Introduction
Since it was first introduced by Matouschek
(1) in 1981 and independently
proposed by O’Donnell and Puri (2)
in 1984, endoscopic injection has become popular for treating
vesicoureteral reflux (VUR) due to its simplicity. The spread of
endoscopic treatment accelerated with the introduction of
dextranomer/hyaluronic acid (Dx/HA). Due to its advantages,
including technical ease, minimal invasiveness, low complication
rate and short hospital stay, endoscopic treatment is a viable
alternative to open ureteral reimplantation. However, the rate of
VUR resolution following injection treatment is lower than that
following open ureteral reimplantation. In a review, it was
reported that the overall success rate of injection ranged between
68 and 92% (3). Open ureteral
reimplantation has a high success rate (94–99%) in correcting VUR,
regardless of technique (4).
Therefore, certain urologists insist that routine postoperative
voiding cystourethrography (VCUG) is not necessary following open
ureteral reimplantation (5–8).
VCUG is an invasive procedure that requires a urethral catheter.
However, the American Urologic Association (AUA) recommends a
postoperative VCUG subsequent to injection treatment (9).
The AUA also recommends that following open surgical
or endoscopic procedures for VUR, a renal ultrasound should be
performed 1 month postoperatively to determine whether there are
any obstructions (9). On the
postoperative ultrasound, the echogenic injection material can
often be visualized in the bladder. When an injection nodule is
detected, it is hypothesized that the injection and maintenance of
materials is successful. The present study evaluated whether the
presence of an injection nodule on ultrasound can predict the
resolution of VUR and replace invasive VCUG. The prognostic factors
for success were also investigated.
Materials and methods
Patients
Patients who received an injection of endoscopic
bulking agent for VUR at the Samsung Medical Centre (Seoul, Korea)
between January 2005 and December 2010 were evaluated
retrospectively. The research protocol was approved by the Samsung
Medical Center Institutional Review Board. Patients with neurogenic
bladder; posterior urethral valve; cloacal anomaly; previous open
anti-refluxing surgery; ureteric abnormality such as duplication,
diverticulum and ureterocele; insufficient medical records; or
those who had not participated in an imaging study were excluded.
The medical records were reviewed for each patient and the age at
surgery, gender, affected side, VUR grade, injection material and
treatment success were evaluated. VUR was graded according to the
grading system of the International Reflux Study Committee
