Introduction
Myocardial ischemia refers to the absolute or
relative lack of coronary blood supply, or transient or chronic
myocardial ischemia caused by interruption to the coronary blood
supply and hypoxia. This ischemia leads to metabolic disorder of
myocardial cells and the accumulation of metabolites, thus causing
myocardial injury, or even myocardial necrosis, and thereby
affecting cardiac function. Myocardial ischemia clinically
manifests as syndromes such as angina pectoris and myocardial
infarction. Long-term myocardial ischemia can result in cardiac
fibrosis and enlargement of the heart, causing arrhythmia or heart
failure, and even resulting in mortality; therefore, it is a
serious threat to human health (1).
For hundreds of years, it has been believed that
hydrogen sulfide (H2S) is a colorless toxic gas with a
smell of rotten eggs, which, when over-inhaled, can suppress the
central nervous and respiratory systems. Studies on H2S
have been confined to its toxic effect (2,3);
however, since the mid-1990s, when it began to be recognized that
H2S could promote long-term potentiation in the
hippocampus (4,5), there has been increasing evidence
that the gas has an important physiological role in the body
(6), particularly in the
cardiovascular and central nervous systems (7,8).
H2S is the third novel gaseous signaling molecule,
following nitric oxide and carbon monoxide (9,10).
In mammals, the endogenous H2S is mainly generated by
the metabolism of sulfur-containing amino acids, such as
L-cysteine; cystathionine-β-synthase (CBS) and
cystathionine-γ-lyase (CSE) are the key enzymes in H2S
generation (7). It has been found
that H2S not only exerts cardiovascular effects in the
cardiovascular system, such as relaxation of vascular smooth
muscle, lowering blood pressure, inhibition of vascular smooth
muscle cell proliferation and regulation of cardiac contractility,
but also is involved in pathophysiological processes, such as
hypertension, pulmonary hypertension, acute myocardial infarction
and ischemia/reperfusion injury. The incidence and development of
myocardial ischemia are complex (11–17).
In a previous model, H2S was found to exert
anti-inflammatory effects (18).
It has been reported that, in a myocardial ischemia/reperfusion
model, the protective effect of sodium hydrosulfide (NaHS) on
myocardial tissues is associated with its anti-inflammatory effects
(19; however, it remains unclear whether the protective effect of
H2S in rats with acute myocardial ischemia is associated
with its regulation of inflammatory cytokines. In the present
study, therefore, an animal model of acute myocardial ischemia was
established in rats by ligation of the coronary artery, in order to
observe the effects of the H2S donor NaHS and the CSE
inhibitor propargylglycine (PPG) on inflammatory cytokines, such as
tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and
nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB),
and intercellular adhesion molecule-1 (ICAM-1) in the presence of
myocardial ischemia. Furthermore, the effect of H2S in
rats with acute myocardial ischemia was explored, as well as the
possible underlying mechanism.
Materials and methods
Drugs and reagents
NaHS and PPG were purchased from Sigma (St. Louis,
MO, USA); primers for ICAM-1 and β-actin were obtained from
Shanghai Generay Biological Engineering Co., Ltd. (Shanghai,
China); the SV Total RNA Isolation system, as well as TaqDNA
polymerase, agarose and ethidium bromide, were purchased from
Promega Corp. (Madison, WI, USA); the RevertAid First Strand cDNA
Synthesis kit used for reverse transcription (RT) was purchased
from Thermo Scientific (Waltham, MA, USA); DNA marker was obtained
from Beijing SBS Genetech Co., Ltd. (Beijing, China). The
polymerase chain reaction (PCR) primers, which were synthesized by
Shanghai Generay Biological Engineering Co., Ltd., were as follows:
ICAM-1 sense, 5′-AAGGTGTGATATCCGGTAGA-3′ and antisense,
5′-CCTTCTAAGTGGTTGGAACA-3′, β-actin sense,
5′-CGTTGACATCCGTAAAGAC-3′ and antisense, 5′-CTGGAAGGTGGACAGTGAG-3′.
A nuclear protein/plasma protein extraction kit was purchased from
Beijing Chong League International Biological Gene Technology Co.,
Ltd. (Beijing, China); rabbit anti-rat NF-κB p65 polyclonal
antibody was obtained from Santa Cruz Biotechnology, Inc. (sc-109;
1,100; Santa Cruz, CA, USA); rat β-actin polyclonal antibody
(sc-130657) was also obtained from Santa Cruz Biotechnology, Inc.
and rat serum TNF-α, IL-1β and IL-6 ELISA detection kits were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Experimental animals
Healthy male Sprague Dawley (SD) rats weighing
270±20 g were provided by the Experimental Animal Center of Hebei
Province (Shijiazhuang, China). The present study was approved by
the Ethics Committee of The Fourth Hospital of Hebei Medical
University (Shijiazhuang, China).
Experimental models and animal
grouping
Thirty-six male SD rats were randomly divided into
sham surgery, ischemia, ischemia + low-, middle- and high-dose NaHS
and ischemia + PPG groups (n=6). The acute myocardial ischemia
model was established by ligating the left anterior descending
coronary artery (LAD) of the rats. In the sham surgery group, the
LADs were not ligated but only threaded. Saline was
intraperitoneally administered to the rats in the ischemia group.
In the ischemia + low-, middle- and high-dose NaHS groups and the
ischemia + PPG group, NaHS (0.78, 1.56 or 3.12 mg/kg) or PPG (30
mg/kg), respectively, was intraperitoneally injected 3 h after the
induction of ischemia. The rats were sacrificed 6 h after the
surgery.
Detection indicators and methods
Observation of morphological changes
in myocardial tissue by transmission electron microscopy (TEM)
At the end of the ischemia, apical tissues were
taken rapidly, rinsed with normal saline to remove the blood, cut
into small slices measuring 1×1×1 mm and placed on ice. The samples
were then fixed in 4% glutaraldehyde, rinsed twice with 0.1 mol/l
cacodylate buffer (Yongda Chemical Reagent Co., Ltd., Tianjin
China), fixed with 1% osmium tetroxide and then washed with buffer.
