
during implantation were determined using western blot analysis.
The LIF and Ang-2 protein expression levels were normalized against
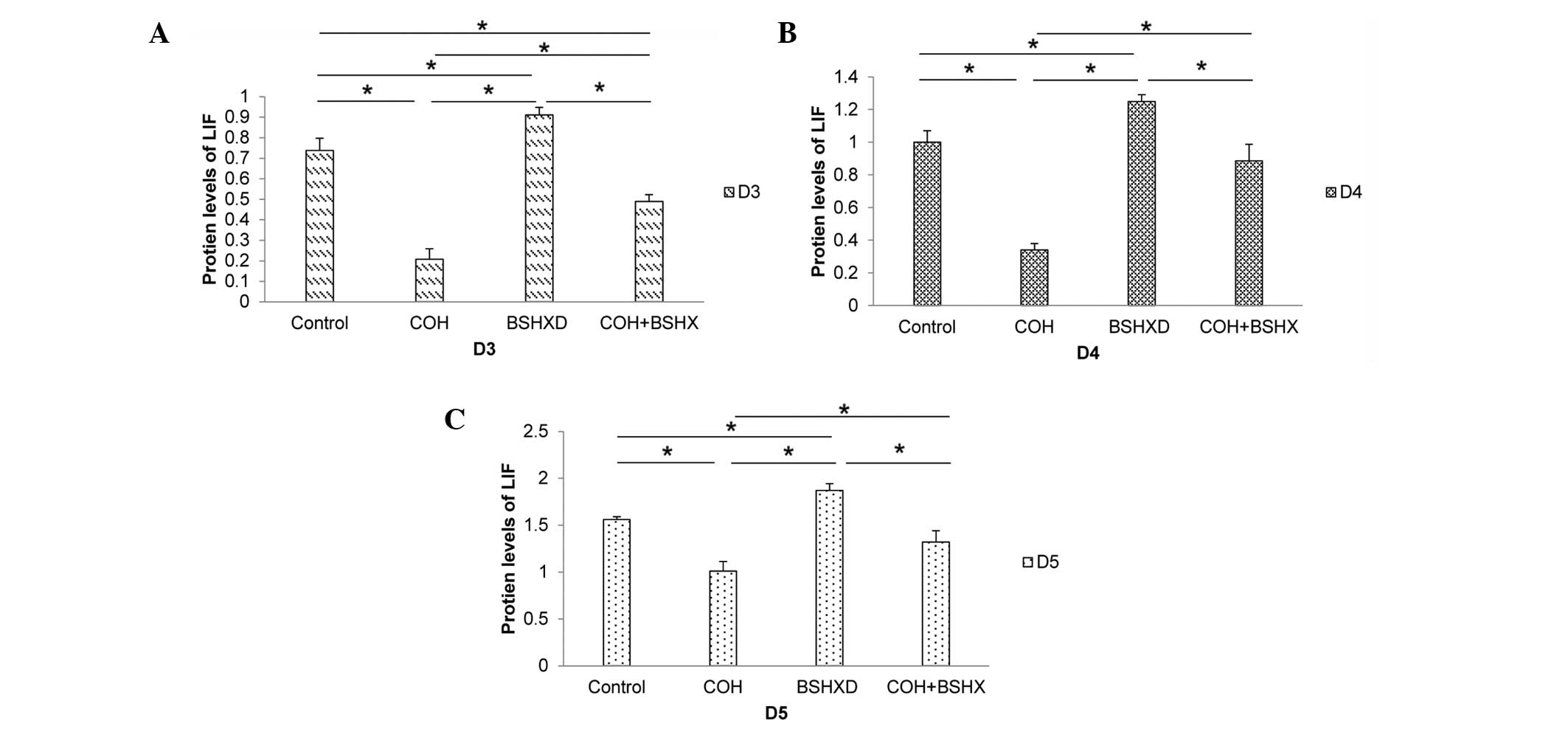
those of GAPDH (Fig. 1). The LIF
protein expression levels were found to be increased during the
implantation period in the four groups (Fig. 2). However, COH treatment
significantly reduced the level of LIF protein expression in the
COH group compared with that in the other groups. No significant
difference was identified between the LIF protein levels in the
control and the COH+BSHXD groups on D4 and D5. Compared with the
control and the COH+BSHXD treatment, BSHXD treatment significantly
increased the level of LIF protein expression in the BSHXD group.
However, no significant differences in the level of Ang-2 protein
expression were observed from D3 to D5.
