Introduction
Autograft and allograft transplantations are the
primary treatments for large bone defects caused by trauma, tumor
resection and infection; however, the problems of limited bone
sources and immune rejection or infection persist. By contrast,
metallic biomedical materials are playing an increasingly important
role in the treatment of bone defects. However, since these metals
and their alloys do not exhibit the same elastic modulus as bone,
stress shielding occurs, resulting in relative displacement of
metal implants and friction along the metal-bone interface
(1,2). This friction generates debris,
inducing a macrophage response, which causes deterioration or
absorption of the bone tissue and ultimately leads to loosening of
the implant or fracture of the host bone (3–5).
Therefore, further research is required to enhance the in
vivo longevity of metallic biomedical materials.
To date, research in this area has focused primarily
on porous titanium and its alloys. However, the clinical
application of porous metal materials has remained limited due to
their low porosity, small surface friction coefficient and concerns
regarding their elastic modulus (6–8).
Porous tantalum has been reported as a promising material for use
in bone tissue engineering. However, only Zimmer Corporation
(Warsaw, IN, USA) has produced porous tantalum material with high
porosity (75–80%), good tissue compatibility, a large surface
friction coefficient and the proper elastic modulus that closely
matches that of human bone. As a result, porous tantalum has been
widely applied in arthroplasty, spinal fusion surgery and the
treatment of femoral head necrosis (9–12).
However, the widespread use of porous tantalum in China is limited
by the high price. Cooperating with numerous domestic research
institutions, Chongqing Runze Pharmaceutical Co., Ltd. (Chongqing,
China) have successfully developed porous tantalum materials, which
were prepared by slip-casting powder through teeming technology.
The porous tantalum selected was −250 mesh pure tantalum powder
with a certain amount of additives and added sponge carrier to
control the pore diameter of porous materials, porosity and pore
distribution, and approved by slip-casting forming and through
1,500–2,100°C high temperature sintered and post-treatment
technology of necessary preparation (preparation method has been
submitted for patenting). The biomechanical indexes showed that
Chinese porous tantalum possessed high porosity, an appropriate
pore diameter size and exhibited biomechanical performance
characteristics that were comparable to human bone, suggesting that
tantalum may be an ideal bone graft material (Table I).
 | Table IMaterial physical and chemical
performance indicators. |
Table I
Material physical and chemical
performance indicators.
| Indicators | Range |
|---|
| Density
(g/cm3) | 3.5–7 |
| Porosity (%) | 65–80 |
| Pore diameter
(μm) | 400–600 |
| Elasticity modulus
(GPa) | 2.0–4.6 |
| Ultimate strength
(MPa) | 110–210 |
| Yield strength
(MPa) | 75–120 |
| Compressive strength
(MPa) | 100–170 |
| Tensile strength
(MPa) | 80–120 |
| Bending strength
(MPa) | 80–150 |
The present study investigated the physical
properties and cytotoxicity of Chinese porous tantalum and examined
osteogenesis at the tantalum-host bone interface following
implantation in the lateral epicondyle of a rabbit femora. The aim
of the study was to assess the possibility of the wider application
of Chinese porous tantalum material.
Materials and methods
Materials
Eight fetal New Zealand rabbits and 24 adult male
rabbits (weight, 2.5–3 kg) were provided by the Experimental Animal
Center of Hebei United University (Tangshan, China). Porous
tantalum was obtained from Chongqing Runze Pharmaceutical Co., Ltd.
The porous tantalum selected was −250 mesh pure tantalum powder
with a certain amount of additives and added sponge carrier to
control the pore diameter of porous materials, porosity and pore
distribution, and approved by slip-casting forming and through
1,500–2,100°C high temperature sintered and post-treatment
technology of necessary preparation (preparation method has been
submitted for patenting). Low-glucose Dulbecco’s modified Eagle’s
medium (DMEM) was purchased from Gibco Life Technologies (Carlsbad,
CA, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) reagent was purchased from Sigma-Aldrich (St. Louis,
MO, USA) and an inverted phase contrast microscope was obtained
from Nikon Corporation (Tokyo, Japan). In addition, a scanning
electron microscope (JSM-6030LV; JEOL, Tokyo, Japan), a Leica
SP1600 hard tissue slicer (Leica Microsystems GmbH, Wetzlar,
Germany), a laser scanning confocal microscope (Olympus
Corporation, Tokyo, Japan) and a Bio-Rad 680 microplate reader
(Bio-Rad Laboratories, Hercules, CA, USA) were employed. This study
was approved by the Institutional Animal Care and Use Committee of
Hebei United University. Postoperative care was professionally
managed by trained personnel and supervised by veterinarians.
Osteoblast cultivation
The periosteum and soft tissue surrounding the
crania of the fetal New Zealand rabbits were removed and cut into
1xl mm pieces prior to digestion in a solution of 0.25% trypsin and
0.1% type II collagenase. Following centrifugation at 2,012 × g for
10 min at 37°C, the supernatant was discarded and DMEM containing
10% fetal bovine serum (Gibco Life Technologies) was added to the
white precipitate, which was pipetted into a cell suspension for
counting the total number of cells and then seeded into cell
culture dishes. The cells were cultured with DMEM supplemented with
10% fetal bovine serum in a humidified incubator containing 5%
CO2 at 37°C. Primary osteoblasts were passaged after
reaching 80–90% confluence. Osteoblasts were identified by alkaline
phosphatase (ALP) staining, collagen type I immunocytochemical
staining and calcium nodule staining.
Cytotoxicity evaluation
Porous tantalum extract was prepared according to
ISO 10993-5, 2009 for high pressure disinfection of porous tantalum
extract preparation (13).