The samples were subsequently progressively dehydrated in acetone,
impregnated in epoxy, embedded, cut into ultrathin slices and then
stained in uranyl acetate-lead citrate. Changes in the myocardial
ultrastructure were observed through TEM.
Determination of TNF-α, IL-6 and IL-1β
levels in the serum
At the end of the ischemia, blood was taken from
rats in each group via the right carotid artery, and serum was
separated through centrifugation at 1,006 × g for 15 min at 4°C.
Double-antibody sandwich ELISA was employed for the detection of
TNF-α, IL-6 and IL-1β levels in the serum, in accordance with the
manufacturer’s instructions (R&D Systems, Inc.). Optical
density values were determined by ELISA and the standard curve was
drawn to calculate TNF-α, IL-6 and IL-1β concentrations in the
sample.
Detection of ICAM-1 mRNA expression in
myocardial tissue by semi-quantitative RT-PCR
The RNA extraction kit was used to extract total RNA
from the myocardial tissues, and RNA then served as a template to
obtain cDNA by RT with the RT-PCR kit (Promega Corp.). β-actin
served as a reference gene. The 50-μl PCR reaction system comprised
25 μl Go Taq® Green Master Mix, 1 μl upstream primer, 1
μl downstream primer, 4 μl DNA template and 19 μl nuclease-free
water. The reaction conditions were as follows: Initial
denaturation at 94°C for 4 min; 35 cycles of 94°C for 45 sec, 60°C
for 60 sec and 72°C for 90 sec; 72°C for a further 7 min. The PCR
product was analyzed using electrophoresis in a 1% agarose gel and
then placed in a gel image analysis system (T-05×20-2A; Vilber
Lourmat Co., Marne-la-Vallee, France) for an absorbance scan.
β-actin served as a reference for calibration, and the ratio of the
absorbance of the target genes to that of β-actin suggested the
relative expression levels of the target genes.
Detection of NF-κB expression in
myocardial tissues by western blotting
The cell lysate was added into myocardial tissues
that had been cut and nuclear proteins were extracted in accordance
with the kit manufacturer’s instructions (Beijing Chong League
International Biological Gene Technology Co., Ltd.). The
bicinchoninic acid assay was used to measure protein concentration.
Nuclear protein samples were taken, analyzed by the method of gel
electrophoresis in 10% sodium dodecyl sulfate-polyacrylamide, and
then electrically transferred to a polyvinylidene difluoride
membrane. The samples were subsequently mixed with anti-NF-κB p65
polyclonal antibody (1:100 dilution)/β-actin polyclonal antibody
(1:500) and kept at 4°C overnight. Following incubation,
chemiluminescence, developing and fixing were performed.
AlphEaseFC™ software (Alpha Innotech, San Leandro, CA, USA) was
employed to analyze the results, and the ratio of the gray value of
each target band to that of β-actin protein was provided to analyze
the protein of interest.
Statistical analysis
Experimental data are presented as the mean ±
standard error of the mean. SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA) processing was used for statistical analysis.
Comparisons were conducted using one-way analysis of variance, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
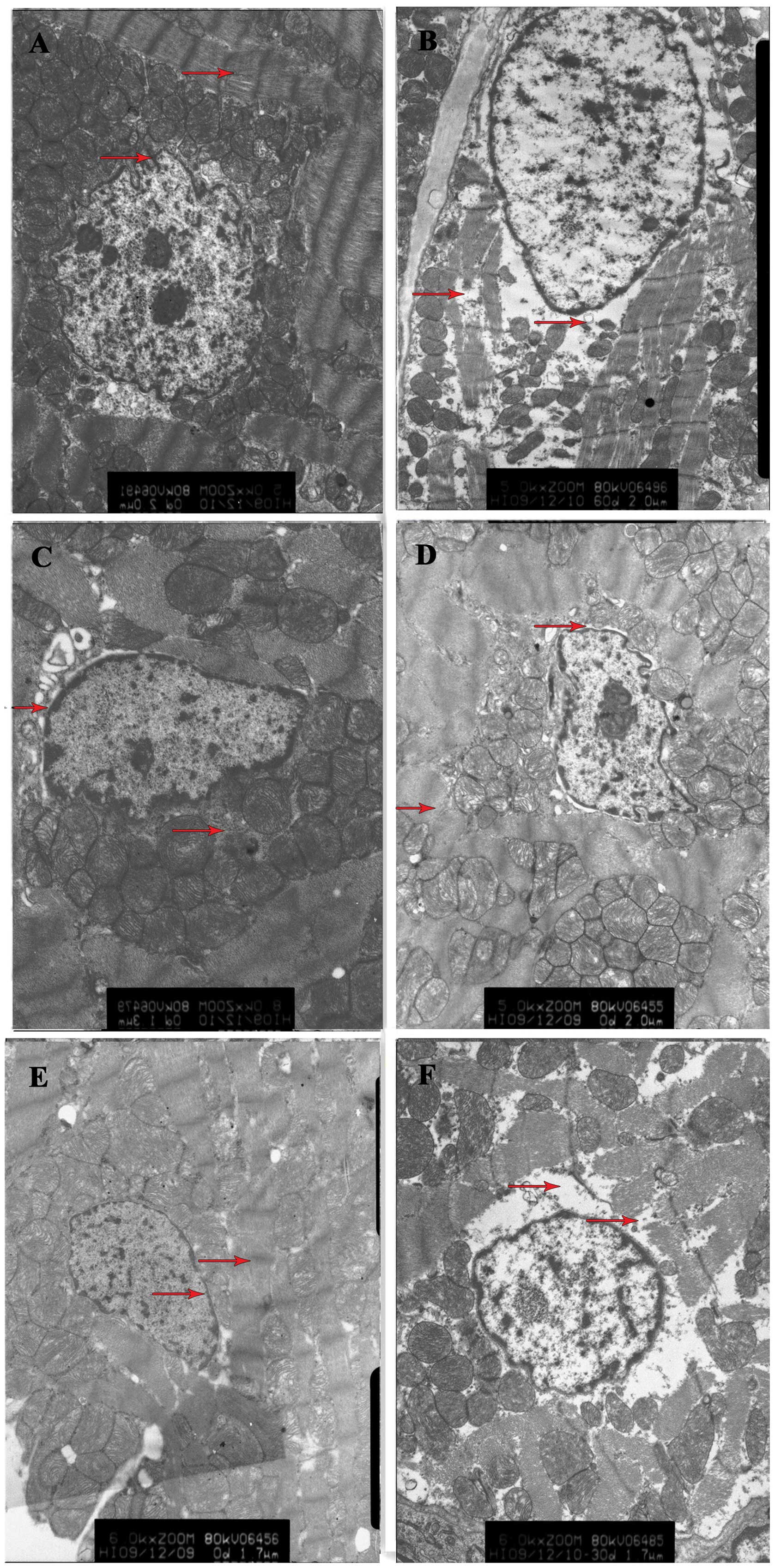
Ultrastructural changes in the myocardial
tissue
In the rats from the sham surgery group, neatly
arranged myocardial fibers, integrated mitochondrial cristae and
membranes and a slight expansion of the perinuclear space were
observed. In rats from the ischemia group, it was noted that there
was myocardial fiber disarray, severe edema in the karyoplasm and
perinuclear space and partial disappearance of the nuclear
membrane; there was also severe swelling, deformation and
dissolution and disappearance of the mitochondrial cristae and
membrane. Compared with that in the ischemia group, the cardiac
damage in the ischemia + low-, medium- and high-dose NaHS groups
was significantly reduced, particularly in the high-dose group;
slightly disordered muscle fiber arrangement and mild edema in the
mitochondrial matrix were also observed. In the ischemia + PPG
group, the degree of myocardial injury was aggravated compared with
that in the ischemia group (Fig.