(10).
Injection procedure
All procedures were performed under general
anesthesia with the patient in the lithotomy position. In the
initial period, the subureteric transurethral technique (STING) was
used. After 2007, the injection technique was changed to the
hydrodistention-implantation technique (HIT). If the coaptation was
insufficient following HIT, STING was also used.
Polydimethylsiloxane (Macroplastique®; Uroplasty,
Minnetonka, MN, USA) was injected until June 2006 and Dx/HA
copolymer (Deflux®; Q-Med Scandinavia, Uppsala, Sweden)
was injected thereafter.
Postoperative examination
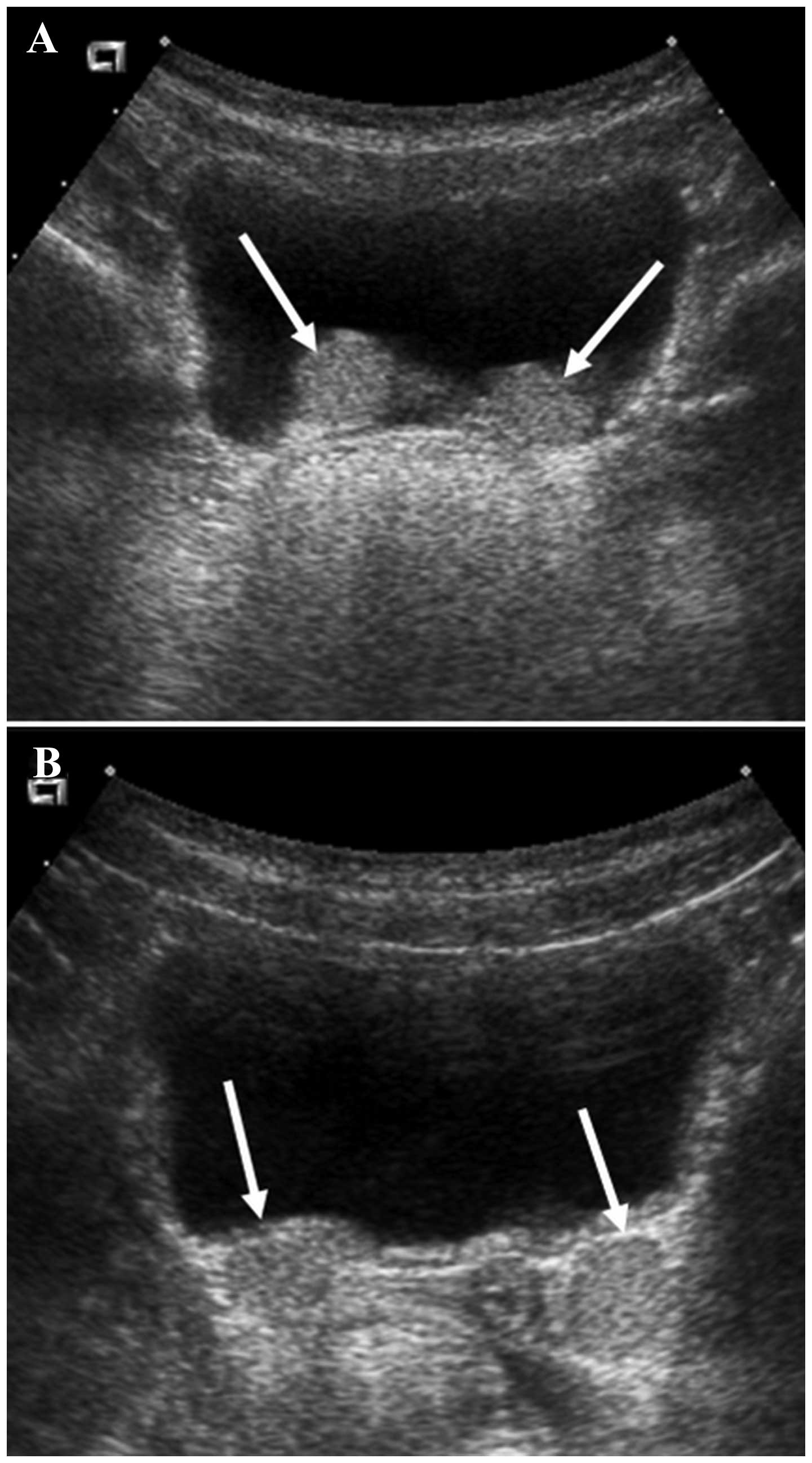
Ultrasound was used to evaluate whether a nodule was
present. To assess postoperative hydronephrosis, ultrasound was
performed routinely in nearly all patients one month post-surgery.
The ultrasound was performed on a full bladder by a pediatric
radiologist. A nodule was defined as a protruding mass lesion
(Fig. 1A) or echogenic mass
(Fig. 1B) distinguished from the
bladder at the ureteral orifice. At three months post-surgery, VCUG
was performed to determine whether the VUR had been resolved. The
injection treatment was considered successful if the VUR had
disappeared on the postoperative VCUG at three months.
Statistical analysis
The success rate in the group with nodules was
compared with that in the group without nodules. To determine the
value of an injection nodule as a diagnostic tool to predict VUR
resolution, the sensitivity, specificity, positive predictive value
(PPV), negative predictive value (NPV) and accuracy were
calculated. The difference in treatment success according to
non-numeric variables was assessed on univariate analysis with
Pearson’s Chi-square test, Fisher’s exact test or the
Cochran-Armitage test. Numeric variables were compared between
treatment success and treatment failure by the Mann-Whitney test. A
logistic regression analysis was conducted to evaluate the
correlation between the variables and success. Variables are
reported with 95% confidence intervals. Data were analyzed using
PASW® 18.0 (SPSS, Inc., Chicago, IL, USA) and P<0.05
was considered statistically significant.
Results
Patient data
Of 186 total patients, 149 patients (220 ureters)
met the inclusion criteria. The mean age at surgery was 3.5 years
(range, 0.6–18 years). Seventy-nine patients (53.0%) were male and
70 patients (47.0%) were female. Unilateral VUR was performed in 78
patients (52.3%) and bilateral VUR was performed in 71 patients
(47.7%). There were 122 patients (81.9%) injected with Dx/HA and 27
patients (18.1%) injected with polydimethylsiloxane. The median
injection volume was 1.13 ml (range, 0.1–4.2 ml).