Extracts were prepared based on the principle of ‘material
weight/extraction transmitter = 0.2 g/ml’, which represented 100%
concentration of the extract. Extraction conditions were 37°C in 5%
CO2 for 72 h, and metal ions were precipitated. Once the
extraction was completed, sterilization was achieved by passing the
extract through a 0.22-μm filter.
An MTT assay was conducted to determine the effects
of porous tantalum on osteoblast viability, growth and
proliferation (14). Third passage
osteoblasts were suspended and seeded in a 96-well plate at
2×104 cells per well. Porous tantalum extract
(experimental group) or complete medium (control group) was added
to the wells until the volume in each well was 200 μl. Next, 20 μl
MTT (5 mg/ml) solution was added to each well (pH 7.4) and the
plate was incubated for 4 h in the CO2 incubator.
Dimethyl sulfoxide was added and low-speed oscillation was applied.
A Bio-Rad enzyme-linked immunometric meter (measurement wavelength,
490 nm; reference wavelength, 650 nm) was used to detect the
optical density (OD) value of the solution in each well, from which
a cell growth curve was prepared.
Osteoblast culture on porous tantalum to
evaluate cytocompatibility
Pancreatin was used to digest a proportion of the
osteoblasts in order to adjust the cell concentration to
1×106 cells/ml. Subsequently, a 30-μl cell suspension
was seeded onto each presterilized porous tantalum sample in the
culture plates. The porous tantalum was turned over and the cells
were seeded onto the other side. DMEM containing 10% fetal bovine
serum was added, and samples were cultured at 37°C in 5%
CO2 and saturated humidity. Cell growth and adhesion to
the tantalum samples was observed on an inverted phase contrast
microscope every day.
Sterilized tantalum samples and those on which cells
were seeded were collected on days 3, 5, 7 and 10. The samples were
washed with phosphate-buffered saline, fixed in 2.5%
glutaraldehyde, dehydrated in 30–100% t-butyl alcohol solutions,
dried in a 37°C electric thermostatic drier (JEOL JFC-1600) for 24
h and underwent ion sputter metal spraying for scanning electron
microscopy (SEM) observation of cell adherence and growth on the
material.
Preparation of rabbits for porous
tantalum implantation
A total of 24 male adult New Zealand white rabbits
(weight, 2.5–3.0 kg) were selected. The model of porous tantalum
implantation in the femoral condyle was established using improved
surgical methods, as reported in a previous study (15). Briefly, 20% urethane (3–4 ml/kg)
was injected into the ear vein for anesthesia. The vastus lateralis
muscle was cut from the outside part of the lower femur, along the
femoral shaft, to expose the femoral condyle. A 3-mm Kirschner
skeletal wire [Association for Osteosynthesis(AO)/Association for
the Study of Internal Fixation (ASIF), Davos, Switzerland]was used
to drill into the femoral lateral condyle, which was ~1 cm from the
knee, perpendicular to the femoral shaft. The drilling resulted in
a cylindrical bone tunnel with a diameter of ~3 mm and a depth of 5
mm. A porous tantalum rod (diameter, 3 mm; height, 5 mm) was
implanted in the bone along the tunnel, with the tantalum rod
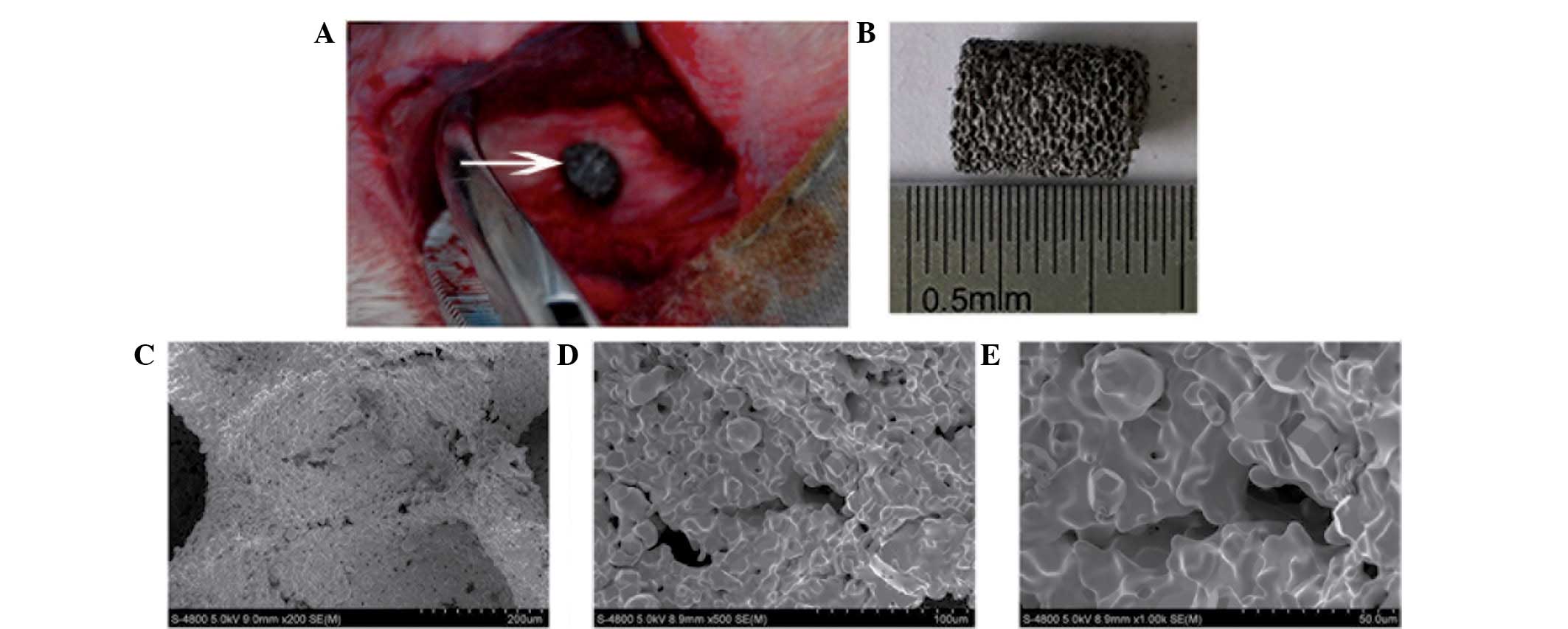
contacting as closely as possible with the tunnel wall of the host
bone (Fig. 1A). The wound was
sutured and marked with adequate hemostasis. Three days after
surgery, the wound was disinfected and penicillin was injected
intramuscularly. Four animals were injected with luciferin calcein
solution (10 mg/kg) in the gluteus maximus on day 5 after surgery.
