Introduction
Sepsis is one of the most common and serious
complications associated with a number of diseases; it is systemic
inflammatory response syndrome (SIRS) caused by infection with
pathogenic microorganisms. SIRS can subsequently develop into
severe sepsis, septic shock and even multiple organ dysfunction
syndrome (MODS). Patients suffering from sepsis utilize numerous
medical resources, and the mortality rate of the disease is high,
and is continuing to rise (1).
The pathogenesis of sepsis is very complex, and
several medications have been used to alleviate the symptoms. It
has been reported that analgesic and/or sedative medications can
relieve pain and anxiety in septic patients, and reduce stress
responses (such as increased myocardial oxygen consumption,
hypercoagulable state and immune suppression), making these
patients feel more comfortable and coordinated (2). Specifically, midazolam, a sedative
agent, has been found to inhibit the release of interleukin (IL)-6
mediated by IL-1β in mouse glioma cells, affecting the immune
function of the central nervous system (3). Moreover, midazolam has been
demonstrated to suppress the inflammatory reaction and stress
response in patients with gastric cancer following surgery,
contributing to postoperative rehabilitation (4). Fentanyl, an analgesic agent, is able
to inhibit the expression of proinflammatory cytokines, including
IL-6, IL-10 and tumor necrosis factor (TNF)-α, in
lipopolysaccharide (LPS)-induced inflammation (5). However, the effects of fentanyl
and/or midazolam on inflammation-related cytokines in sepsis have
not yet been fully elucidated.
In the present study, mouse models of sepsis were
established to investigate the effects of midazolam and fentanyl,
as well as their combination, on immune function and mortality. The
aim was to provide a theoretical basis for the clinical application
of these analgesic and sedative medications.
Materials and methods
Animal grouping and modeling
Ninety specific pathogen-free Kunming mice, half
male and half female, weighing 20±2.24 g, were included in the
study (SCXK Xin2011-0001; Animal Center of Centers for Disease
Control of Xinjiang Uygur Autonomous Region, Ürümqi, China). These
mice were randomly divided into the sham-operated (n=10), sepsis
model (n=20), fentanyl-treated (n=21), midazolam-treated (n=19) and
fentanyl/midazolam combination (n=20) groups. The study and the
experimental procedures were approved by the Clinical Ethics
Committee and the Animal Care Committee of the People’s Hospital of
Xinjiang Uygur Autonomous Region (Ürümqi, China).
The cecal ligation and puncture (CLP) method was
used for the establishment of the sepsis model, according to a
previously described procedure (6). Briefly, after 8 h of fasting prior to
surgery, these mice were weighed and then subjected to anesthesia
by the intraperitoneal injection of 10% chloral hydrate (3.5 ml/kg
body weight). Following abdominal disinfection, an incision along
the abdominal midline was made to open the peritoneal cavity. The
cecum was ligated with a silk suture at 0.5–1 cm from the end, and
a through-and-through cecal puncture was achieved with a needle.
The intestines were then placed back into the peritoneal cavity,
and the incision was sutured. For the sham-operated group, the
surgery was carried out only with flipping of the cecum.
Drug administration
Drugs were administrated via intraperitoneal
injection, at 10 and 2 h before, as well as 3, 9, 15 and 21 h after
the surgery. The dose of fentanyl was 0.0005 mg/kg (Renfu
Pharmaceutical Co. Ltd., Yichang, China) and that of midazolam was
1 mg/kg (Enhua Pharmaceutical Co. Ltd., Suzhou, China). For the
combination group, 0.0005 mg/kg fentanyl plus 1 mg/kg midazolam
were administered. In addition, physiological saline was used in
the sham-operated and model groups.
Locomotor activity detection
Locomotor activity was assessed at 3 h before and 15
h after the surgery in each group. Total activities were detected
automatically with an autonomous movement instrument (ZZ-6; Chengdu
TME Technology Co., Ltd., Chengdu, China). Each mouse was placed in
a box (14.2×11.2×11.4 cm) for 5 min in light.
General observation
After the mice were sacrificed by cervical
dislocation, the peritoneal and thoracic cavities were cut open.
Pathogenic changes in the organs, including the heart, thymus,
lungs, spleen, liver, kidney, intestine, stomach and brain were
observed. The organ weights were measured and the corresponding
coefficients were calculated, as previously prescribed (7).
Leukocyte counting
Three hours after the last drug administration, 4
and 2 ml phosphate-buffered saline (PBS; Tecom Science and
Technology Co., Ltd., Nanchang, China) was injected into the
peritoneal and thoracic cavities, respectively. After gently
rubbing the abdomen, the peritoneal and thoracic cavities were cut
open to collect the lavage fluid for leukocyte counting analysis
with a fully automated blood cell analyzer (BC-2800Vet; Mindray
Medical International Ltd., Shenzhen, China).