1).
Changes in TNF-α, IL-1β and IL-6 levels
in the serum
Compared with the sham surgery group, the TNF-α,
IL-1β and IL-6 serum levels in the rats were significantly elevated
in the ischemia group (P<0.01). Compared with the ischemia
group, the IL-1β and IL-6 serum levels were significantly reduced
in the ischemia + low-, medium- and high-dose NaHS groups; in the
ischemia + medium- and high-dose NaHS groups the TNF-α level in the
serum was significantly reduced. In the ischemia + PPG group, the
serum levels of TNF-α, IL-1β and IL-6 were significantly increased
compared with those in the ischemia group (P<0.05 or P<0.01)
(Fig. 2 and Table I).
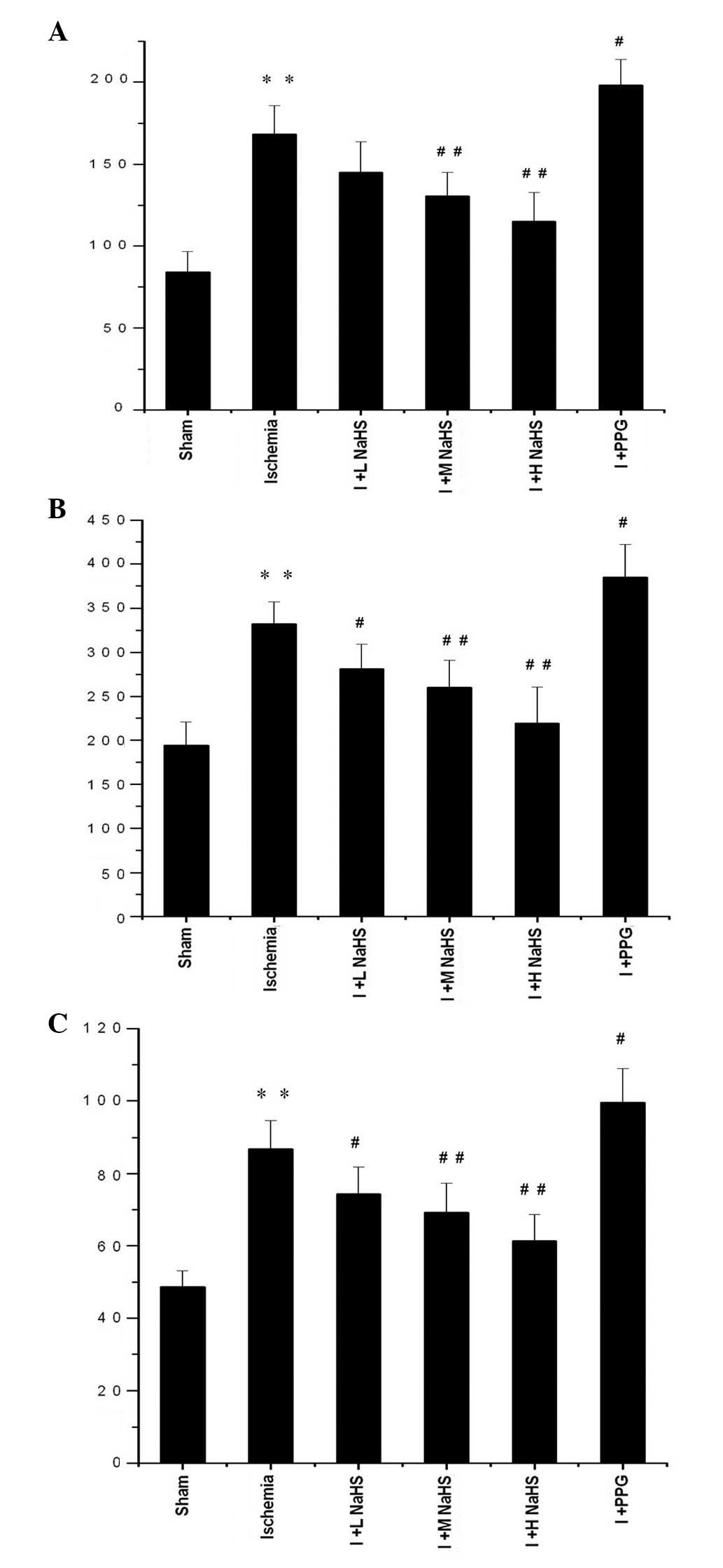 | Figure 2Effect of hydrogen sulfide on the
change in the serum levels of (A) tumor necrosis factor-α, (B) IL-6
and (C) IL-1β in rats. Data are presented as the mean ± standard
error of the mean (n=6) with the units of ng/l.