Fluorescein alizarin red solution (40 mg/kg) was injected in the
same manner on day 19 after surgery, in order to observe
osteogenesis. The animals were separated into cages, without
limitation of movement, and euthanized at week ten after
surgery.
Porous tantalum-bone hard tissue slicing
and staining
In total, 20 rabbits were euthanized by air embolism
at weeks 2, 4, 8 and 12 after surgery (five at each time point).
The bone tissue in the femoral condyle was removed along with the
porous tantalum rod, and then washed, trimmed and fixed in 10%
formaldehyde. Following dehydration, infiltration, embedding (20 g
benzoyl peroxide in 800 ml methacrylic acid methyl ester, phthalic
acid and 200 ml dibutyl) and polymerization, a metal slicer was
used to slice the material along the direction parallel to the
longitudinal axis of the porous tantalum rod, fully exposing its
plane. The plane was polished and 90-μm slices were prepared, which
were ground down to 20 μm, dried and exposed to glycol ether ester
to remove any plastic. Sections were stained with methylene blue
and osteogenesis was observed under a light microscope. The
materials acquired at week 10 after the surgery were processed into
porous tantalum-bone hard tissue slices using the same method and
were used to evaluate osteogenesis at the porous tantalum-bone
interface via laser scanning confocal microscopy with a 488-nm
laser (calcein excitation wavelength) and a 543 nm laser (alizarin
red excitation wavelength).
Statistical analysis
Data were analyzed using SPSS 20.0 statistical
software (IBM, Armonk, NY, USA). Experimental data are expressed as
the mean ± standard deviation. In the cytotoxicity and cell growth
experiments, comparisons between the experimental and control
groups were analyzed using the Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Surface characteristics of porous
tantalum
Under microscopic observation, the appearance of the
porous tantalum scaffolds was gray and smooth, with pinpoint-sized
pores distributed evenly in a honeycomb pattern on the surface and
fracture surface (Fig. 1B).
SEM revealed that the material surface and
cross-section had an interconnected pore distribution, with a pore
diameter size of 400–600 μm. The trabecular pillars had a
microporous structure, where the diameter of the micropores was
~100 μm. Pore distribution was uniform and the pores were
interconnected. The microporous structure with interconnected pores
had a diameter of 200–400 μm, the particle diameter was 20–50 μm
and there were 50–200 μm interstices between the particles
(Fig. 1C–E).
Cultivation of isolated fetal rabbit
osteoblasts
Primary osteoblasts were observed as translucent
spheres under an inverted phase contrast microscope. The cells grew
adherently after 24 h, presenting a variety of forms, which were
