Introduction
Cervical cancer is one of the most common
gynecologic malignant tumors. It mainly results from chronic
infection with human papillomavirus (HPV), which leads to abnormal
metaplasia and subsequent cancer. In recent years, the age of
incidence of cervical cancer has been decreasing (1,2).
Currently, the clinical treatment of cervical cancer is focused on
surgery and chemotherapy, sometimes in combination with traditional
Chinese medicine treatment. However, the cure rate in patients with
advanced cervical cancer remains very low and the recurrence rate
is high (3). Cancer patients often
have severe cellular immune function defects, and cervical cancer
patients are no exception. Therefore, improving the cellular
immunity functions of cancer patients is essential to improve their
survival rate and cure rate. In recent years, the application of
biological treatment technology in tumor immunotherapy has been
constantly expanding and gradually accepted by medical personnel
and patients. This technology has become the fourth main treatment
option for tumors, following surgery, radiotherapy and
chemotherapy. Cytokine-induced killer (CIK) cells have become the
preferred type of cells used in antitumor adoptive cell
immunotherapy. CIK cells are heterogeneous cells that can be
generated from human peripheral blood mononuclear cells (PBMCs) by
culturing in the presence of a variety of cytokines in vitro
(4). CIK cells have the advantages
having the strong antitumor activity of T lymphocytes and a
non-major histocompatibility complex (MHC)-restricted tumoricidal
effect (5–7). Dendritic cells (DCs) are currently
the most powerful antigen-presenting cells (APCs) that have been
discovered. They can induce antigen-specific immune responses. A
co-culture of DCs and CIK cells has the advantages of fast
proliferation, high cytotoxicity and a broad tumor-killing spectrum
(8). In recent years, clinical
studies concerning the use of biological immunotherapy in the
treatment of cancer have been increasing; however, few of them have
focused on cervical cancer (9).
Through comparing the efficacy of chemotherapy alone with that of
biological immune therapy combined with chemotherapy on patients
with cervical cancer, the current study explored the effectiveness
of immunotherapy combined with chemotherapy on the immune function
of patients with cervical cancer and the recurrence rate of the
cancer.
Subjects and methods
Clinical data
A total of 79 patients with cervical cancer in
Suzhou Kowloon Hospital (Suzhou, China) from March 2008 to March
2010 participated in the study. The selection criteria of the
patients were as follows: i) diagnosed as cervical cancer by
pathology; ii) between the age of 30 and 70 years; iii) without
organ lesions, e.g., of the liver, kidney or heart; iv) without
cardiovascular and psychiatric disorders; and v) signed an
acknowledgment agreement. The patients were randomly divided into a
control group and an experimental group. The patients of the
control group were treated with chemotherapy, while the patients of
the experimental group were treated with biological immune therapy
combined with chemotherapy. The control group consisted of 39 cases
(age, 51.9±16.8 years), 30 of whom had squamous cell carcinoma and
nine of whom had adenocarcinoma. According to International
Federation of Gynecology and Obstetrics (FIGO) stage
classification, there were 16 cases in Stage IIa, 11 cases in Stage
IIb, seven cases in Stage IIIa, four cases in Stage IIIb, and one
case in Stage IV. The experimental group consisted of 40 cases
(age, 52.4±17.1 years), 31 of whom had squamous cell carcinoma and
nine of whom had adenocarcinoma. According to FIGO stage
classification, there were 15 cases in Stage IIa, 12 cases in Stage
IIb, eight cases in Stage IIIa, four cases in Stage IIIb and one
case in Stage IV. There were no significant differences between the
two groups in age, clinical stage or the degree of tumor invasion
(P>0.05). This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Suzhou Kowloon Hospital, Shanghai Jiao Tong University School of
Medicine. Written informed consent was obtained from all
participants.
Preparation of DC-CIK cells
Prior to chemotherapy, 1.5–4.0×109 PBMCs
were collected from each patient. During the collection of the
PBMCs, 60 ml plasma was isolated to prepare for cell reinfusion.