**P<0.01 vs. the sham group; #P<0.05
and ##P<0.01 vs. the ischemia group. Sham, rats
underwent the surgical procedures but without the ischemic insult,
followed by treatment with saline; Ischemia, rats underwent the
surgical procedures and were then treated with saline; I + L NaHS,
ischemic rats treated with 0.78 mg/kg NaHS; I + M NaHS, ischemic
rats treated with 1.56 mg/kg NaHS; I + H-NaHS, ischemic rats
treated with 3.12 mg/kg NaHS; I + PPG, ischemia rats treated with
30 mg/kg PPG; NaHS, sodium hydrosulfide; PPG, propargylglycine; IL,
interleukin. |
 | Table IEffect of hydrogen sulfide on the
serum levels of TNF-α, IL-6 and IL-1β (n=6). |
Table I
Effect of hydrogen sulfide on the
serum levels of TNF-α, IL-6 and IL-1β (n=6).
| Group | TNF-α (ng/l) | IL-6 (ng/l) | IL-1β (ng/l) |
|---|
| Sham | 84.03±12.49 | 194.36±26.32 | 48.67±4.50 |
| Ischemia |
168.47±17.13a |
332.47±24.88a | 86.79±7.82a |
| I + L NaHS | 145.00±18.65 |
281.15±28.34b | 74.41±7.43b |
| I + M NaHS |
130.56±14.37c |
260.15±30.94c | 69.22±8.18c |
| I + H NaHS |
114.93±17.85c |
219.25±41.50c | 61.32±7.34c |
| I + PPG |
198.06±15.85b |
384.71±37.55b | 99.45±9.48b |
Changes in ICAM-1 mRNA expression in the
myocardial tissue
In the ischemia group the ICAM-1 mRNA expression in
the myocardial tissues of the rats was significantly increased
compared with that in the sham surgery group (P<0.01). Compared
with the ischemia group, the ICAM-1 mRNA expression in the
myocardial tissues of the rats was reduced in the ischemia + low-,
medium- and high-dose NaHS groups (P<0.05 or P<0.01); ICAM-1
mRNA expression was increased markedly, but not significantly, in
the ischemia + PPG group (P>0.05) (Fig. 3 and Table II).
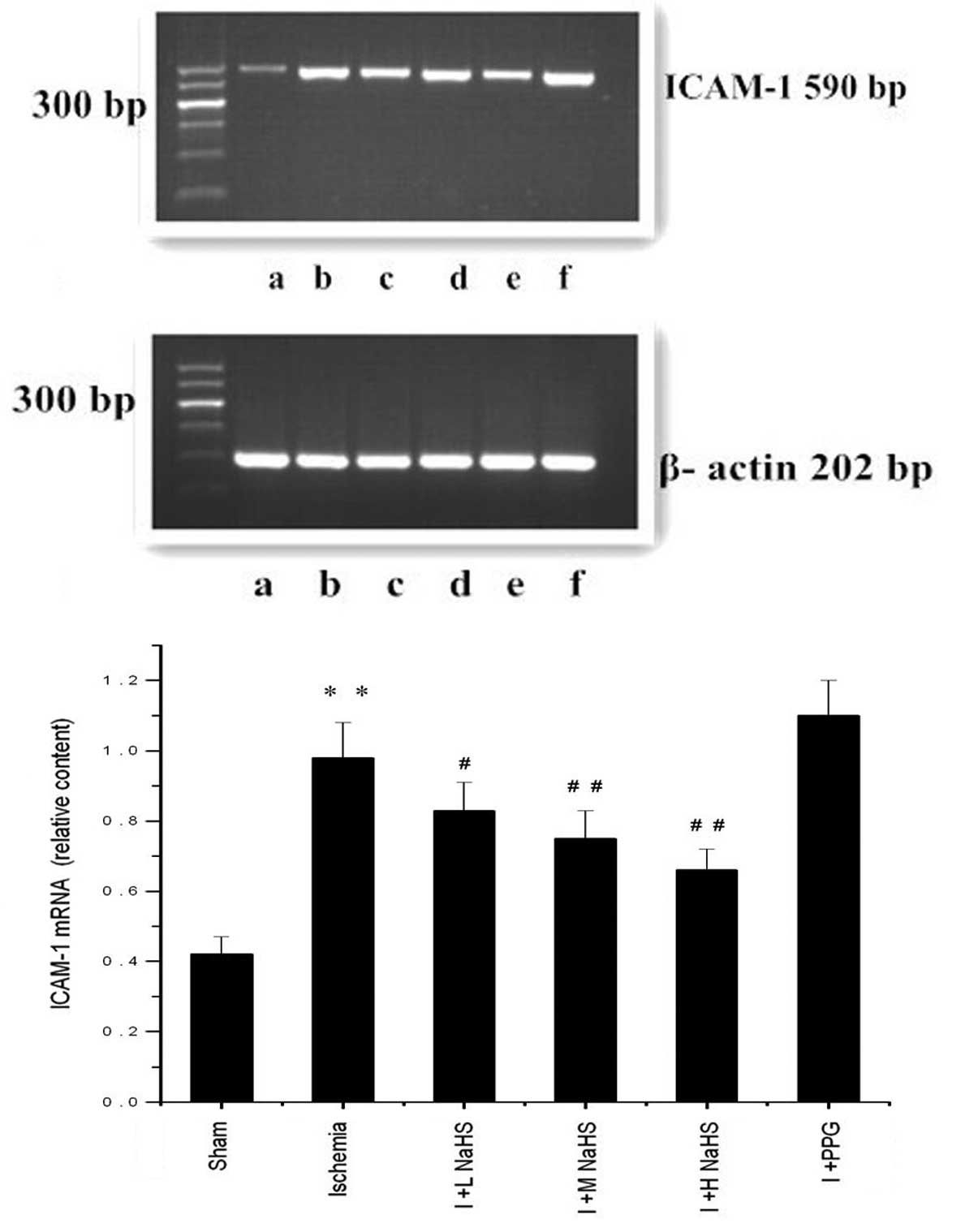 | Figure 3Effect of hydrogen sulfide on the
change in the expression of ICAM-1 mRNA in myocardial tissue in
rats, as assessed using reverse transcription-polymerase chain
reaction analysis: (a) Sham, (b) ischemia, (c) I + L NaHS, (d) I +
M NaHS, (e) I + H NaHS, (F) I + PPG. Data are presented as the mean
± standard error of the mean (n=5). **P<0.01 vs. the
sham group; #P<0.05 and ##P<0.01 vs.