primarily short fusiform and triangular with the cytoplasm
extending outward to form protrusions. The cell volume was enlarged
by day 3, with cells connecting and increasing in number. By day 7,
the cell groups fused to form a monolayer covering the bottom of
the plate, and the cell morphology became polygonal or irregular.
The cells were passaged when they reached 80–90% confluence. The
majority of the cells exhibited triangular or long fusiform shapes,
with the cell volumes and growth rate increasing. The third passage
cells exhibited a long fusiform morphology. The results of ALP
staining, collagen type I immunocytochemical staining and calcium
nodule staining were positive, confirming that the cells were
osteoblasts.
Effects of porous tantalum extract on
osteoblast viability and growth
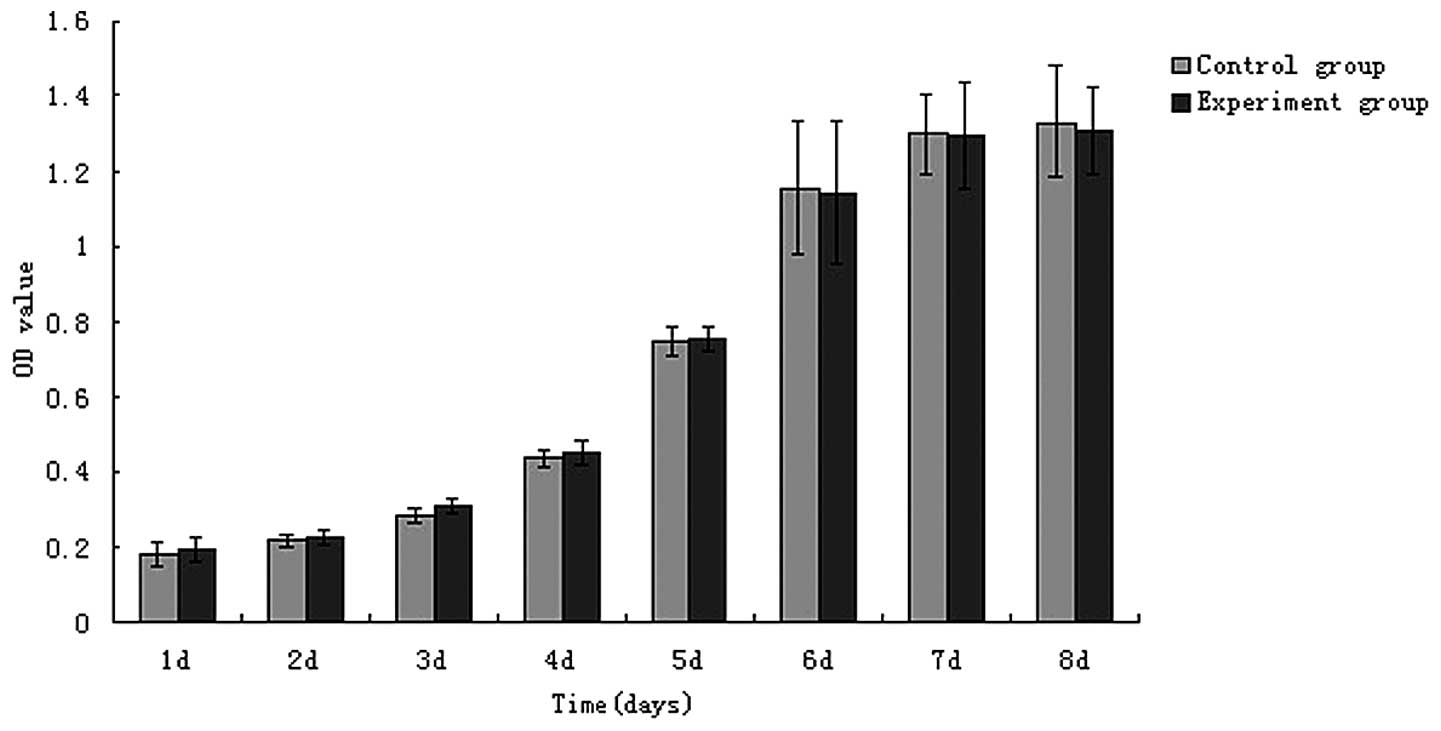
Absorbance values in the MTT assay showed that
between days 1 and 8 of osteoblast culture, osteoblast growth
changed from slow to rapid, and then to slow again, ultimately
entering a stable phase. Absorbance values for the control cells
and those exposed to porous tantalum extract at the same time
points were analyzed using an independent sample t-test, and the
results showed that the cell proliferation did not statistically
differ between the groups with extended cultivation time
(P>0.05). A cell growth curve was constructed based on the OD
values (Fig. 2). The results
demonstrated that tantalum metal ions were not cytotoxic to
osteoblasts, achieving the basic requirement of implant
materials.
Osteoblast culture on porous tantalum
samples for evaluation of cytocompatibility
Observation by inverted phase contrast microscopy
showed that in osteoblasts cultured on porous tantalum for one day,
a small number of cells began to adhere to the edge of the
material. In addition, cells of different sizes were dispersed on
the bottom of the culture plates, with cell processes exhibiting
fusiform or polygonal shapes. The number of cells adhering to the
edge increased and the cells proliferated rapidly between days 3
and 5, aggregating and fusing into flakes. Monolayers of cells were
formed in the bottom of the wells after seven days.
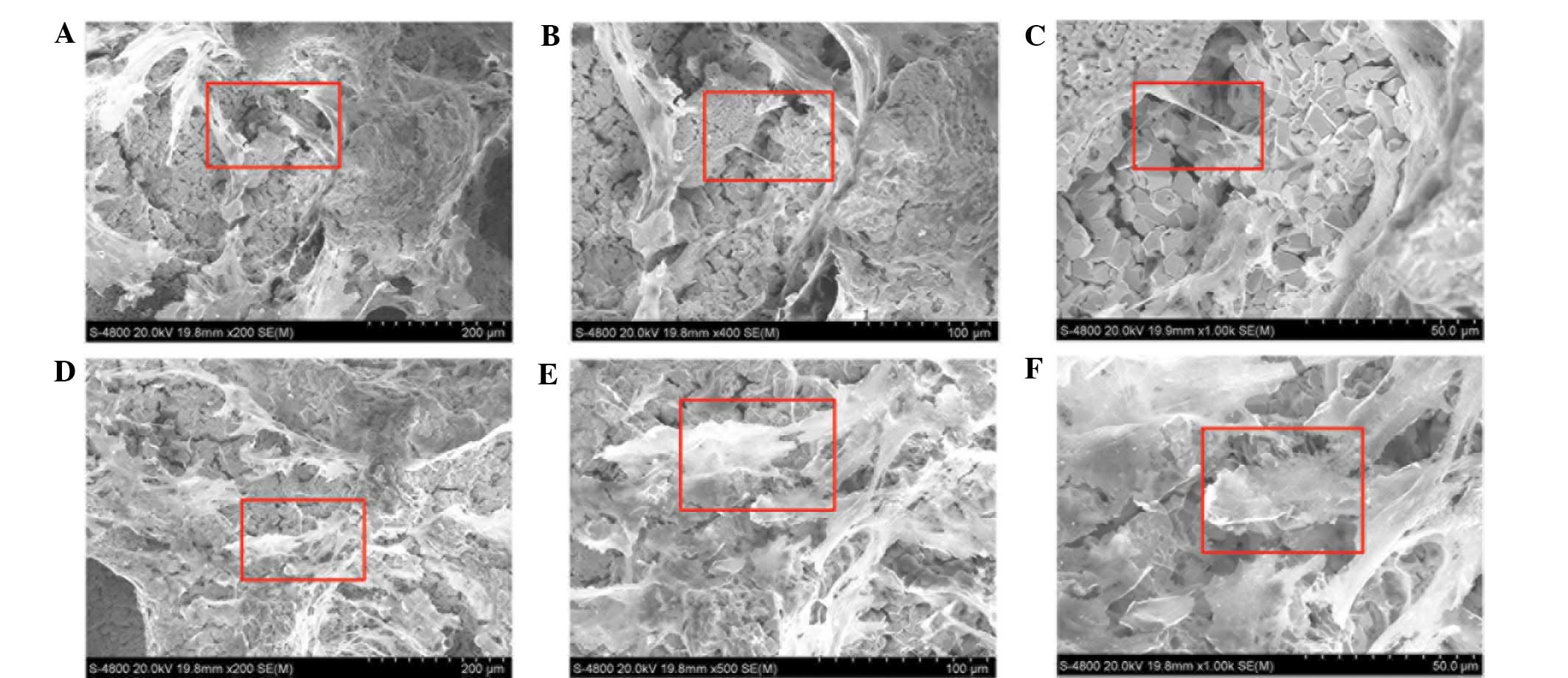
SEM revealed that the porous tantalum had numerous
pores. A small number of cells with varying morphologies were
sparsely arranged on the tantalum samples. By day 3, a few
protrusions grew adherently on the surface of the scaffolds and
within the scaffold pores. The cells stretched, lengthened and
extended pseudopodia gradually over time. By day 5, the processes
of adjacent cells connected with each other across the pores,
creating a flake with burr-like processes extending into the
surroundings. On day 10, cells on the surface and in the pores grew
into multiple layers, secreting matrix and covering the surface
completely (Fig. 3).