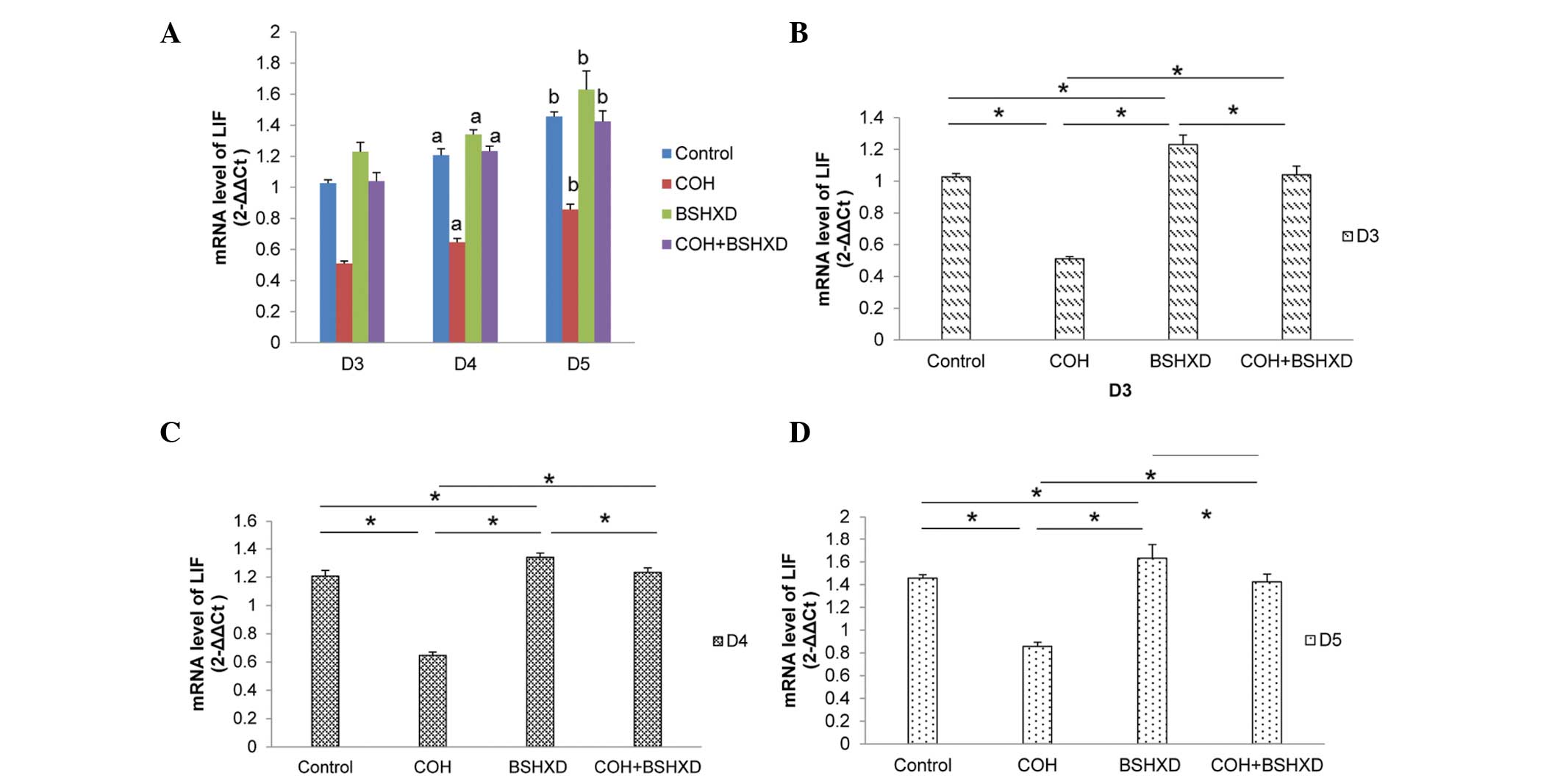
qPCR
To verify the changes in expression levels, LIF and
Ang-2 transcript levels were measured using qPCR. The differences
in the qPCR results were statistically significant, and substantial
differences in expression were observed using western blot
analysis. LIF and Ang-2 mRNA were expressed in the rat endometrium
during implantation. LIF mRNA expression levels were increased from
D3 to D5 (Fig. 3). COH treatment
significantly reduced the LIF mRNA expression levels compared with
those in the control, BSHXD and COH+BSHXD groups, and the BSHXD
group had significantly increased LIF mRNA expression levels
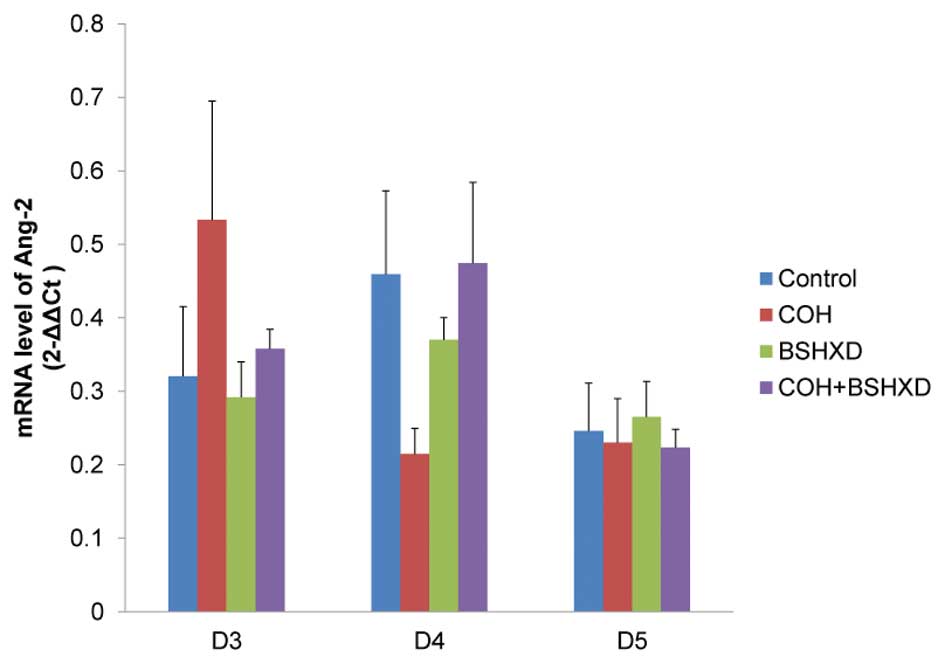
compared with the control and COH+BSHXD groups. However, no
significant differences were identified in Ang-2 mRNA expression
levels from D3 to D5 (Fig. 4).
Number of implantation sites and live
births
The effects of COH and BSHXD on the number of
implantation sites and live births are summarized in Table III. The number of implantation
sites and live births in the COH group was significantly lower
compared with those in the other groups. No significant difference
was identified among the control, BSHXD and COH+BSHXD groups;
however, the implantation site and live-birth number was highest in
the BSHXD group.
 | Table IIINumber of implantation sites and live
births in each group. |
Table III
Number of implantation sites and live
births in each group.
| Variables | Control | COH | BSHXD | COH+BSHXD |
|---|
| Implantation
sites | 8.67±1.93 | 3.60±0.51a | 9.14±1.18 | 7.83±0.48 |
| Live births | 9.00±1.90 | 3.17±0.40a | 9.50±0.56 | 7.33±0.49 |
Discussion
The aim of the present study was to investigated the
effect of BSHXD in a COH rat model during the implantation window