the ischemia group. Sham, rats underwent the surgical procedures
but without the ischemic insult, followed by treatment with saline;
Ischemia, rats underwent the surgical procedures and were then
treated with saline; I + L NaHS, ischemic rats treated with 0.78
mg/kg NaHS; I + M NaHS, ischemic rats treated with 1.56 mg/kg NaHS;
I + H-NaHS, ischemic rats treated with 3.12 mg/kg NaHS; I + PPG,
ischemia rats treated with 30 mg/kg PPG; NaHS, sodium hydrosulfide;
PPG, propargylglycine; ICAM-1, intercellular adhesion
molecule-1. |
 | Table IIChanges in the expression of ICAM-1
mRNA and NF-κB protein in myocardial tissue in rats (n=5). |
Table II
Changes in the expression of ICAM-1
mRNA and NF-κB protein in myocardial tissue in rats (n=5).
| Group | ICAM-1 mRNA
(relative content) | NF-κB (relative
density) |
|---|
| Sham | 0.42±0.05 | 0.72±0.062 |
| Ischemia | 0.98±0.10a | 1.08±0.040a |
| I + L NaHS | 0.83±0.08b | 1.01±0.052 |
| I + M NaHS | 0.75±0.08c | 0.98±0.033c |
| I + H NaHS | 0.66±0.06c | 0.90±0.036c |
| I + PPG | 1.10±0.10 | 1.16±0.025b |
Changes in NF-κB expression in the
myocardial tissue
Western blotting results showed a trace amount of
NF-κB expression in the myocardial tissues of rats in the sham
surgery group; in the ischemia group the NF-κB expression in the
myocardial tissues of the rats was significantly increased compared
with the sham surgery group (P<0.01). Compared with the ischemia
group, NF-κB expression in the myocardial tissues of the rats was
significantly reduced in the ischemia + medium- and high-dose NaHS
groups; in the ischemia + PPG group, NF-κB expression in the
myocardial tissues of the rats was increased (P<0.05 or
P<0.01) (Fig. 4 and Table II).
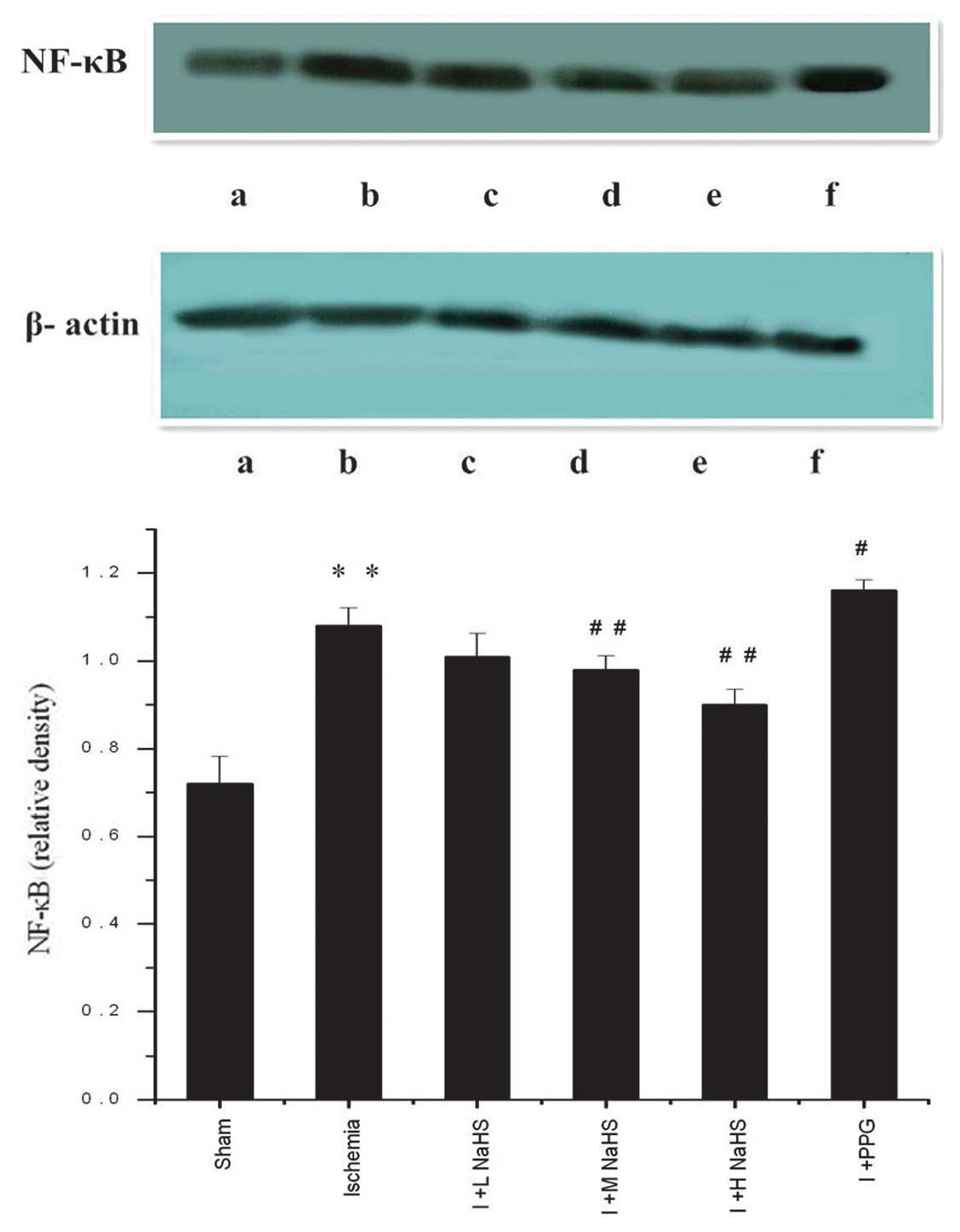 | Figure 4Effect of hydrogen sulfide on the
change in the expression of NF-κB in myocardial tissue in rats, as
assessed by western blotting: (a) Sham, (b) ischemia, (c) I + L
NaHS, (d) I + M NaHS, (e) I + H NaHS, (F) I + PPG. Data are
presented as the mean ± standard error of the mean (n=5).