The collected PBMCs were centrifuged at 2,500 × g for 10 min, and
the supernatant was decanted. The sediment was washed with
phosphate-buffered saline (PBS) twice and then resuspended. After
that, the PBMCs were mixed with lymphocyte separation medium
(Dingguo Biotechnology Co., Beijing, China) in a 1:1 ratio,
centrifuged at 3,000 × g for 15 min and the supernatant was
decanted. The PBMCs were washed with normal saline three times, and
then resuspended in serum-free medium to modulate the cells. The
suspension was divided into two parts in the ratio of 1:9. The cell
density of the small part of the suspension was adjusted to
1–2×107/ml. Then, the suspension was seeded in 6-well
plates (2 ml/plate), adherently cultured for 2 h in an incubator
and then the non-adherent cells were sucked out. Serum-free medium
containing granulocyte-macrophage colony-stimulating factor (GMCSF;
800 U/ml; Dingguo Biotechnology Co.) and interleukin (IL)-4 (500
U/ml; Dingguo Biotechnology Co.) was added for culturing DCs, and
replaced every 3 days. On day 7, tumor necrosis factor (TNF)-α
(1,000 U/ml; Dingguo Biotechnology Co.) was added to promote
maturation. The large part of the suspension was mixed with
interferon (INF)-γ (1,000 U/ml; Dingguo Biotechnology Co.) and used
as CIK serum-free medium, in which IL-1α (100 U/ml), IL-2 (500
U/ml) and CD3 monoclonal mouse anti-human antibodies (Boster
Biotechnology Co., Wuhan, China) were added from day 2, and
replaced every 3 days. On day 8, the mature DCs were collected and
divided into two portions. One portion of the DCs was washed with
normal saline 3 times and then resuspended in 2 ml autologous
plasma. This was then used for administration to patients by
superficial lymph node or subcutaneous injection. The other portion
of the DCs was mixed and co-cultured with CIK cells at a ratio of
1:10 for 7 days to construct the DC-CIK cell mixture. Starting from
day 8, DC-CIK cells were reinfused to patients once a day for a
total of four times and the total number of cells was
2–3.5×1010. Prior to reinfusion, all cells underwent
bacterial, fungal and endotoxin detection tests and were found to
be negative.
Therapeutic methods
Patients in the control group were given
conventional chemotherapy with cisplatin (20 mg per day; Haoshen
Pharmaceutical Co., Nanjing, China). This involved dissolving 20 mg
cisplatin in 250 ml normal saline, and then administered by
intravenous drip within 2 h with the avoidance of light. One course
of treatment lasted for 10 days. Patients in the experimental group
underwent the collection of PMBCs 1 day prior to chemotherapy,
which were used for co-culture to generate DC-CIK cells. From the
second day, the experimental patients were given the same
chemotherapy as that given to the patients in the control group.
However, the experimental patients were also given a reinfusion of
DC-CIK cells after the chemotherapy. Three months later, all
patients from these two groups were given a second course of
treatment.
Observation indices and judgment of
curative effect
Prior to treatment and 2 weeks after treatment, 4 ml
peripheral blood was collected from each patient of the two groups.
The CD3+, CD4+, CD8+,
CD16+, CD56+ and
CD4+CD25+ cell ratios in peripheral blood
were detected by flow cytometry and the expression levels of
perforin, granzyme B (GraB) and CD107a in the PBMCs were observed.