using western blot and qPCR analyses and by measuring the average
number of implantation sites and live births.
In numerous mammalian species, including mice,
humans and sheep, uterine LIF expression is upregulated during the
onset of embryo implantation (24), suggesting that LIF may be of
general significance to implantation in mammals. Previous studies
on early murine pregnancy have demonstrated that LIF expression
levels are highest in the uterus between the fourth and fifth day
of pregnancy (25). These data are
consistent with the results from the present study that
demonstrated that LIF expression increased during implantation in
the four groups and peaked on D5. A role for LIF in implantation
regulation and embryonic development was proposed. The observation
that recombinant LIF inhibits murine embryonic stem cell
differentiation (26,27) indicates that LIF may have a role in
early embryonic growth and development. LIF mRNA is expressed in
murine embryos from the fertilized egg to the blastocyst stage
(28,29). These data likely explain why lower
LIF protein and mRNA expression in the COH group was associated
with lower implantation site numbers. Furthermore, LIF may also be
important during placentation and subsequent fetal development. A
potential role for LIF in placentation was first suggested when the
LIF receptor (LIFR) was cloned from a human placental cDNA library
(30), which was further
strengthened by a study demonstrating that normal placentation was
disrupted in LIFR−/− mouse embryos (31). A possible explanation for the lower
numbers of live births in the COH group in the present study is
that the establishment of decidualization and placentation requires
LIF; however, COH treatment significantly disturbed LIF expression.