**P<0.01 vs. the sham group; #P<0.05
and ##P<0.01 vs. the ischemia group. Sham, rats
underwent the surgical procedures but without the ischemic insult,
followed by treatment with saline; Ischemia, rats underwent the
surgical procedures and were then treated with saline; I + L NaHS,
ischemic rats treated with 0.78 mg/kg NaHS; I + M NaHS, ischemic
rats treated with 1.56 mg/kg NaHS; I + H-NaHS, ischemic rats
treated with 3.12 mg/kg NaHS; I + PPG, ischemia rats treated with
30 mg/kg PPG; NaHS, sodium hydrosulfide; PPG, propargylglycine;
NF-κB, nuclear factor κ-light-chain-enhancer of activated B
cells. |
Discussion
Previously it has been found that numerous mammalian
cells and tissues can produce H2S, a novel type of gas
neurotransmitter in the body with a wide range of biological
effects (4,20,21).
H2S is predominantly generated by L-cysteine under the
action of CBS and CSE (13).
Numerous mammalian cells, tissues, organs and systems can produce
H2S, which is mainly synthesized by tissue-specific
metabolic enzymes utilizing endogenous methionine, homocysteine and
L-cysteine; a small amount of H2S is generated by
non-enzymatic synthesis (7,23).
Endogenous H2S is generated in mammals in three main
ways, two of which are pyridoxal 5′-phosphate-dependent enzyme
regulating pathways. In these pathways, two key enzymes, CBS and
CSE, generate H2S, pyruvate and ammonium via a transfer
action with L-cysteine and homocysteine serving as a substrate
(7). The third method of
generating H2S is through the zinc-dependent
3-mercaptopyruvate sulfurtransferase (3MST) catalytic pathway:
Aspartate aminotransferase metabolizes L-cysteine to produce
3-mercaptopyruvate, which is then desulfurized by 3MST to generate
H2S (24). 3MST is
present in the cytoplasm and mitochondria, while CBS and CSE exist
only in the cytoplasm. In mammals, the distribution of CBS and CSE
is tissue-specific, with CSE found mainly in the cardiac and
vascular smooth muscle (14,25)
and CBS mainly in the nervous system (26); however CBS and CSE may be expressed
simultaneously in the small intestine, liver and kidney (25,27).
One-third of the total H2S is present in
gaseous form in the body while two-thirds are present in the form
of NaHS, which combines with H+ in the body to generate
H2S. A dynamic equilibrium exists between NaHS and
HS−, so as to ensure the stable presence of
H2S and the maintenance of the pH of the environment
(27). Under physiological
conditions, levels of H2S in SD rat plasma are ~46
μmol/l (28).
According to the literature (29,30)
and the results of the preliminary experiment, the rats in the
present study were intraperitoneally injected with 0.78, 1.56 or
3.12 mg/kg NaHS or 30 mg/kg PPG (CSE inhibitor) 3 h after acute
myocardial ischemia. NaHS, an H2S donor, dissociates
into Na+ and HS− in aqueous solution, and
HS− binds with H+ to generate H2S
(31). Preliminary experiments
showed that NaHS and PPG in the above-mentioned doses exerted
superior treatment and aggravation effects on acute myocardial
ischemia injury, respectively. These doses were therefore selected
for the investigation into the effects of NaHS and PPG on acute
myocardial ischemia injury. Three hours after the rats with acute
myocardial ischemia were administered NaHS, the myocardial
ultrastructural damage was significantly reduced, and increases in
the NaHS dose led to more significantly reduced myocardial
ultrastructural damage. This suggested that H2S could
reduce acute myocardial ischemia injury and had a protective effect
on myocardial structure subsequent to ischemia.
NF-κB, an important nuclear transcription factor, is
widely found in eukaryotic cells and is a member of the Rel protein
family. To date, five members of this family have been identified
in mammals: p65 (RelA), RelB, C-Rel, p50/p105 (NF-κB1) and p52/p100
(NF-κB2) (32). These proteins are
usually present in the form of homo-or heterodimers, wherein the
heterologous dimer generated from p65 and p50 is the most common
form. In a resting state, NF-κB binds with its inhibiting factor,
inhibitor of NF-κB (IκB), and exists in a non-activated state in
the cytoplasm. When the cells are under the influence of certain
stimuli, such as ischemia, hypoxia, oxygen radicals, cytokines and
certain viruses, IκB is phosphorylated, ubiquitinated, identified
by the proteasome and then rapidly degraded, so as to expose the
nuclear localization signal located on the p50 subunit. NF-κB is
thus activated and translocates to the nucleus, where the
transcription of numerous genes, including TNF-α, ICAM-1,
cyclooxygenase-2, inducible nitric oxide synthase and phospholipase
A2, is activated, (33,34).
When myocardial ischemia occurs, vascular endothelial cells are
stimulated first; following the activation of NF-κB the expression
of a variety of substances, including TNF-α and vascular cell
adhesion molecule-1 (VCAM-1), is initiated. Under the action of
these neurotransmitters, leukocyte adhesion, migration, invasion
and damage to the heart muscle appear in the ischemic region of the
blood vessels. Further accumulation of white blood cells enhances
the release of inflammatory mediators and oxygen radicals, which
aggravate the ischemia, resulting in vascular and myocardial damage
(35,36). In addition, the TNF-α
neurotransmitter produced in the above process induces significant
metabolic and hemodynamic changes in the body, and leads to an
inflammatory factor ‘cascade effect’ (10). TNF-α can induce the generation of
other inflammatory mediators, such as IL-1 and ICAM-1, and further
activate NF-κB, so as to increase the degree of ischemic
injury.