Enzyme-linked immunosorbent assay
(ELISA)
Blood samples were collected from carotid arteries
prior to sacrifice. These blood samples were centrifuged at 1,000 ×
g for 10 min, and the harvested serum was subjected to the
detection of IL-1, IL-10, TNF-α, procalcitonin (PCT) and C-reactive
protein (CRP) with ELISA kits (CRP and PCT kits from Shanghai
XiTang Biotechnology Co., Ltd., Shanghai, China, and IL-1, IL-10
and TNF-α kits from Shenzhen Dakewe Biotechnology Co., Ltd.
Shenzhen, China), according to the manufacturers’ instructions.
Statistical analysis
Data are expressed as mean ± standard deviation.
SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA) was used
for the statistical analysis. χ2 test was performed for
the comparison of the leukocyte counting analysis, and the
Student’s t-test was used for pairwise comparisons. Variance
analysis was applied for multiple comparisons. P<0.05 was
considered to indicate a statistically significant result.
Results
Effects of fentanyl and/or midazolam on
body weights, locomotor activities and mortality rates in septic
mice
Septic mouse models were established by CLP, and the
medications were administered at 10 and 2 h before, and 3, 9, 15,
and 21 h after the surgery. In the sham-operated group, mice awoke
from anesthesia at 2–3 h after surgery, with normal physiological
functions, including eating, breathing and bowel movement. However,
in the septic models, with or without medications, mice awoke at 3
h after surgery. At 6 h, the model mice begun to suffer from
sickness, with decreased motility, lethargy, piloerection, reduced
consumption, tachypnea, pyuria, diarrhea and eye exudates. As these
symptoms progressed, the mice exhibited shivering, stiff limbs,
dyspnea and even neck stiffness, and finally death occurred. In the
sham-operated and model groups, body weights were increased
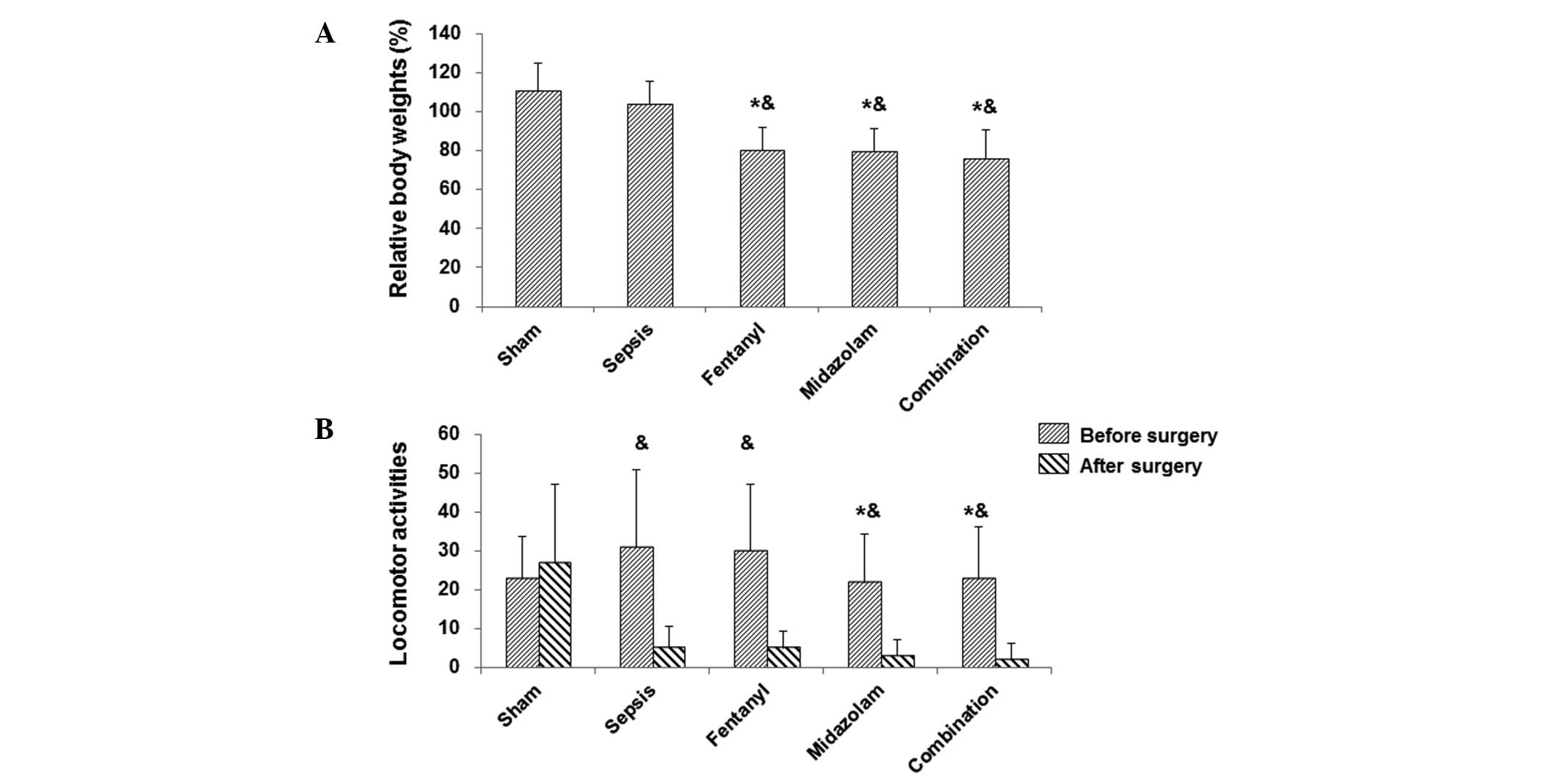
following surgery (Fig. 1A).