The cumulative relapse rate, the cumulative survival rate and the
median survival time of the patients were also analyzed.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 13.0 (SPSS Inc., Chicago, IL, USA). Continuous
variables are presented as the mean ± standard deviation and were
tested with the Student’s t-test. Categorical variables were tested
with the Chi-square test. Cumulative recurrence rate and cumulative
survival rate were compared with the Kaplan-Meier method and
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
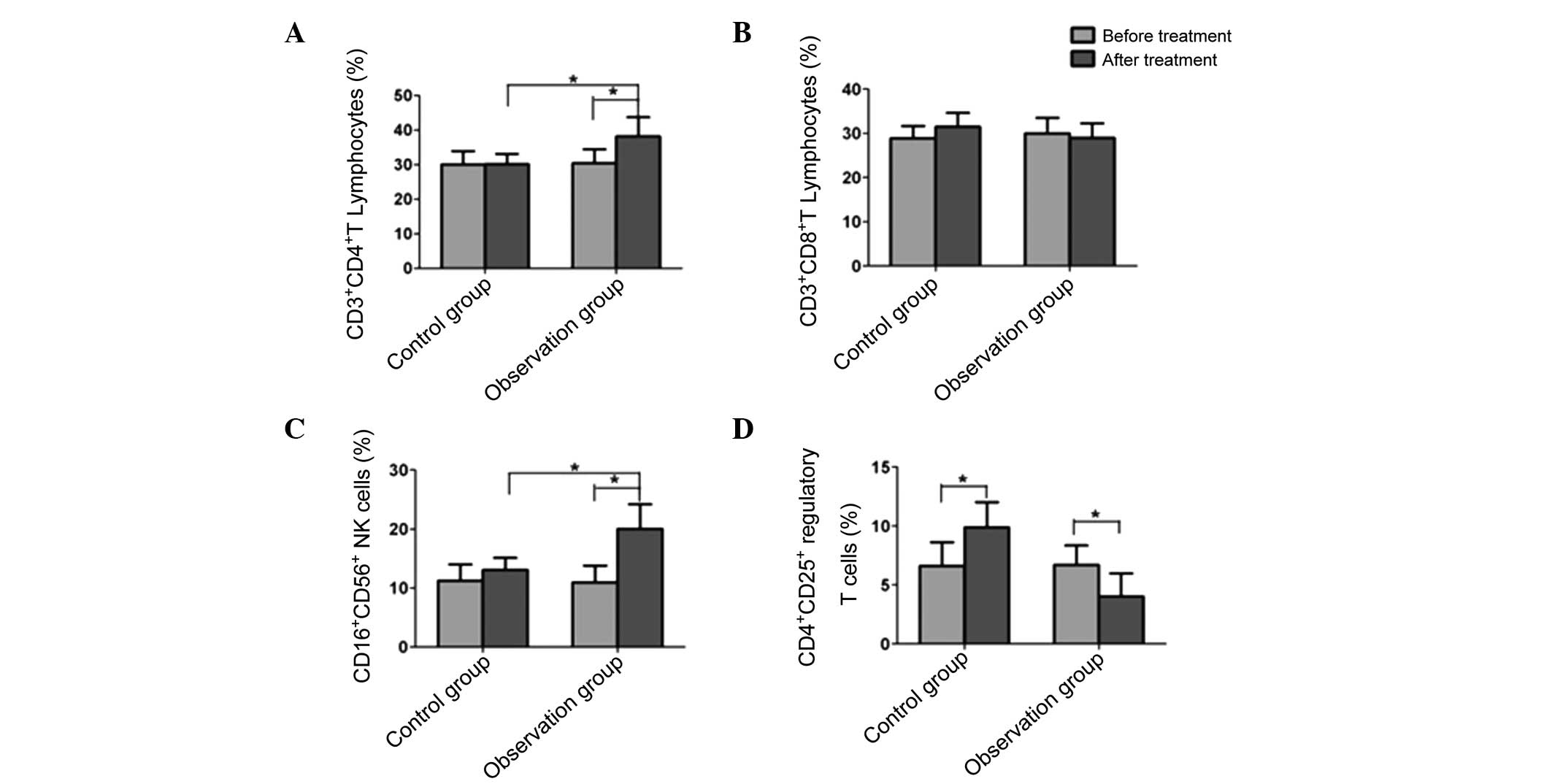
Changes in peripheral blood T-lymphocyte
subsets in the two groups prior to and following treatment
Prior to treatment, the
CD3+CD4+, CD3+CD8+,
CD16+CD56+ and
CD4+CD25+ cell levels exhibited no
significant differences between the experimental and control groups
(P>0.05). In the patients of the control group, the
CD3+CD4+, CD3+CD8+ and
CD16+CD56+ cell levels had no significant
differences prior to and following treatment, but the
CD4+CD25+ regulatory T-cell ratio
significantly increased after treatment (P<0.05). Following
treatment, the CD3+CD4+ and
CD16+CD56+ cell levels in the experimental
group were significantly higher than those prior to treatment
(P<0.05), but the CD3+CD8+ cell level did
not significantly differ from that prior to treatment. In addition,
the CD4+CD25+ regulatory T-cell ratio
following treatment was significantly reduced in the experimental
group compared with that prior to treatment (Fig. 1).