Morphological observations of the porous
tantalum implanted in the rabbit femoral condyle
Animals were conscious 30 min after the surgery and
exhibited recovery of knee movement. The incisions exhibited no
swelling, bleeding or oozing, and the wounds healed well. Loosening
of implantation materials was not observed. Implanted porous
tantalum rods were closely combined with the host bone at each time
point. No gap between the host bone and the tantalum implant was
visible by the naked eye and no adverse reactions, such as
infection, occurred.
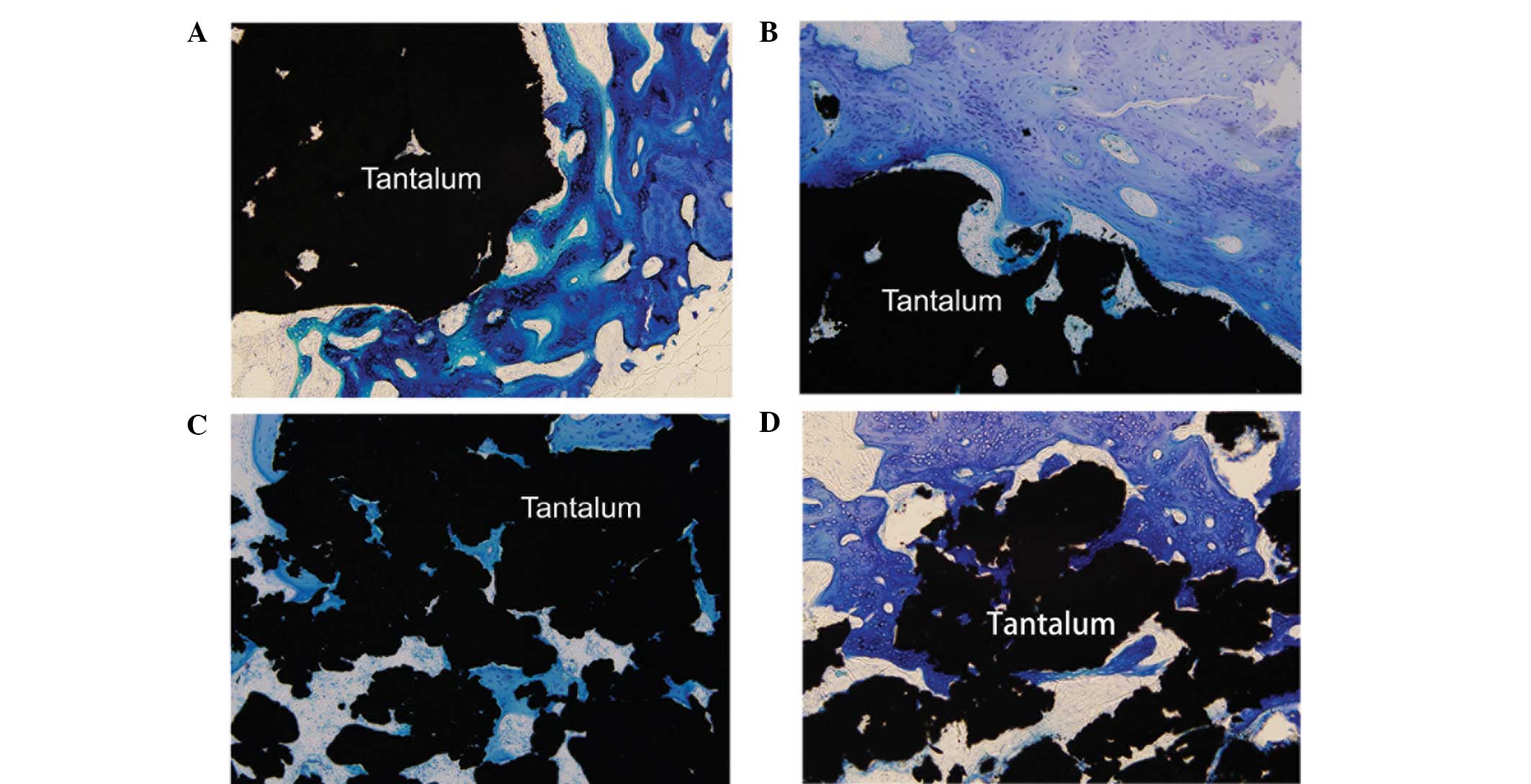
Observation of osteogenesis at the tantalum-host
bone interface in the hard tissue slices revealed that the porous
tantalum was in close contact with the bone tissue, as shown by
methylene blue staining.