Univariate analysis of success rate
Among the 220 ureters, 161 ureters (73.2%) exhibited
a complete resolution of VUR on postoperative VCUG. The success
rates of VUR were 82.6% (19/23) for Grade I, 89.3% (25/28) for
Grade II, 72.0% (67/93) for Grade III, 66.7% (44/66) for Grade IV
and 60.0% (6/10) for Grade V. As the VUR grade increased, the
success rate tended to decrease (P=0.018).
Injection nodules were present in 152 ureters
(69.1%). Of these, VUR resolved in 128 ureters (84.2%). The group
with injection nodules had a higher success rate than the group
without injection nodules (84.2 vs. 48.5%, respectively;
P<0.001). There was a positive correlation between the presence
of the injection mounds and VUR resolution. On univariate analysis,
gender, laterality and injection material did not significantly
influence the success rate (Table
I). The mean injection volume in the group with VUR resolution,
however, was significantly smaller than that in the group with
persistent VUR (1.05 vs. 1.38 ml, respectively; P=0.001).
 | Table ISuccess rate according to each
variable and univariate analysis between variables and success. |
Table I
Success rate according to each
variable and univariate analysis between variables and success.
| Variables | No. of ureters, n
(%) | Success rate, n
(%) | P-value |
|---|
| Nodule | | | <0.001 |
| Present | 152 (69.1) | 128 (84.2) | |
| Absent | 68 (30.9) | 33 (48.5) | |
| Gender | | | 0.378 |
| Male | 116 (52.7) | 82 (70.7) | |
| Female | 104 (47.3) | 79 (76.0) | |
| Laterality | | | 0.184 |
| Right | 102 (46.4) | 79 (77.5) | |
| Left | 118 (53.6) | 82 (66.5) | |
| Injection
material | | | 0.327 |
| Dx/HA | 181 (82.3) | 130 (71.8) | |
|
Polydimethylsiloxane | 39 (17.7) | 31 (79.5) | |
| Grade | | | 0.018a |
| I | 23 (10.5) | 19 (82.6) | |
| II | 28 (12.7) | 25 (89.3) | |
| III | 93 (42.3) | 67 (72.0) | |
| IV | 66 (30.0) | 44 (66.7) | |
| V | 10 (4.5) | 6 (60.0) | |
Multivariate analysis of success
rate
On multivariate analysis, injection nodules were
predictive of endoscopic injection success (odds ratio, 6.050;
P<0.001). The failure rate increased with increasing injection
volume (odds ratio, 0.428; P=0.004; Table II). Sonographic injection nodules
had 79.5% sensitivity, 59.3% specificity, 84.2% positive predictive
value, 51.5% negative predictive value and 74.1% accuracy as a
diagnostic tool for success rate. These values increased slightly
with increasing VUR grade (Table
III).
 | Table IIMultivariate analysis between
variables and success. |
Table II
Multivariate analysis between
variables and success.
| Risk factors | Odds ratio | 95% confidence
interval | P-value |
|---|
| Nodule | 6.050 | 2.998–12.209 | <0.001 |
| Age | 1.079 | 0.969–1.201 | 0.164 |
| Gender (female) | 1.051 | 0.519–2.131 | 0.890 |
| Laterality
(left) | 0.671 | 0.334–1.350 | 0.264 |
| Injection material
(polydimethylsiloxane) | 1.079 | 0.430–3.170 | 0.761 |
| Injection volume | 0.428 | 0.240–0.761 | 0.004 |
| Grade |
| I | 1 | – | – |
| II | 1.555 | 0.272–8.906 | 0.620 |
| III | 0.541 | 0.146–2.004 | 0.358 |
| IV | 0.569 | 0.144–2.254 | 0.422 |
| V | 0.694 | 0.107–4.515 | 0.702 |
 | Table IIIDiagnostic values of sonographic
injection nodules (%). |
Table III
Diagnostic values of sonographic
injection nodules (%).
| Grade | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|
| I | 68.4 | 50.0 | 86.7 | 25.0 | 65.2 |
| II | 80.0 | 66.7 | 95.2 | 28.6 | 78.6 |
| III | 80.6 | 50.0 | 80.6 | 50.0 | 72.0 |
| IV | 81.8 | 63.6 | 81.8 | 63.6 | 75.8 |
| V | 83.3 | 100.0 | 100.0 | 90.0 | 90.0 |
| Total | 79.5 | 59.3 | 84.2 | 51.5 | 74.1 |
Discussion
As endoscopic injection has a lower success rate
than open surgical reimplantation, there have been numerous efforts
to identify good candidates for endoscopic injection treatment and
predictive factors for success (11–15).