Changes in the expression levels of
perforin, GraB and CD107a in the PBMCs of the two groups prior to
and following treatment
The expression levels of perforin, GraB and CD107a
in the PBMCs of the control group after treatment were
significantly decreased compared with those prior to treatment
(P<0.05). However, in the experimental group, the expression
levels of perforin, GraB and CD107a significantly increased after
treatment compared with those prior to treatment (P<0.05;
Fig. 2).
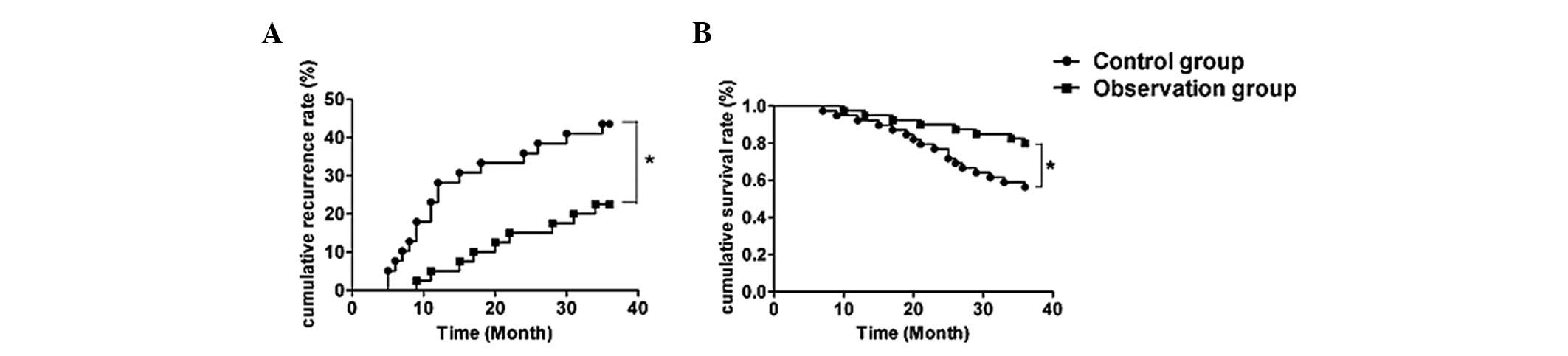
Comparison of the cumulative recurrence
rate and cumulative survival rate between the two groups
Patients from the two groups were followed up for
more than 3 years after treatment. Eighteen recurrent cases were
found in the control group but only nine recurrent cases were found
in the experimental group. Kaplan-Meier curves demonstrated that
the cumulative 1-year, 2-year and 3-year recurrence rates in the
control group were 28.2% (11 cases), 35.9% (14 cases) and 46.2% (18
cases), respectively. In the experimental group, the cumulative
recurrence rates were 5% (2 cases), 15% (6 cases) and 22.5% (9
cases), respectively. The cumulative recurrence rates between the
control and experimental groups were significantly different when
compared by log-rank test (P<0.05). The cumulative 1-year,
2-year and 3-year survival rates in the control group were 92.31,
76.92 and 56.41%, respectively. In the experimental group, the
cumulative survival rates were 97.25, 90 and 80%, respectively.
Log-rank test showed that the cumulative survival rate of the
experimental group was significantly different from that of the
control group (P<0.05; Fig.
3).
Discussion
Cervical cancer is one of the most common malignant
tumors of females. The incidence rate of this cancer is second only
to that of breast cancer, but the mortality rate is the highest
among the malignant gynecological tumors (10). In the treatment of cervical cancer,
the high recurrence rate following treatment is a major problem.
Currently, platinum chemotherapy-based comprehensive treatment is
the preferred method for treating recurrent cervical cancer, but
the total efficiency is only 20–30% (11). Moreover, since the tumor volume is
relatively large in the advanced stages, patients are susceptible
to immune suppression during the chemotherapy process (12,13).
Therefore, a feasible and acceptable treatment strategy is urgently
required for the clinical treatment of advanced cervical cancer. In
recent years, numerous clinical data have shown that the immune
function of patients with cervical cancer is suppressed
significantly due to disorders of cellular immune function
(14). This phenomenon is
particularly common in patients with advanced cancer (15).