This indicates that LIF may have an important role in pregnancy
establishment.
The other major finding of the present study was
that there were no significant differences in Ang-2 expression
among the four groups. Angiopoietins are a family of growth factors
that promote vessel maturation and remodeling (32,33),
which are important processes during implantation (34–36).
Ang-2 causes loosening of cell-matrix and cell-to-cell contacts,
which allows access to angiogenic inducers (37). Therefore, Ang-2 expression may
promote angiogenesis (38).
Furthermore, Ang-2 is selectively expressed in the ovary, uterus
and placenta of mice and humans (32). Thus, it was hypothesized in the
present study that Ang-2 expression would demonstrate a similar
increase to LIF during implantation. However, this association was
not observed. The lack of a difference in endometrial Ang-2
expression from D3 to D5 is that angiogenic mechanisms during
implantation may not require Ang-2. In a previous study, Ang-2
expression levels were determined using in situ
hybridization and it was demonstrated that Ang-2 was expressed at
low levels between the first and fifth days of pregnancy
(peri-implantation) and was then expressed at higher levels from
the sixth to seventh day in mice (11). These results clearly demonstrate
that Ang-2 may not be a dominant angiogenic factor during the early
stages of pregnancy. The results of the present study support the
theory that maternal regulation of endometrial angiogenesis prior
to implantation is different from the regulation that occurs once
the embryo has implanted (39).
It has been hypothesized that using COH with GnRHa
in a long protocol to induce multifollicular development may also
affect endometrial receptivity (40,41).
The endometrium undergoes a morphological advancement prior to
implantation, and it is not surprising that a side-effect of COH is
the regulation of supraphysiological levels of steroid hormones and
paracrine mediators produced and received by the endometrium.
Genomic analyses of human endometrial receptivity have been
previously performed (42,43), and these analyses demonstrate that
numerous genes are aberrantly expressed in the COH endometrium, and
the expression levels are similar to those in a non-receptive
endometrium (44). These previous
studies highlight the necessity for modifying COH treatment to
achieve an endometrium that resembles that of the natural
endometrium cycle morphologically and functionally, which is likely
to improve pregnancy outcomes. Therefore, BSHXD was used along with
COH treatment to promote the recovery of the impaired endometrium
in rats. In a previous study, the authors of the present study
demonstrated the effects of BSHXD on endometrial morphology and LIF
expression in non-pregnant rats (45). The results from the present study,
including increased LIF expression, and restored implantation site
and live-birth numbers in the COH+BSHXD group, suggest that BSHXD
treatment may be a clinical option for patients with an impaired
endometrium following COH treatment. TCM uses a holistic and
synergistic approach to restore homeostasis (46); however, TCM does not translate the
normal state into a super-normal one, which accounts for a lack of
significant differences between the control and BSHXD groups.
In conclusion, it was demonstrated that: i) COH
treatment significantly decreased LIF protein and mRNA expression
levels and the number of implantation sites and live births in rats
compared with those in the natural cycle; ii) integrating BSHXD and
COH treatment restores LIF expression and significantly increases
the probability of implantation and the number of live births; iii)
no significant differences in LIF expression or the average number
of implantation sites and live births were observed among the
control, BSHXD and COH+BSHXD groups; iv) LIF and Ang-2 were
expressed in mature female rat endometrium during the implantation
window; however, no association was found between LIF and Ang-2
expression. The results from the present study provide novel
insights into a TCM approach for infertility treatment and assisted
reproductive technology. However, further clinical studies are
required to confirm the proposed approach.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (no. 81173292).
References
|
1
|
Psychoyos A: Hormonal control of
ovoimplantation. Vitam Horm. 31:201–256. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nikas G: Endometrial receptivity: changes
in cell-surface morphology. Semin Reprod Med. 18:229–235. 2000.
View Article : Google Scholar
|
|
3
|
Simón C, Martín JC and Pellicer A:
Paracrine regulators of implantation. Baillieres Best Pract Res
Clin Obstet Gynaecol. 14:815–826. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escary JL, Perreau J, Duménil D, Ezine S
and Brûlet P: Leukaemia inhibitory factor is necessary for
maintenance of haematopoietic stem cells and thymocyte stimulation.
363:361–364. 1993.PubMed/NCBI
|
|
5
|
Cavagna M and Mantese JC: Biomarkers of
endometrial receptivity - a review. Placenta. 24(Suppl B): S39–S47.