However, in the medication-treated groups (fentanyl, midazolam and
their combination), the body weights were significantly declined
compared with those prior to the surgery (P<0.0; Fig. 1A). With regard to locomotor
activities, there were no significant differences among these
groups at 3 h prior to the surgery. At 15 h after the surgery,
locomotor activity did not markedly change in the sham-operated
group, while in septic mice (with or without medications) the
activities were reduced, with no significant differences among
these groups (Fig. 1B).
To investigate the effects of fentanyl, midazolam
and their combination on the mortality of septic mice, the
conditions of the mice were monitored and the survival rates were
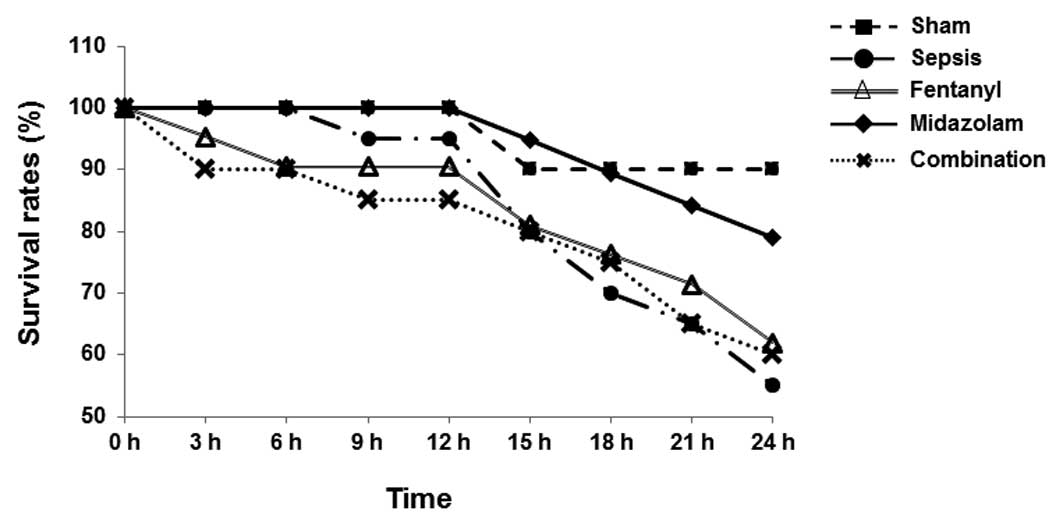
recorded at hourly intervals. As shown in Fig. 2, in the model group, mice started
to die at 9 h after surgery. The first death occurred at 3 h after
surgery for the fentanyl group, at 3 h for the combination group,
and at 15 h for the midazolam group (Fig. 2). Among the mice subjected to CLP,
at 24 h after surgery the mortality rate was highest in the model
group (45.0%), while the lowest mortality rate was in the midazolam
group (21.1%, 4/19; P<0.05). The mortality rates in the fentanyl
and combination groups were 38.1 and 40.0%, respectively. These
results suggest that the mortality rate of septic mice was reduced
by treatment with fentanyl and/or midazolam.