Cell adhesion molecules (CAMs) are a class of
glycoprotein receptors present on the cell surface, and their main
function is to promote cell-cell adhesion and cell-tissue matrix
adhesion. CAMs play an important role in maintaining the
stabilization of normal tissues, mediation of inflammatory
responses, thrombosis, damage repair and immunoregulation (37). ICAM-1, a transmembrane protein
antigen on the cell surface, is widely distributed in various
tissues, and can activate T cells, endothelial cells, fibroblasts
and tissue macrophages. There is only a low level of ICAM-l
expression in myocardial cells under normal conditions, and its
expression and activation are strictly regulated. Under the action
of hypoxia and cytokines, large amounts of ICAM-1 are generated on
the membrane surface of myocardial cells (38). NF-κB binding sites can be found on
the ICAM-1 gene promoter (39):
NF-κB is activated to enter the nucleus and promote the expression
of ICAM-1; ICAM-1 can then in turn further activate NF-κB, thereby
forming a positive feedback loop and continuously amplifying the
inflammation.
IL-1, an inflammatory cytokine, is produced by
activated leukocytes, particularly monocytes/macrophages, and is
the initiating factor in the body’s inflammatory cytokine cascade;
IL-1β is the main form of secretion. IL-1 may have a toxic effect
through direct action on the cells, acting to destroy the structure
and function of vascular endothelial cells and release large
amounts of inflammatory cytokines, mediated by inflammatory cell
adhesion, resulting in the excessive release of oxygen radicals,
damaged vascular endothelial cells and decreased myocardial
contractility. IL-1 can also activate platelets to stimulate
platelet aggregation and thrombosis; in addition, it can produce
vasoconstrictors, such as endothelin, to increase coronary vascular
resistance (40). IL-6, an
important inflammatory immune reaction medium, is involved in
atherosclerosis formation and development, which is an important
risk factor for coronary heart disease. IL-6 activation stimulates
neutrophil and myocardial cell adhesion, so as to release plasmin
and produce large amounts of oxygen radicals to damage myocardial
cells. Simultaneously, IL-6 stimulates the expression of ICAM-1 on
the surface of endothelial cells, leading to the increased
permeability of the endothelium (41,42).
The results of the present study showed that,
following acute myocardial ischemia, TNF-α, IL-1β and IL-6 levels
in the serum in the myocardial tissue of rats were increased, and
ICAM-1 mRNA and NF-κB expression in the myocardial tissues was
significantly increased. Li et al (43) also reported that, following simple
transient cardiac ischemia, NF-κB activity was rapidly and
significantly increased; this may be the molecular mechanism
underlying the rapid expression of a series of early inflammatory
genes. The activation of NF-κB following myocardial ischemia could
induce the production of TNF-α by myocardial tissues (36) in addition to regulating the
expression of numerous genes, including IL-1β, IL-6, ICAM-l and
VCAM-1. This indicates that the complex interaction of cytokines
with NF-κB and inflammatory adhesion molecules can lead to further
amplified and enhanced inflammation, resulting in myocardial
inflammation, injury or even death.
This study showed that, following the administration
of PPG, TNF-α, IL-1β and IL-6 levels in the serum and myocardial
tissues of rats, as well as ICAM-1 mRNA expression and the
expression of NF-κB protein, were increased. Following the
administration of NaHS, however, TNF-α, IL-1β and IL-6 levels in
the serum and myocardial tissues of rats, as well as ICAM-1 mRNA
expression in myocardial tissues and NF-κB protein expression, were
decreased, indicating that exogenously supplemented H2S
inhibited the synthesis of inflammatory cytokines (such as IL-1β),
nuclear transcription factors (such as TNF-α) and adhesion
molecules in the serum and myocardial tissues of rats following the
development of myocardial ischemia, thereby reducing myocardial
injury and protecting myocardial tissues. In conclusion, the
findings of the present study provide novel evidence for the
exogenous supplement of H2S on acute myocardial ischemia
injury via the modulation of inflammatory factors.
Acknowledgements
The authors would like to thank Dr Yun Xie (Disease
Prevention and Control Center of Hebei Province, Shijiazhuang,
China) for technical assistance. This study was supported by grants
from the Key Program of Hebei Provincial Health Department Medical
Research, China (no. 20130247), the Key Basic Research Project of
Hebei Province, China (no. 13967602D) and the Natural Science
Foundation of Hebei Province, China (no. C2009001458).
References
|
1
|
Chen X and Chen WZ: Cardiovascular
Pharmacology. 3rd Edition. People’s Medical Publishing House;
Beijing, China: pp. 4052002
|
|
2
|
Prior MG, Sharma AK, Yong S and Lopez A:
Concentration-time interactions in hydrogen sulphide toxicity in
rats. Can J Vet Res. 52:375–379. 1988.PubMed/NCBI
|
|
3
|
Reiffenstein RJ, Hulbert WC and Roth SH:
Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol.
32:109–134. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abe K and Kimura H: The possible role of
hydrogen sulfide as an endogenous neuromodulator. J Neurosci.
16:1066–1071. 1996.PubMed/NCBI
|
|
5
|
Kimura H: Hydrogen sulfide induces cyclic
AMP and modulates the NMDA receptor. Biochem Biophys Res Commun.
267:129–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szabó C: Hydrogen sulphide and its
therapeutic potential. Nat Rev Drug Discov. 6:917–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang R: Two’s company, three’s a crowd:
can H2S be the third endogenous gaseous transmitter? FASEB J.
16:1792–1798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu X, Li T, Bi S, et al: Possible role of
hydrogen sulfide on the preservation of donor rat hearts.
Transplant Proc. 39:3024–3029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li XH, Du JB and Tang CS: Impact of
hydrogen sulfide donor on pulmonary vascular structure and
vasoactive peptides in rats with pulmonary hypertension induced by
high pulmonary blood flow. Zhongguo Yi Xue Ke Xue Yuan Xue Bao.