2003. View Article : Google Scholar
|
|
6
|
Hoozemans DA, Schats R, Lambalk CB,
Homburg R and Hompes PG: Human embryo implantation: current
knowledge and clinical implications in assisted reproductive
technology. Reprod Biomed Online. 9:692–715. 2004. View Article : Google Scholar
|
|
7
|
Stewart CL, Kaspar P, Brunet LJ, et al:
Blastocyst implantation depends on maternal expression of leukaemia
inhibitory factor. Nature. 359:76–79. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dimitriadis E, Nie G, Hannan NJ, Paiva P
and Salamonsen LA: Local regulation of implantation at the human
fetal-maternal interface. Int J Dev Biol. 54:313–322. 2010.
View Article : Google Scholar
|
|
9
|
Fiedler U, Reiss Y, Scharpfenecker M, et
al: Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and
has a crucial role in the induction of inflammation. Nat Med.
12:235–239. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maisonpierre PC, Suri C, Jones PF, et al:
Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo
angiogenesis. Science. 277:55–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsumoto H, Ma WG, Daikoku T, et al:
Cyclooxygenase-2 differentially directs uterine angiogenesis during
implantation in mice. J Biol Chem. 277:29260–29267. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Mouzon J, Goossens V, Bhattacharya S,
et al; European IVF-monitoring (EIM) Consortium, for the European
Society of Human Reproduction and Embryology (ESHRE). Assisted
reproductive technology in Europe, 2006: results generated from
European registers by ESHRE. Hum Reprod. 25:1851–1862. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papanikolaou EG, Bourgain C, Kolibianakis
E, Tournaye H and Devroey P: Steroid receptor expression in late
follicular phase endometrium in GnRH antagonist IVF cycles is
already altered, indicating initiation of early luteal phase
transformation in the absence of secretory changes. Hum Reprod.
20:1541–1547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fauser BC and Devroey P: Reproductive
biology and IVF: ovarian stimulation and luteal phase consequences.
Trends Endocrinol Metab. 14:236–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saadat P, Boostanfar R, Slater CC,
Tourgeman DE, Stanczyk FZ and Paulson RJ: Accelerated endometrial
maturation in the luteal phase of cycles utilizing controlled
ovarian hyperstimulation: impact of gonadotropin-releasing hormone
agonists versus antagonists. Fertil Steril. 82:167–171. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kolibianakis E, Bourgain C, Albano C, et
al: Effect of ovarian stimulation with recombinant
follicle-stimulating hormone, gonadotropin releasing hormone
antagonists, and human chorionic gonadotropin on endometrial
maturation on the day of oocyte pick-up. Fertil Steril.
78:1025–1029. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pellicer A, Valbuena D, Cano F, Remohí J
and Simón C: Lower implantation rates in high responders: evidence
for an altered endocrine milieu during the preimplantation period.
Fertil Steril. 65:1190–1195. 1996.PubMed/NCBI
|
|
18
|
Check JH, Choe JK, Katsoff D,
Summers-Chase D and Wilson C: Controlled ovarian hyperstimulation
adversely affects implantation following in vitro
fertilization-embryo transfer. J Assist Reprod Genet. 16:416–420.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toth B, Würfel W, Germeyer A, Hirv K,
Makrigiannakis A and Strowitzki T: Disorders of implantation - are
there diagnostic and therapeutic options? J Reprod Immunol.
90:117–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu XT, Fu P and Wang YP: Use of
traditional Chinese medicine in assisted reproductive technology.
Chinese Archives of Traditional Chinese Medicine. 27:120–123.
2009.(In Chinese).
|
|
21
|
Zhang JW, Lian F, Zheng S and Yan HL:
Effect of Erzhi Tiangui granules (traditional Chinese medicine) on
follicular fluid interleukin-1β, interleukin-6 concentration and
embryo quality in patients undergoing controlled ovarian
hyperstimulation. Reprod Contracept. 27:714–717. 2007.(In
Chinese).
|
|
22
|
Lian F, Teng YL, Zhang JW, et al: Clinical
study on Erzhi Tiangui granules combined with in vitro
fertilization-embryo transplant for treatment of 61 cases of
infertility. J Tradit Chin Med. 47:439–441. 2006.(In Chinese).