During week 2, new bone tissue grew at the interface
of the tantalum and the host bone, on the surface of the material
and in the pores, with small blood vessels also growing in certain
areas. By week 4, the number of new bone cells at the interface
increased, and the cells connected with the host bone as flakes.
Bone collagen and the lacuna increased around the new trabecula, in
which bone cells were clearly observed. Between weeks 8 and 12, the
tantalum surface and pores were fully covered with new bone tissue.
New trabecula had matured and contacted the material directly,
presenting an interwoven, parallel arrangement, which covered the
surface and filled the pores. The tantalum and bone formed a solid
tantalum-bone direct bond, with no soft tissue layer at the
interface (Fig. 4).
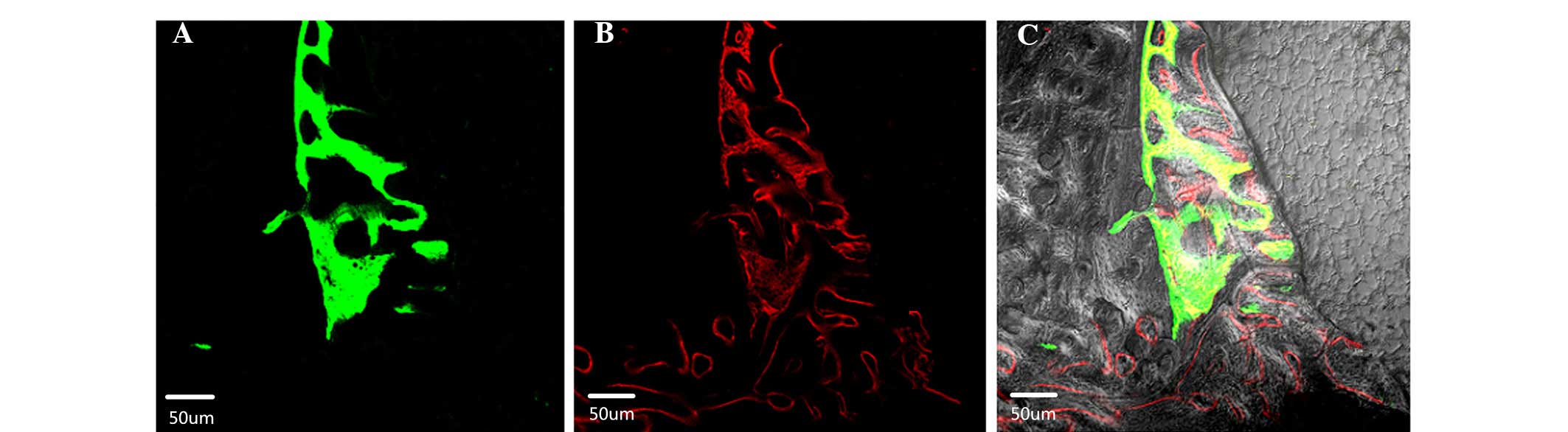
Observation by laser scanning confocal microscopy at
week 10 after the porous tantalum implantation revealed new bone
tissue, which was visible by green fluorescence (calcein) and red
fluorescence (alizarin red) at the tantalum-host bone interface and
within the pores. The red fluorescence band surrounded the green
band. The green and red fluorescent bands were discontinuous in the
early stage, but integrated by the late stage (Fig. 5).
Discussion
The porous tantalum used in the present study was
developed by Chongqing Runze Medical Devices Co. Ltd. The
appearance of the material is gray, bright and clean. Under SEM,
the material exhibits a three-dimensional spatial structure. Under
×500 magnification, pores with a diameter of 200–400 μm are visible
on the material surface, which are evenly distributed and
interconnected. The trabecula between the pores is rough,
presenting a micropore structure with a diameter of 100 μm. The
micropore structure is clearly visible under ×1,000 magnification,
with a diameter ranging between 50 and 200 μm. Since porous
tantalum has a three-dimensional geometric scaffold structure and
interconnected honeycomb pores, it offers a large internal surface
area, a three-dimensional structure and a good material-cell
interface on which osteoblasts are able to adhere, grow and develop
(16–18).
The roughness of the porous tantalum increases the
cell contact area, improving the adhesive strength of the
osteoblasts on the material surface. Tantalum surfaces ground by
grit and etched by acid have been shown to promote osteogenesis
(19,20). A study by Sagomonyants et al
(21) indicated that tantalum has
useful physical, chemical, mechanical and biological properties,
including a fully interconnected porous structure. This nanopore
structure has a large impact on osteoblast proliferation, and its
rough surface may absorb more large molecules, promoting cell
adhesion, proliferation and osteogenic capability. Tantalum also
facilitates the expression of osteoblast ALP and collagen type I.
In addition, bone tissue ingrowth and pore size are closely
associated. The microcolumn structure on the tantalum surface
promotes the overall osteogenic response. The high density per
volume of the column with a small diameter affords stronger
mechanical properties, resulting in more complete ingrowth of bone
tissue and better integration with the host tissue following
implantation. Thus, tantalum is particularly suitable for clinical
application as it cannot be degraded and supports bone function for
a long time (8).
In the present study, osteoblasts cultured in
vitro were seeded onto porous tantalum samples. Observation by
SEM showed that the cells adhered to the tantalum surface and pore
walls by day 3 of the culture, presenting various morphologies. The
cells proliferated further and numerous cells had merged into
flakes by day 7, while a number of cells secreted matrix, covering
the surface of the material. By day 10, the cells covering the
surface had secreted large amounts of matrix, which almost
completely covered the material. This result demonstrated that the
three-dimensional porous structure of the tantalum material
provided not only an ideal space for adhesion and proliferation,
but also promoted secretion and infiltration of nutrients and
metabolites, which demonstrates that Chinese porous tantalum offers
good biocompatibility.