The prognostic factors can be divided into preoperative factors and
treatment-associated factors. The preoperative factors are
patient-dependent factors, such as VUR grade, anatomic bladder and
ureteral abnormalities and dysfunctional voiding; preoperative VUR
grade is a chief prognostic factor. Higher VUR grades are
associated with lower success rates (13,14,16).
Although there are discrepancies among studies, the
known treatment-associated factors are surgeon-dependent factors
such as surgeon experience, injection technique, mound morphology
and location, and injective volume. These factors are associated
with the outcome of the endoscopic injection treatment. The goal of
endoscopic injection treatment is to create a subureteral mound
that is able to elevate and coapt the ureteral orifice. A
satisfactory mound is the most important factor in the success of
Dx/HA injection, following adjustment for other factors such as VUR
grade and the volume injected (13).
Whether the presence of an injection nodule on a
postoperative ultrasound can predict the resolution of VUR has
remained uncertain until now. Few studies have investigated the
association between sonographic injection nodules and the success
of endoscopic injection (17–19).
A polydimethylsiloxane implant was identifiable in 84% of
ultrasounds in one study and 86% of these had corrected VUR on
postoperative VCUG (19). In
addition, ultrasound had a sensitivity of 89% and specificity of
86% for VUR correction. Another study, however, reported no
correlation between the presence of a Dx/HA nodule and the
resolution of VUR on VCUG (17).
In the present study, patients with an injection nodule had a
higher success rate than patients without an injection nodule. On
multivariate analysis, injection nodules were predictive factors
for the success of endoscopic injection. Contrary to the results of
Ellsworth et al (17), the
sensitivity and specificity were relatively low in the present
study. Therefore, it is concluded that the presence of an injection
nodule on postoperative ultrasound cannot replace VCUG.
In the present study, 24 ureters (10.9% of the total
cases) with injection mounds had sustained VUR following endoscopic
injection, which may have several explanations. The injection
material could have been in the wrong position. When performing a
second injection for failed cases, implants were observed in
improper locations. Another explanation may be insufficient
coaptation of the ureter.
In 68 cases (30.9% of the total cases), injection
mounds were not detected. These injection mounds were likely to
have been missed by the radiologist either because they were too
small or because they were absent. An insufficient volume could be
due to too little material being injected or the injection material
being spilled. If the bladder mucosa overlying the injection
material was eroded, the material may have been expelled during
voiding.
The VUR resolved in certain cases without
sonographic injection nodules. This finding may be explained by
tissues reacting with the injection materials. Hydrolysis of
dextranomer microspheres reduces the volume of the injected
materials, but endogenous collagen production between the
microspheres results in tissue augmentation (20).
In 2002, Oswald et al compared a single
endoscopic injection of polydimethylsiloxane with Dx/HA for the
treatment of VUR in children (21). VUR was corrected in 86.2% of the
children injected with polydimethylsiloxane and in 71.4% of the
children injected with Dx/HA at the three-month follow-up visit. No
postoperative complications were observed in either group. The
success rates in the current study, which were 79.5% for the
children injected with polydimethylsiloxane and 71.8% for the
children injected with Dx/HA, are consistent with the data from the
previous study. Also, no significant differences were identified
between the two groups in the present study (P=0.327).
In the present study, increased injection volumes
were identified to be associated with injection failure. This is
comparable to the findings of a previous report (13) and may be due to difficulty in
creating a proper mound with larger injection volumes. If the
ureteral orifice is wide or the distal ureter is dilated, a greater
volume might be necessary to coapt the ureteral orifice. These
cases are also more likely to fail than low grade VURs. By
contrast, smaller volumes indicated success in creating a mound and
an increased likelihood of reflux resolution.
In conclusion, the presence of a postoperative
injection nodule is able to predict resolution of VUR. However, the
sensitivity and specificity are relatively low. If a postoperative
injection mound is present on ultrasound examination, the child’s
parents should be informed of the high probability of success prior
to performing VCUG.