|
|
23
|
Huang ST and Chen AP: Traditional Chinese
medicine and infertility. Curr Opin Obstet Gynecol. 20:211–215.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimber SJ: Leukaemia inhibitory factor in
implantation and uterine biology. Reproduction. 130:131–145. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhatt H, Brunet LJ and Stewart CL: Uterine
expression of leukemia inhibitory factor coincides with the onset
of blastocyst implantation. Proc Natl Acad Sci USA. 88:11408–11412.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith AG, Heath JK, Donaldson DD, et al:
Inhibition of pluripotential embryonic stem cell differentiation
by. Nature. 336:688–690. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams RL, Hilton DJ, Pease S, et al:
Myeloid leukaemia inhibitory factor maintains the developmental
potential of embryonic stem cells. Nature. 336:684–687. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conquet F and Brûlet P: Developmental
expression of myeloid leukemia inhibitory factor gene in
preimplantation blastocysts and in extraembryonic tissue of mouse
embryos. Mol Cell Biol. 10:3801–3805. 1990.PubMed/NCBI
|
|
29
|
Murray R, Lee F and Chiu CP: The genes for
leukemia inhibitory factor and interleukin-6 are expressed in mouse
blastocysts prior to the onset of hemopoiesis. Mol Cell Biol.
10:4953–4956. 1990.PubMed/NCBI
|
|
30
|
Gearing DP, Thut CJ, VandeBos T, et al:
Leukemia inhibitory factor receptor is structurally related to the
IL-6 signal transducer, gp130. EMBO J. 10:2839–2848.
1991.PubMed/NCBI
|
|
31
|
Ware CB, Horowitz MC, Renshaw BR, et al:
Targeted disruption of the low-affinity leukemia inhibitory factor
receptor gene causes placental, skeletal, neural and metabolic
defects and results in perinatal death. Development. 121:1283–1299.
1995.PubMed/NCBI
|
|
32
|
Gale NW and Yancopoulos GD: Growth factors
acting via endothelial cell-specific receptor tyrosine kinases:
VEGFs, angiopoietins, and ephrins in vascular development. Genes
Dev. 13:1055–1066. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Charnock-Jones DS, Kaufmann P and Mayhew
TM: Aspects of human fetoplacental vasculogenesis and angiogenesis.
I Molecular regulation. Placenta. 25:103–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaufmann P, Mayhew TM and Charnock-Jones
DS: Aspects of human fetoplacental vasculogenesis and angiogenesis.
II Changes during normal pregnancy. Placenta. 25:114–126. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Red-Horse K, Drake P and Fisher S: Human
pregnancy: the role of chemokine networks at the fetal-maternal
interface. Expert Rev Mol Med. 6:1–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hur SE, Lee JY, Moon HS and Chung HW:
Angiopoietin-1, angiopoietin-2 and Tie-2 expression in eutopic
endometrium in advanced endometriosis. Mol Hum Reprod. 12:421–426.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanahan D: Signaling vascular
morphogenesis and maintenance. Science. 277:45–50. 1997. View Article : Google Scholar
|
|
39
|
Rabbani ML and Rogers PA: Role of vascular
endothelial growth factor in endometrial vascular events before
implantation in rats. Reproduction. 122:85–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Simón C, Cano F, Valbuena D, Remohí J and
Pellicer A: Implantation: Clinical evidence for a detrimental
effect on uterine receptivity of high serum oestradiol
concentrations in high and normal responder patients. Hum Reprod.
10:2432–2437. 1995. View Article : Google Scholar
|
|
41
|
Simón C, Garcia Velasco JJ, Valbuena D, et
al: Increasing uterine receptivity by decreasing estradiol levels
during the preimplantation period in high responders with the use
of a follicle-stimulating hormone step-down regimen. Fertil Steril.
70:234–239. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carson DD, Lagow E, Thathiah A, et al:
Changes in gene expression during the early to mid-luteal
(receptive phase) transition in human endometrium detected by
high-density microarray screening. Mol Hum Reprod. 8:871–879. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kao LC, Tulac S, Lobo S, et al: Global
gene profiling in human endometrium during the window of
implantation. Endocrinology. 143:2119–2138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Horcajadas JA, Riesewijk A, Polman J, et
al: Effect of controlled ovarian hyperstimulation in IVF on
endometrial gene expression profiles. Mol Hum Reprod. 11:195–205.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gong X, Yu Y, Tong Q, Ren Y and Jin Z:
Effects of ‘Bu Shen Huo Xue Decoction’ on the endometrial
morphology and expression of leukaemia inhibitory factor in the rat
uterus during the oestrous cycle. Evid Based Complement Alternat
Med. 2013:4960362013. View Article : Google Scholar
|
|
46
|
Efferth T, Li PC, Konkimalla VSB and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|