Impacts of fentanyl and/or midazolam on
organ weights and coefficients in septic mice
To further investigate the effects of fentanyl,
midazolam and their combination on the pathology of sepsis, the
organ weights were measured and the coefficients were calculated in
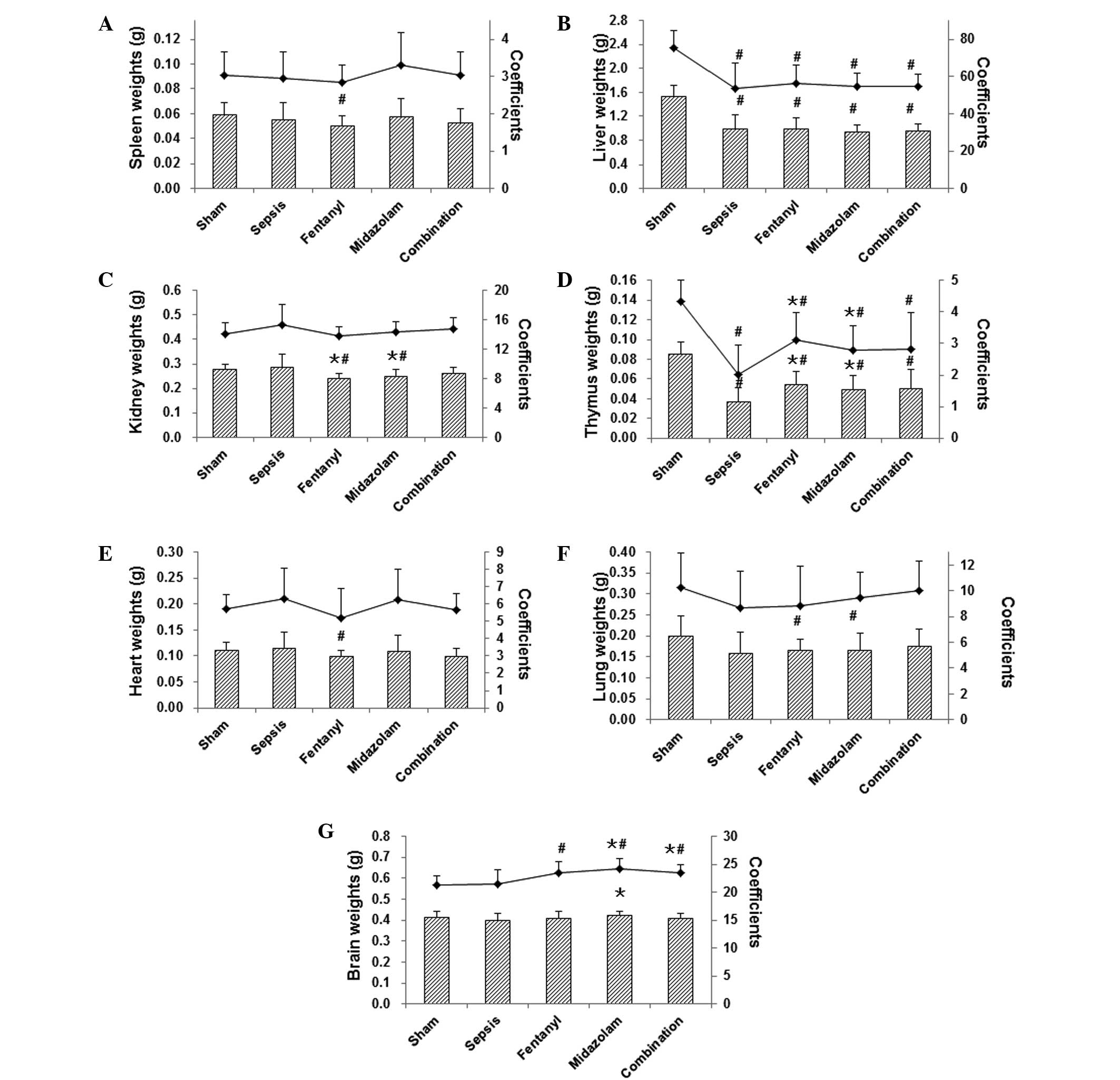
these mice. Observation of the organs indicated that, in the
sham-operated group, no abnormalities were present in the heart,
thymus, lungs, spleen, liver, kidney, intestine, stomach or brain.
However, in the model group, there were evident bloody exudates in
the peritoneal cavity, and distension of the jejunum, swelling of
the cecum and gangrene. Livers with uneven or dark color, reddish
lungs and meningeal venous congestion were also observed. The
weights of the liver and thymus were significantly decreased in
septic mice compared with those in the sham-operated group, while
the weights of spleen, kidney, heart, lung, and brain did not
change markedly. In the medication-treated groups, the
administration of fentanyl and midazolam led to reduced kidney and
increased thymus weights, while the brain weight was elevated in
the midazolam group compared with those in the sepsis model group
(Fig. 3).
Concerning organ coefficients, the liver and thymus
coefficients were clearly declined in the model group compared with
those in the sham-operated group. Following drug administrations,
the brain coefficient was significantly elevated for all treatments
(Fig. 3G). Furthermore, the thymus
coefficient was increased for both fentanyl and midazolam
treatments, and the lung coefficient was increased for midazolam
treatment and the combination therapy, but no significance was
reached (Fig. 3F). These results
suggest that the histopathological changes (as indicated by the
organ weights and coefficients) were influenced by the
administration of fentanyl and/or midazolam in septic mice.