Bio-immunotherapy has been widely applied to treat
different types of tumors. Its effectiveness in killing and
inhibiting tumors and advantage in improving the immune function of
patients undergoing chemotherapy have been demonstrated by clinical
researchers (16–18). Yang et al (19) applied autologous CIK cell infusion
combined with cisplatin therapy to treat 20 patients with advanced
cervical cancer and observed the results after one month. The
authors found that the TH1/TH2 immune index and the number of
natural killer cells increased significantly following the combined
treatment, compared with those following cisplatin chemotherapy
alone. Their study demonstrated that combined treatment with CIK
cells and chemotherapy was able to improve the immune function of
patients with cervical cancer; however, follow-up results and the
3-year recurrence rate were lacking. Long-term follow-up of the
effectiveness of CIK cell treatment combined with chemotherapy is
necessary. Zhu et al (20)
applied a combined treatment comprising autoimmune cells and
radiotherapy to treat cervical cancer. The results demonstrated
that the immune function of the patients that received the
combination therapy was significantly higher than that of the
patients treated with radiotherapy alone. In addition, 5-year
follow-up results showed that the quality of life in the majority
of the patients that received the combined treatment was
significantly improved, and the Karnofsky score and the overall
1-year, 2-year and 5-year survival rates of the patients treated
with the combination therapy were significantly higher than those
of the patients treated with radiotherapy alone. A study conducted
by Zhu et al (20)
demonstrated that radiotherapy combined with immune cell therapy
for the treatment of cervical carcinoma was able to enhance the
immune function of patients and prolong their survival periods.
Laurin et al (21) observed that CIK cells expressed
high levels of perforin and GraB, and so were able to induce tumor
cell apoptosis through an extracellular apoptosis pathway.
Hackstein and Thomson (22)
revealed that the cytotoxic activity of CIK cells was remarkably
enhanced following co-culture with DCs and stimulation by DCs. It
was demonstrated that co-cultures of DCs and CIK cells not only
have non-MHC-restricted CIK cell cytotoxicity but also are able to
stimulate the MHC-restricted cytotoxic effect mediated by
antigen-loaded DC cells. Therefore, the specific killing effects
were enhanced. In the present study, the effectiveness of a DC-CIK
cell co-culture combined with cisplatin chemotherapy in the
treatment of cervical cancer was observed, and it was found that
the CD3+CD4+ and
CD16+CD56+ cell levels in patients following
treatment were significantly higher than those prior to treatment,
but the proportion of CD4+CD25+ regulatory T
cells significantly decreased following treatment (P<0.05),
which corresponds well with the results of Yang et al
(23). Moreover, in the present
study, it was observed that the perforin, GraB and CD107a positive
expression rates were significantly higher in the combined
treatment group than in the control group (P<0.05), and the
cumulative 1-year, 2-year and 3-year recurrence rates were
significantly reduced in the combined treatment group compared with
those in the control group (P<0.05).
In conclusion, this study confirmed the
effectiveness of biological immune treatment, particularly
biological immune treatment combined with chemotherapy, in the
treatment of tumors, and provides further evidence to support its
clinical application in the treatment of cervical cancer.
References
|
1
|
Bynum SA, Brandt HM, Sharpe PA, Williams
MS and Kerr JC: Working to close the gap: identifying predictors of
HPV vaccine uptake among young African American women. J Health
Care Poor Underserved. 22:549–561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang XC and Wu MY: Progress. on
DC-CIK-based adoptive cellular immunotherapy for uterine cervical
neoplasm. Guangdong Yao Xue Yuan Xue Bao. 29:575–579. 2013.(In
Chinese).
|
|
3
|
Tewari KS and Monk BJ: New strategies in
advanced cervical cancer: from angiogenesis blockade to
immunotherapy. Clin Cancer Res. 20:5349–5358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang FS, Liu MX, Zhang B, et al: Antitumor
activities of human autologous cytokine-induced killer (CIK) cells
against hepatocellular carcinoma cells in vitro and in vivo. World
J Gastroenterol. 8:464–468. 2002.PubMed/NCBI
|
|
5
|
Itsumi M and Tatsugami K: Immunotherapy
for renal cell carcinoma. Clin Dev Immunol. 2010:2845812010.