28:159–163. 2006.(In Chinese). PubMed/NCBI
|
|
10
|
Chen YH, Yao WZ, Geng B, et al: Endogenous
hydrogen sulfide, in patients with COPD. Chest. 128:3205–3211.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao W, Zhang J, Lu Y and Wang R: The
vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP)
channel opener. EMBO J. 20:6008–6016. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan H, Du J and Tang C: The possible role
of hydrogen sulfide on pathogenesis of spontaneous hypertension in
rats. Biochem Biophys Res Commun. 313:22–27. 2004. View Article : Google Scholar
|
|
13
|
Hongfang J, Bailin Cong, Bin Zhao, et al:
Effects of hydrogen sulfides on hypoxic pulmonary vascular
structural remodeling. Life Sci. 78:1299–1309. 2006. View Article : Google Scholar
|
|
14
|
Geng B, Yang J, Qi Y, et al: H2S generated
by heart in rat and its effect on cardiac function. Biochem Biophys
Res Commun. 313:362–368. 2004. View Article : Google Scholar
|
|
15
|
Yang G, Sun X and Wang R: Hydrogen
sulfide-induced apoptosis of human aorta smooth muscle cells via
the activation of mitogen-activated protein kinases and caspase-3.
FASEB J. 18:1782–1784. 2004.PubMed/NCBI
|
|
16
|
Chunyu Z, Junbao D, Dingfang B, et al: The
regulatory effect of hydrogen sulfide on hypoxic pulmonary
hypertension in rats. Biochem Biophys Res Commun. 302:810–816.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johansen D, Ytrehus K and Baxter GF:
Exogenous hydrogen sulfide (H2S) protects against regional
myocardial ischemia-reperfusion injury - evidenve for a role of K
ATP channels. Basic Res Cardiol. 101:53–60. 2006. View Article : Google Scholar
|
|
18
|
Zanardo RC, Brancaleone V, Distrutti E, et
al: Hydrogen sulfide is an endogenous modulator of
leukocyte-mediated inflammation. Faseb J. 20:2118–2120. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sivarajah A, Collino M, Yasin M, et al:
Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in
a rat model of regional myocardial I/R. Shock. 31:267–274. 2009.
View Article : Google Scholar
|
|
20
|
Mitchell TW, Savage JC and Gould DH:
High-performance liquid chromatography detection of sulfide in
tissues from sulfide-treated mice. J Appl Toxicol. 13:389–394.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang GD and Wang R: H(2)S and cellular
proliferation and opoptosis. Sheng Li Xue Bao. 59:133–140.
2007.PubMed/NCBI
|
|
22
|
Stipanuk MH and Beck PW: Characterization
of the enzymic capacity for cysteine desulphhydration in liver and
kidney of the rat. Biochem J. 206:267–277. 1982.PubMed/NCBI
|
|
23
|
Stipanuk MH: Sulfur amino acid metabolism:
pathways for production and removal of homocysteine and cysteine.
Annu Rev Nutr. 24:539–577. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamoun P: Endogenous production of
hydrogen sulfide in mammals. Amino Acids. 26:243–254. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao W, Zhang J, Lu Y and Wang R: The
vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP)
channel opener. EMBO J. 20:6008–6016. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eto K, Ogasawara M, Umemura K, et al:
Hydrogen sulfide is produced in response to neuronal excitation. J
Neurosci. 22:3386–3391. 2002.PubMed/NCBI
|
|
27
|
Hosoki R, Matsuki N and Kimura H: The
possible role of hydrogen sulfide as an endogenous smooth muscle
relaxant in synergy with nitric oxide. Biochem Biophys Res Commun.
237:527–531. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang R: The gasotransmitter role of
hydrogen sulfide. Antioxid Redox Signal. 5:493–501. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu YZ, Wang ZJ, Ho P, et al: Hydrogen
sulfide and its possible roles in myocardial ischemia in
experimental rats. J Appl Physiol (1985). 102:261–268. 2007.
View Article : Google Scholar
|
|
30
|
Johansen D, Ytrehus K and Baxter GF:
Exogenous hydrogen sulfide (H2S) protects again stregional
myocardial ischemia-reperfusion injury - Evidence for a role of K
ATP channels. Basic Res Cardiol. 101:53–60. 2006. View Article : Google Scholar
|
|
31
|
Łowicka E and Bełtowski J: Hydrogen
sulfide (H2S) - the third gas of interest for pharmacologists.
Pharmacol Rep. 59:4–24. 2007.PubMed/NCBI
|
|
32
|
Siebenlist U, Fronzoso G and Brown K:
Structure regulation and function of NF-kappa B. Annu Rev Cell
Biol. 10:405–455. 1994. View Article : Google Scholar
|
|
33
|
Lee JI and Burckart GJ: Nuclear factor
kappa B: important transcription factor and therapeutic target. J
Clin Pharmacol. 38:981–993. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Atreya I, Atreya R and Neurath MF:
NF-kappaB in inflammatory bowel disease. J Intern Med. 263:591–596.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fiorucci S, Antonelli E, Distrutti E, et
al: Inhibition of hydrogen sulfide generation contributes to
gastric injury caused by anti-inflammatory nonsteroidal drugs.
Gastroenterology. 129:1210–1224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meldrum DR: Tumor necrosis factor in the
heart. Am J Physiol. 274:R577–R595. 1998.PubMed/NCBI
|
|
37
|
Otterbein LE, Bach FH, Alam J, et al:
Carbon monoxide has anti-inflammatory effects involving the
mitogen-activated protein kinase pathway. Nat Med. 6:422–428. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marino M, Scuderi F, Mazzarelli P, et al:
Constitutive and cytokine-induced expression of MHC and
intercellular adhesion molecule-l (ICAM-l) on human myoblasts. J
Neuroimmunol. 116:94–101. 2003. View Article : Google Scholar
|
|
39
|
Kupatt C, Habazettl H, Goedecke A, et al:
Tumor necrosis factor-alpha contributes to ischemia- and
reperfusion-induced endothelial activation in isolated hearts. Circ
Res. 84:392–400. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roivainen M, Vilk-Kajander M, Palosuo T,
et al: Infections, inflammation and the risk of coronary heart
disease. Circulation. 101:252–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hansen PR: Role of neutrophils in
myocardial ischemia and reperfusion. Circulation. 91:1872–1875.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vanden Berghe W, Vermeulen L, De Wilde G,
et al: Signal transduction by tumor necrosis factor and gene
regulation of the inflammatory cytokine interleukin-6. Biochem
Pharmacol. 60:1185–1195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li C, Browder W and Kao RL: Early
activation of transcription factor NF-kappaB during ischemia in
perfused rat heart. Am J Physiol. 276:H543–H552. 1999.PubMed/NCBI
|