Effects of fentanyl and/or midazolam on
the expression of inflammation-related cytokines in septic
mice
To further elucidate the mechanism through which
fentanyl and/or midazolam exert beneficial effects in these septic
mice, the leukocyte numbers in peritoneal and thoracic cavity
lavage fluid were counted, and inflammation was evaluated by
detecting the expression of pro-inflammatory cytokines by ELISA.
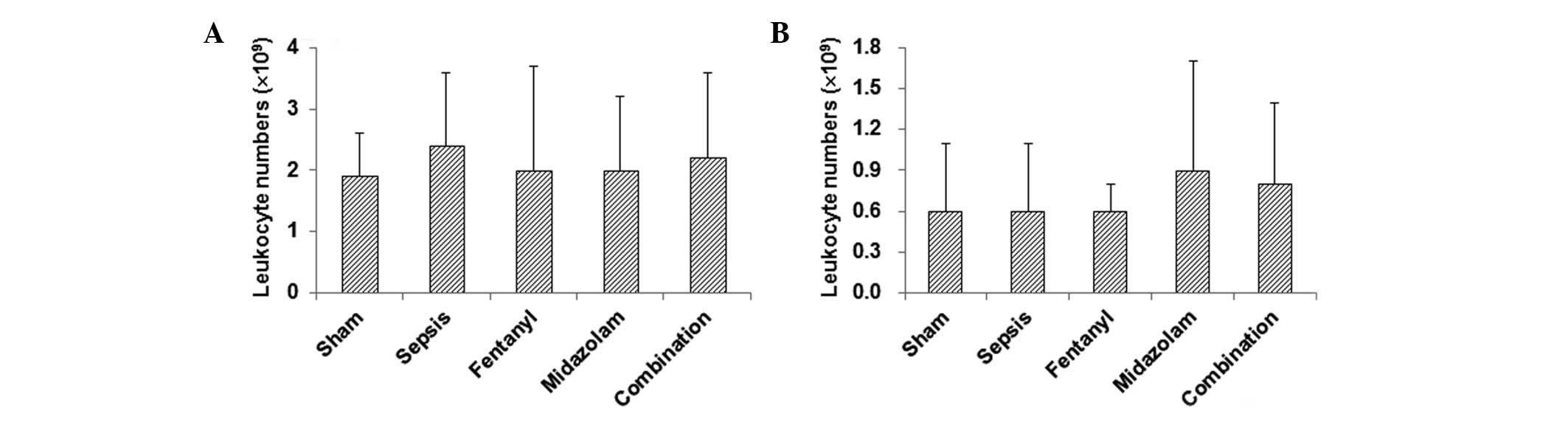
The results indicate that leukocyte numbers in the peritoneal
cavity lavage fluid were elevated in the model group compared with
those in the sham-operated group, without significant differences
(Fig. 4A). In the
medication-treated groups, the leukocyte numbers in the peritoneal
cavity lavage fluid were lower than those in the model group
(Fig. 4A). For the thoracic cavity
lavage fluid, there were no significant differences in leukocyte
numbers between the sham-operated and model groups, while the
administration of fentanyl and/or midazolam slightly increased the
number of leukocytes (Fig.
4B).
Results from ELISA demonstrated that, compared with
those in the sham-operated group, the expression levels of PCT,
CRP, TNF-α, IL-1β and IL-10 were significantly elevated in the
model group (P<0.05; Fig. 5).
In the midazolam-treated group, the expression levels of CRP,
IL-10, TNF-α and PCT were decreased, while the IL-1β level was
increased, compared with those in the model group (P<0.05 for
CRP, IL-10 and IL-1β; Fig. 5). In
the fentanyl-treated group, the expression level of TNF-α was
significantly reduced (P<0.05), while the expression level of
IL-1β was significantly increased (P<0.05), compared with those
in the model group (Fig. 5). In
the combination treatment group, only the expression level of CRP
was significantly reduced compared with that in the model group
(P<0.05; Fig. 5). These results
suggest that the levels of proinflammatory cytokines were reduced
by the administration of fentanyl and/or midazolam, which may
contribute to the beneficial effects of these medications in septic
mice.