References
|
1
|
Matouschek E: Treatment of vesicorenal
reflux by transurethral teflon-injection (author’s transl). Urologe
A. 20:263–264. 1981.(In German). PubMed/NCBI
|
|
2
|
Puri P and O’Donnell B: Correction of
experimentally produced vesicoureteric reflux in the piglet by
intravesical injection of Teflon. Br Med J (Clin Res Ed). 289:5–7.
1984. View Article : Google Scholar
|
|
3
|
Chertin B, Kocherov S, Chertin L, et al:
Endoscopic bulking materials for the treatment of vesicoureteral
reflux: a review of our 20 years of experience and review of the
literature. Adv Urol. 2011:3096262011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grossklaus DJ, Pope JC, Adams MC and Brock
JW: Is postoperative cystography necessary after ureteral
reimplantation? Urology. 58:1041–1045. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Ghoneimi A, Odet E, Lamer S, et al:
Cystography after the Cohen ureterovesical reimplantation: is it
necessary at a training center? J Urol. 162:1201–1202. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bomalaski MD, Ritchey ML and Bloom DA:
What imaging studies are necessary to determine outcome after
ureteroneocystostomy? J Urol. 158:1226–1228. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bisignani G and Decter RM: Voiding
cystourethrography after uncomplicated ureteral reimplantation in
children: is it necessary? J Urol. 158:1229–1231. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barrieras D, Lapointe S, Reddy PP, et al:
Are postoperative studies justified after extravescial ureteral
reimplantation? J Urol. 164:1064–1066. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters CA, Skoog SJ, Arant BS Jr, et al:
Summary of the AUA Guideline on Management of Primary
Vesicoureteral Reflux in Children. J Urol. 184:1134–1144. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
No authors listed. Medical versus surgical
treatment of primary vesicoureteral reflux: report of the
International Reflux Study Committee. Pediatrics. 67:392–400.
1981.PubMed/NCBI
|
|
11
|
Routh JC and Reinberg Y: Predicting
success in the endoscopic management of pediatric vesicoureteral
reflux. Urology. 76:195–198. 2010. View Article : Google Scholar
|
|
12
|
Routh JC, Kramer SA, Inman BA, et al:
Utility of dextranomer/hyaluronic acid injection in setting of
bladder and ureteral anomalies. Urology. 71:435–438. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yucel S, Gupta A and Snodgrass W:
Multivariate analysis of factors predicting success with
dextranomer/hyaluronic acid injection for vesicoureteral reflux. J
Urol. 177:1505–1509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elder JS, Diaz M, Caldamone AA, et al:
Endoscopic therapy for vesicoureteral reflux: a meta-analysis. I
reflux resolution and urinary tract infection. J Urol. 175:716–722.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lavelle MT, Conlin MJ and Skoog SJ:
Subureteral injection of Deflux for correction of reflux: analysis
of factors predicting success. Urology. 65:564–567. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Routh JC, Inman BA and Reinberg Y:
Dextranomer/hyaluronic acid for pediatric vesicoureteral reflux:
systematic review. Pediatrics. 125:1010–1019. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ellsworth PI, Yates JK and Caldamone AA:
Presence of dextranomer-hyaluronic acid (DxHA) mound on
postoperative ultrasound does not predict resolution of
vesicoureteral reflux. J Pediatr Urol. 7:438–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozcan C, Ergün R, Ozbek SS, Avanoğlu A and
Ulman I: Bladder ultrasound in the evaluation of the efficacy of
dextranomer/hyaluronic acid injection for treatment of
vesicoureteral reflux. J Clin Ultrasound. 35:357–362. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herz D, Hafez A, Bagli D, Capolicchio G,
McLorie G and Khoury A: Efficacy of endoscopic subureteral
polydimethylsiloxane injection for treatment of vesicoureteral
reflux in children: a north american clinical report. J Urol.
166:1880–1886. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stenberg A, Larsson E, Lindholm A, Ronneus
B, Stenberg A and Läckgren G: Injectable dextranomer-based implant:
histopathology, volume changes and DNA-analysis. Scand J Urol
Nephrol. 33:355–361. 1999. View Article : Google Scholar
|
|
21
|
Oswald J, Riccabona M, Lusuardi L, Bartsch
G and Radmayr C: Prospective comparison and 1-year follow-up of a
single endoscopic subureteral polydimethylsiloxane versus
dextranomer/hyaluronic acid copolymer injection for treatment of
vesicoureteral reflux in children. Urology. 60:894–898. 2002.
View Article : Google Scholar : PubMed/NCBI
|