The interaction between osteoblasts and implants is
an important focus of research into the compatibility of bone graft
materials. The basis of this interaction is the adhesion of cells
to the implant material. Cells must interact with the surface of
the scaffold, including adhering, extending, migrating,
differentiating and proliferating. Adhesion to a material,
including metal, can affect the proliferation and differentiation
of cells. The interaction between osteoblasts and the metallic
material depends largely on the surface properties of the material,
which include the geometric structure, porosity and chemistry. The
Chinese tantalum implantation material has a three-dimensional
structure and interconnected pores of sufficient size, which is
beneficial for bone cell adhesion and migration, and thus,
appropriate for osteogenesis. Therefore, this material may be used
as a bone graft substitute material.
The cytotoxicity test is one of the most important
detection indexes prior to the clinical application of biological
materials or medical devices, and is also the first test in
biological safety evaluation. Currently, there are two methods for
detecting cytotoxicity. The first is morphological observation,
which involves observing morphological changes and growth to
determine whether the material is toxic. The second method is to
evaluate viability with an MTT assay, which involves calculating
the relative cell proliferation rate based on light absorption
indirectly, in order to determine the level of cytotoxicity. Higher
cell proliferation rate was associated with lower toxicity of the
tantulum material (22). In the
present study, detection based on cytotoxicity test standards
outside the body from an international biological evaluation system
of medical devices showed that osteoblast proliferation increased
slowly in the early stage, increased rapidly in the middle stage
(days 4–7) and then increased slowly again. In the experimental and
control groups, osteoblasts maintained good morphology, with no
statistically significant difference observed in the OD values
between the groups (P>0.05). Therefore, the results indicated
that tantalum ions are not toxic to osteoblast cells. This result
was consistent with international standards; thus, Chinese porous
tantalum may be safe to implant in animals.
Requirements for bone graft materials include
excellent mechanical properties, a porous structure and the
capacity to support ingrowth of the host tissue. Compared with
other metallic materials, porous tantalum is stable and has a
higher porosity, larger pore size and a low elastic modulus
(7). Therefore, it is used in hip
and knee joint reconstruction surgery and in the production of
prostheses (23). In the present
study, hard tissue slicing technology combined with specific
pathological staining was applied. Light microscopy was used to
observe osteogenesis on the surface of the material at the
tantalum-bone interface. The results showed that the porous
tantalum-bone interface was the most active area of osteogenesis
during the early stages of culture. The morphological
characteristics of the new bone indicated that the osteoblasts were
able to adhere, grow and proliferate in the pores of the material
following the implantation of porous tantalum in the host bone, and
was accompanied by the ingrowth of small blood vessels. Bone
lacunae were observed in the new trabecula, in which new bone cells
were clearly visible. By week 12 after implantation, the surface
and the pores of the porous tantalum rods were fully covered with
new bone tissue, presenting an interwoven arrangement. Similarly to
common paraffin slices, hard tissue slices reflect only the local
osteogenic state at a particular point and are unable reveal the
complete osteogenic process. In the present study, rabbit hips were
injected intramuscularly with fluorescein calcein and alizarin red
on days 5 and 19 after implantation, respectively. New bone tissue
at the tantalum-bone interface and in the pores was observed in the
hard tissue slices using laser scanning confocal microscopy at
laser wavelengths of 488 nm (calcein) and 543 nm (alizarin red);
thus, the osteogenesis process was observed dynamically. On day 5,
the green fluorescent band of the immature bone cells marked by
calcein was observed, and a red fluorescent band of new bone tissue
marked by alizarin red was observed on day 19. The bands were in
direct contact with the surface of the tantalum and grew into the
pores. These results showed that osteogenesis occurred on the
surface of the host bone in the early stage after implantation and
that bone formation was time-dependent, which suggests that new
bone tissue at the tantalum-host bone interface matures over time,
with calcium deposition occurring later. The new bone and host bone
integrate and become difficult to distinguish. This form of
osteogenesis may explain the existence of a solid combination of
porous tantalum and bone, as well as bone conduction to a certain
extent.
When the pore diameter of a porous material is 15–40
μm, fibrous tissue has been reported to grow into the interior of
the material (24,25). When the diameter reaches 40–100 μm,
ingrowth of non-calcified bone-like tissue occurs. When the
diameter exceeds 150 μm, the pore structure of the scaffold may
fill completely with bone tissue, potentially facilitating an
improved osseointegrative effect. Chinese porous tantalum has a
high porosity (65–80%) and is able to induce the development of
fibrous tissue with rich blood vessels and bone tissue growth
inside its pores. Implanted tantalum and host bone may produce a
stable connection and integration. These results also further
confirm that porous tantalum offers good tissue compatibility and
promotes the adhesion, proliferation and differentiation of
osteoblasts. Therefore, Chinese porous tantalum is a suitable bone
graft substitute material for the construction of three-dimensional
structures for bone defect repair. In the present study, porous
tantalum was only observed in the animals for three months;
therefore, a longer observation period may be tested in future
studies. Furthermore, various metal implant materials were not
compared in the present study, such as porous titanium material,
which may be studied in future research.
References
|
1
|
Cardaropoli F, Alfieri V, Caiazzo F and
Sergi V: Manufacturing of porous biomaterials for dental implant
applications through selective laser melting. Adv Mat Res.
535–537:1222–1229. 2012. View Article : Google Scholar
|
|
2
|
Balla VK, Bodhak S, Bose S and
Bandyopadhyay A: Porous tamalum structures for bone implants:
fabrication, mechanical and in vitro biological properties. Acta
Biomater. 6:3349–3359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bobyn JD, Poggie RE, Krygier JJ, Lewallen
DG, Hanssen AD, Lewis RJ, Unger AS, O’Keefe TJ, Christie MJ, Nasser
S, Wood JE, Stulberg SD and Tanzer M: Clinical validation of a
structural porous tantalum biomaterial for adult reconstruction. J
Bone Joint Surg Am. 86-A(Suppl 2): 123–129. 2004.
|
|
4
|
Zhang HY, Luo JB, Zhou M, Zhang Y and
Huang YL: Biotribological properties at the stem-cement interface
lubricated with different media. J Mech Behav Biomed Mater.
20:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang HY, Zhang SH, Luo JB, Liu YH, Qian
SH, Liang FH and Huang YL: Investigation of protein adsorption
mechanism and biotribological properties at simulated stem-cement
interface. J Tribol. 135:0323012013. View Article : Google Scholar
|
|
6
|
Pohler OE: Unalloyed titanium for implants
in bone surgery. Injury. 31(Suppl 4): 7–13. 2000. View Article : Google Scholar
|
|
7
|
Matsuno H, Yokoyama A, Watari F, Uo M and
Kawasaki T: Biocompatibility and osteogenesis of refractory metal
implants, titanium, hafnium, niobium, tantalum and rhenium.
Biomaterials. 22:1253–1262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stiehler M, Lind M, Mygind T, Baatrup A,
Dolatshahi-Pirouz A, Li H, Foss M, Besenbacher F, Kassem M and
Bünger C: Morphology, proliferation, and osteogenic differentiation
of mesenchymal stem cells cultured on titanium, tantalum, and
chromium surfaces. J Biomed Mater Res A. 86:448–458. 2008.
View Article : Google Scholar
|
|
9
|
Cohen R: A porous tantalum trabecular
metal: basic science. Am J Orthop (Belle Mead NJ). 31:216–217.
2002.
|
|
10
|
Bobyn JD, Stackpool GJ, Hacking SA, Tanzer
M and Krygier JJ: Characteristics of bone ingrowth and interface
mechanics of a new porous tantalum biomaterial. J Bone Joint Surg
Br. 81:907–914. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zardiackas LD, Parsell DE, Dillon LD,
Mitchell DW, Nunnery LA and Poggie R: Structure, metallurgy, and
mechanical properties of a porous tantalum foam. J Biomed Mater
Res. 58:180–187. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang JC, Yu WD, Sandhu HS, Tam V and
Delamarter RB: A comparison of magnetic resonance and computed
tomographic image quality after the implantation of tantalum and
titanium spinal instrumentation. Spine (Phila Pa 1976).
23:1684–1688. 1998. View Article : Google Scholar
|
|
13
|
International Organization for
Standardization (ISO). Biological evaluation of medical devices -
Part 5: Tests for in vitro cytotoxicity. 3rd edition. ISO 10993-5.
2009
|
|
14
|
Wang N, Li H, Wang J, Chen S, Chen S, Ma Y
and Zhang Z: Study on the anticorrosion, biocompatibility, and
osteoinductivity of tantalum decorated with tantalum oxide nanotube
array films. ACS Appl Mater Interfaces. 4:4516–4523. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mrosek EH, Schagemann JC, Chung HW,
Fitzsimmons JS, Yaszemski MJ, Mardones RM, O’Driscoll SW and
Reinholz GG: Porous tantalum and poly-epsilon-caprolactone
biocomposites for osteochondral defect repair: preliminary studies
in rabbits. J Orthop Res. 28:141–148. 2010.
|
|
16
|
Li Y, Wei SB, Cheng XQ, Zhang T and Cheng
G: Corrosion behavior and surface characterization of tantalum
implanted TiNi alloy. Surf Coat Technol. 202:3017–3022. 2008.
View Article : Google Scholar
|
|
17
|
Christie MJ: Clinical applications of
trabecular metal. Am J Orthop (Belle Mead NJ). 31:219–220.
2002.
|
|
18
|
Jonitz A, Lochner K, Lindner T, Hansmann
D, Marrot A and Bader R: Oxygen consumption, acidification and
migration capacity of human primary osteoblasts within a
three-dimensional tantalum scaffold. J Mater Sci Mater Med.
22:2089–2095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hacking SA, Bobyn JD, Tanzer M and Krygier
JJ: The osseous response to corundum blasted implant surfaces in a
canine hip model. Clin Orthop Relat Res. 364:240–253. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang Z, Xie Y, Yang F, et al: Porous
tantalum coatings prepared by vacuum plasma spraying enhance bmscs
osteogenic differentiation and bone regeneration in vitro and in
vivo. PLoS One. 8:e662632013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sagomonyants KB, Hakim-Zargar M, Jhaveri
A, Aronow MS and Gronowicz G: Porous tantalum stimulates the
proliferation and osteogenesis of osteoblasts from elderly female
patients. J Orthop Res. 29:609–616. 2011. View Article : Google Scholar
|
|
22
|
Shimko DA, Shimko VF, Sander EA, Dickson
KF and Nauman EA: Effect of porosity on the fluid flow
characteristics and mechanical properties of tantalum scaffolds. J
Biomed Mater Res B Appl Biomater. 73:315–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levine BR, Sporer S, Poggie RA, Della
Valle CJ and Jacobs JJ: Experimental and clinical performance of
porous tantalum in orthopedic surgery. Biomaterials. 27:4671–4681.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barrère F, van der Valk CM, Meijer G,
Dalmeijer RA, de Groot K and Layrolle P: Osteointegration of
biomimetic apatite coating applied onto dense and porous metal
implants in femurs of goats. J Biomed Mater Res B Appl Biomater.
67:655–665. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wigfield C, Robertson J, Gill S and Nelson
R: Clinical experience with porous tantalum cervical interbody
implants in a prospective randomized controlled trial. Br J
Neurosurg. 17:418–425. 2003. View Article : Google Scholar : PubMed/NCBI
|