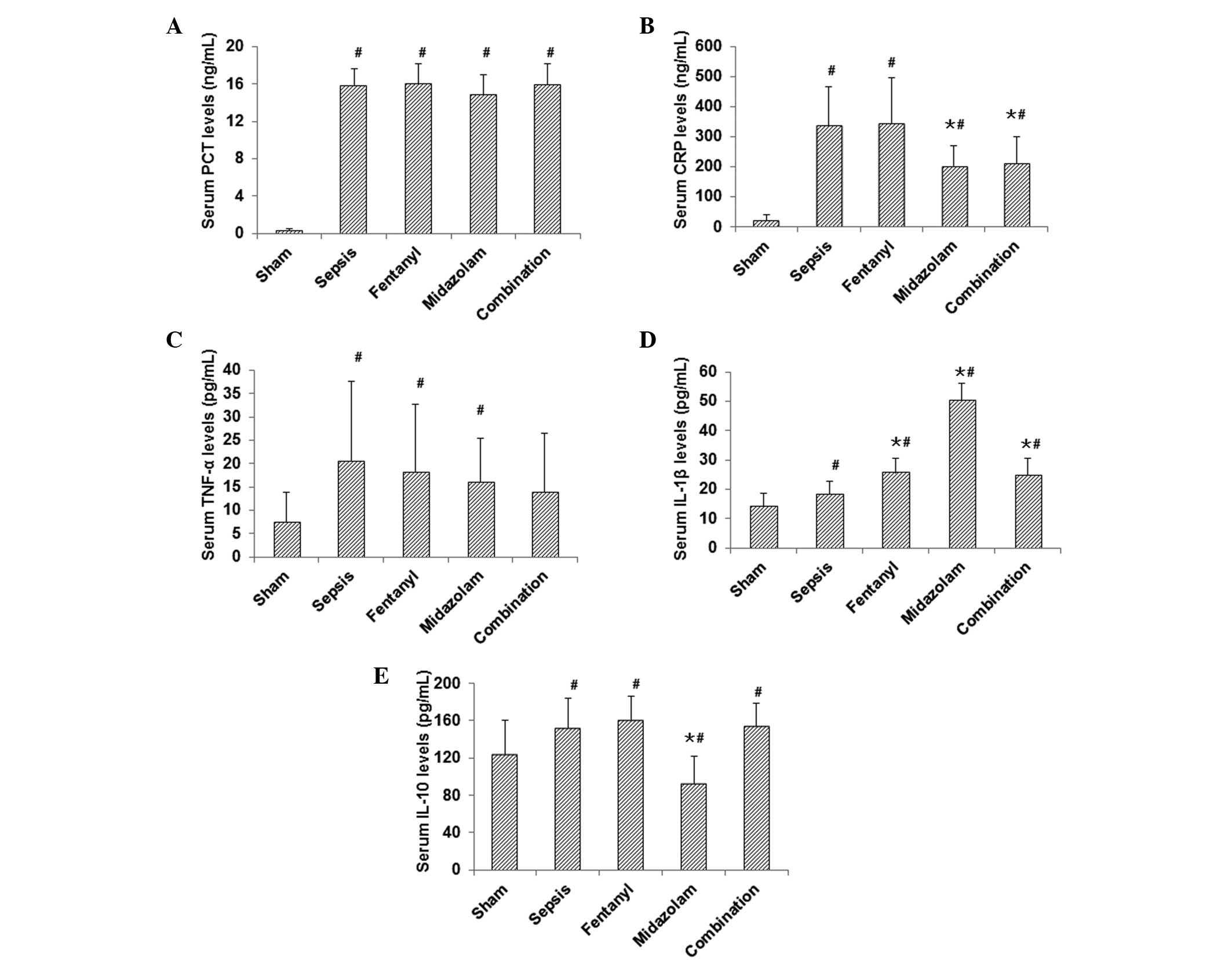 | Figure 5Effects of fentanyl and/or midazolam
on the expression of inflammation-related cytokines in septic mice.
Serum levels of (A) PCT, (B) CRP, (C) TNF-α, (D) IL-1β and (E)
IL-10 were evaluated by ELISA in the sham-operated, model,
fentanyl, midazolam and combination groups. #P<0.05
compared with the sham-operated group; ★P<0.05
compared with the model group. PCT, procalcitonin; CRP, C-reactive
protein; TNF, tumor necrosis factor; Il, interleukin; ELISA,
enzyme-linked immunosorbent assay. |
Discussion
Fentanyl and midazolam are the most commonly
clinically used analgesic and sedative drugs. The concept of
immunosedation in the treatment of sepsis has been proposed
(8), based on the finding that
midazolam and dexmedetomidine can improve the prognosis of septic
mice (9). Accordingly, in the
present study, mouse models of sepsis were established, to
investigate whether analgesia/sedation with fentanyl and/or
midazolam could influence the expression of cytokines and the
prognosis of septic mice. It was found that, at 15 h following the
modeling surgery, the locomotor activity did not clearly change in
the sham-operated group, while in septic mice (with or without
medications), the activities were reduced. In particular, the
reduction of locomotor activity in the midazolam group was less
than that in the fentanyl group, indicating that within 24 h after
surgery, it may be necessary to apply the analgesic agent prior to
the sedative, and that the optimal outcome may not be achieved with
sedative alone.