View Article : Google Scholar
|
|
6
|
Kim HM, Lim J, Kang JS, et al: Inhibition
of human cervical carcinoma growth by cytokine-induced killer cells
in nude mouse xenograft model. Int Immunopharmacol. 9:375–380.
2009. View Article : Google Scholar
|
|
7
|
Hontscha C, Borck Y, Zhou H, Messmer D and
Schmidt-Wolf IG: Clinical trials on CIK cells: first report of the
international registry on CIK cells (IRCC). J Cancer Res Clin
Oncol. 137:305–310. 2011. View Article : Google Scholar
|
|
8
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhargava A, Bunkar N, Khare NK, Mishra D
and Mishra PK: Nanoengineered strategies to optimize dendritic
cells for gastrointestinal tumor immunotherapy: from biology to
translational medicine. Nanomedicine (Lond). 9:2187–2202. 2014.
View Article : Google Scholar
|
|
10
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kastritis E, Bamias A, Bozas G, et al: The
impact of age in the outcome of patients with advanced or recurrent
cervical cancer after platinum-based chemotherapy. Gynecol Oncol.
104:372–376. 2007. View Article : Google Scholar
|
|
12
|
Motz GT and Coukos G: Deciphering and
reversing tumor immune suppression. Immunity. 39:61–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindau D, Gielen P, Kroesen M, Wesseling P
and Adema GJ: The immunosuppressive tumour network: myeloid-derived
suppressor cells, regulatory T cells and natural killer T cells.
Immunology. 138:105–115. 2013. View Article : Google Scholar :
|
|
14
|
Hasan UA, Bates E, Takeshita F, et al:
TLR9 expression and function is abolished by the cervical
cancer-associated human papillomavirus type 16. J Immunol.
178:3186–3197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Yu W, Li H, Yu J, Zhang X, Ren X
and Cao S: Can the dual-functional capability of CIK cells be used
to improve antitumor effects? Cell Immunol. 287:18–22. 2014.
View Article : Google Scholar
|
|
16
|
Wang QJ, Wang H, Pan K, et al: Comparative
study on anti-tumor immune response of autologous cytokine-induced
killer (CIK) cells, dendritic cells-CIK (DC-CIK) and
semi-allogeneic DC-CIK. Chin J Cancer. 29:641–648. 201
|
|
17
|
Zheng YW, Li RM, Zhang XW and Ren XB:
Current adoptive immunotherapy in non-small cell lung cancer and
potential influence of therapy outcome. Cancer Invest. 31:197–205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren J, Di L, Song G, Yu J, et al:
Selections of appropriate regimen of high-dose chemotherapy
combined with adoptive cellular therapy with dendritic and
cytokine-induced killer cells improved progression-free and overall
survival in patients with metastatic breast cancer: reargument of
such contentious therapeutic preferences. Clin Transl Oncol.
15:780–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang HZ, Shi N, Li LN, Kang H and Yang LN:
Clinical research of interventional treatment combined with CIK in
patients with uterine cervix cancer. Haerbin Yi Ke Da Xue Xue Bao.
44:263–266. 2010.(In Chinese).
|
|
20
|
Zhu YH, Liu JQ, Cao H, et al: Clinical
efficiency of radiotherapy combined with self-immune cell therapy
for cervical carcinoma. Zhongguo Zhong Liu Sheng Wu Zhi Liao Za
Zhi. 19:421–427. 2012.(In Chinese).
|
|
21
|
Laurin D, Marin V, Biagi E, et al:
Exploration of the lysis mechanisms of leukaemic blasts by
chimaeric T-cells. J Biomed Biotechnol. 2010:2345402010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hackstein H and Thomson AW: Dendritic
cells: emerging pharmacological targets of immunosuppressive drugs.
Nat Rev Immunol. 4:24–34. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L, Ren B, Li H, Yu J, Cao S, Hao X
and Ren X: Enhanced antitumor effects of DC-activated CIKs to
chemotherapy treatment in a single cohort of advanced
non-small-cell lung cancer patients. Cancer Immunol Immunother.
62:65–73. 2013. View Article : Google Scholar
|