Sepsis, induced by infection, can cause damage to
organs, including the liver, kidney, lung, and brain, leading to
immune disorders, forming a vicious cycle resulting in enhanced
actions of septic bacteria and related toxins (1). The results of the present study
demonstrated that the organ weights and coefficients of the liver
and thymus were significantly reduced, while the spleen weight and
coefficient were not markedly changed in septic mice compared with
those in sham-operated mice. As the thymus is the main organ for
T-lymphocyte development, the CLP-induced sepsis models used in the
study may be associated with immune dysfunction. The organ weights
and coefficients of the thymus were significantly elevated in
fentanyl and/or midazolam groups compared with those in the model
group, indicating that analgesia/sedation may regulate cellular
immunity. Notably, the organ weights and coefficients of the brain
and lung were also influenced by the medications, possibly
indicating the swelling of the brain and lung induced by
hypotension in the model mice. These results suggest that, in the
clinical use of midazolam, hypotension should be carefully
monitored and appropriately treated to avoid damage to the brain
and lung.
Inflammation has been associated with the
pathogenesis of sepsis. A previous study has shown that midazolam
can inhibit the inflammation and stress response in patients with
gastric cancer subsequent to surgery, contributing to their
rehabilitation (4). In the present
study, the administration of analgesic and sedative drugs decreased
the numbers of leukocytes in the peritoneal cavity lavage fluid in
septic mice, indicating that these drugs may inhibit the
inflammatory response in sepsis. It is widely accepted that
infection can trigger an inflammatory cascade in the body, causing
systemic immune dysfunction and finally leading to sepsis (10). Numerous factors are involved in the
inflammatory process in the development of sepsis. In line with
this, the results of the present study demonstrated that, compared
with those in the sham-operated group, the expression levels of
inflammation-related cytokines, including IL-1β, TNF-α and IL-10,
were elevated in the septic mice.
IL-1β and TNF-α are proinflammatory cytokines,
initiating the inflammatory response in the body. IL-1β and TNF-α
are also able to induce hypotension and subsequent hemodynamic
instability (11,12). It has been reported that in
patients with meningitis, IL-1β expression levels are negatively
correlated with prognosis, that is, a higher IL-1β expression level
results in a poorer prognosis (13). However, the association between
IL-1β expression level and prognosis in patients with sepsis has
not yet been established (14).
The present study found that the administration of analgesic and/or
sedative drugs markedly reduced the expression level of TNF-α in
septic mice, indicating that analgesia/sedation reduce the
inflammatory response, which may be associated with the improved
mortality rate in the medication-treated groups compared with the
model group. These results are consistent with the findings from
Babcock et al (15).
Moreover, Qiao et al (9)
found that the application of midazolam and dexmedetomidine
significantly reduced the mortality rate of septic mice, which may
be associated with decreased levels of IL-6 and TNF-α. Wu et
al (5) also reported that
fentanyl caused a marked reduction in the levels of IL-6 and TNF-α
in human peripheral blood induced by LPS. These results demonstrate
the mortality-reducing effects of fentanyl in septic mice.
IL-10 is one of the important immunoregulatory and
anti-inflammatory cytokines, mainly produced by mononuclear
macrophages as well as T and B lymphocytes. The major biological
effects of IL-10 include inhibiting the antigen-presenting function
of macrophages and suppressing the synthesis of TNF-α and other
cytokines. IL-10 can reduce excessive inflammation in sepsis, and
IL-10-deficiency leads to multiple organ failure and increased
mortality rates in mice (16).
However, the excessive release of anti-inflammatory and
immunosuppressive factors may make it difficult to control the
infection. Hypersecretion of the anti-inflammatory mediator IL-10
may cause excessive immunosuppression, and reduce the body’s
resistance to infection, inducing secondary infection and even
leading to sepsis. In the present study, the expression level of
IL-10 was significantly higher in the model group than in the
sham-operated group. In medication-treated groups, only treatment
with midazolam clearly decreased the IL-10 expression, indicating
that midazolam is able to suppress the hypersecretion of IL-10,
reduce immune dysfunction and thereby improve the survival rate in
septic mice.
C-reactive protein (CRP) is an acute phase protein,
which is produced by cells in the liver, kidney and lung under
pathological conditions. CRP is a sensitive marker for non-specific
inflammation in the body, and therefore is also a highly correlated
index of sepsis (17). CRP levels
reflect the severity of tissue injury and infection. Continuously
high-levels of CRP may stimulate SIRS and high metabolic reactions,
resulting in malnutrition and a lack of structural proteins,
exacerbating the disease process. In the present study, the serum
level of CRP was significantly higher in the model group than in
the sham-operated group, indicating severe inflammatory responses
in these models. The administration of midazolam alone or in
combination with fentanyl markedly downregulated the expression of
CRP, indicating inhibition of the inflammatory response in sepsis.
PCT is the 116-amino precursor to calcitonin, which has been
associated with the pathogenesis of sepsis and other inflammatory
injuries. It has been reported that the PCT level is positively
correlated with the severity of sepsis (18). In the present study, the PCT level
was lower in the midazolam group compared with that in all other
septic groups, with or without medications, which may be associated
with the lower mortality in this group.
In conclusion, the results demonstrated that
analgesic and/or sedative drugs are able to reduce inflammatory
responses in septic mice, and that immunosedation contributes to
the improved mortality rate in these models. This study
investigated the modulating effects of analgesia/sedation on the
immune function in sepsis, providing a theoretical basis for
further clinical studies concerning the treatment of sepsis.
Further studies are required to elucidate the detailed mechanism(s)
through which analgesia/sedation exert immune-regulating
effects.
Acknowledgements
This study was supported by a grant from the
People’s Hospital of Xinjiang Uygur Autonomous Region (no.
20100119).
References
|
1
|
Angus DC and van der Poll T: Severe sepsis
and septic shock. N Engl J Med. 369:840–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dellinger RP, Levy MM, Rhodes A, et al;
Surviving Sepsis Campaign Guidelines Committee including the
Pediatric Subgroup. Surviving sepsis campaign: international
guidelines for management of severe sepsis and septic shock: 2012.
Crit Care Med. 41:580–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanabe K, Kozawa O and Iida H: Midazolam
suppresses interleukin-1β-induced interleukin-6 release from rat
glial cells. J Neuroinflammation. 8:682011. View Article : Google Scholar
|
|
4
|
Li SH, Chen YX, Lv HY and Li JZ: Effects
of midazolam on the systemic inflammatory response syndrome in
patients with gastric cancer after radical operation. Shiyong Yixue
Zazhi. 24:3181–3183. 2008.(In Chinese).
|
|
5
|
Wu Y, Wang Y and Zhan J: Effects of
remifentanyl and fentanyl on LPS-induced cytokine release in human
whole blood in vitro. Mol Biol Rep. 36:1113–1117. 2009. View Article : Google Scholar
|
|
6
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun JX, An J and Lian J: The discussion of
influence factors on laboratory animal organ weight and
coefficients. Laboratory Animal Science. 26:49–51. 2009.(In
Chinese).
|
|
8
|
MacLaren R: Immunosedation: a
consideration for sepsis. Crit Care. 13:1912009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiao H, Sanders RD, Ma D, et al: Sedation
improves early outcome in severely septic Sprague Dawley rats. Crit
Care. 13:R1362009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boomer JS, To K, Chang KC, et al:
Immunosuppression in patients who die of sepsis and multiple organ
failure. JAMA. 306:2594–2605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okusawa S, Gelfand J, Ikejima T, et al:
Interleukin 1 induces a shock-like state in rabbits. Synergism with
tumor necrosis factor and the effect of cyclooxygenase inhibition.
J Clin Invest. 81:1162–1172. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van der Poll T, Romijn JA, Endert E, et
al: Tumor necrosis factor mimics the metabolic response to acute
infection in healthy humans. Am J Physiol. 261:E457–E465.
1991.PubMed/NCBI
|
|
13
|
van Deuren M, van der Ven-Jongekrijg H,
Baterlink AK, et al: Correlation between proinflammatory cytokines
and antiinflammatory mediators and the severity of disease in
meningococcal infections. J Infect Dis. 172:433–439. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Munoz C, Misset B, Fitting C, et al:
Dissociation between plasma and monocyte-associated cytokines
during sepsis. Eur J Immunol. 21:2177–2184. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Babcock GF, Hernandez L, Yadav E, et al:
The burn wound inflammatory response is influenced by midazolam.
Inflammation. 35:259–270. 2012. View Article : Google Scholar
|
|
16
|
Han Lu, Tao Ma, Wenquan Hu, et al: The
study on immunologic injury caused by sepsis. Chinese Journal of
Coal Industry Medicine. 15:890–892. 2012.(In Chinese).
|
|
17
|
Mamani M, Hashemi SH, Hajilooi M, et al:
Evaluation of fibronectin and C-reactive protein levels in patients
with sepsis: a case-control study. Acta Med Iran. 50:404–410.
2012.PubMed/NCBI
|
|
18
|
Luzzani A, Polati E, Dorizzi R, et al:
Comparison of procalcitonin and C-reactive protein as markers of
sepsis. Crit Care Med. 31:1737–1741. 2003. View Article : Google Scholar : PubMed/NCBI
|