Introduction
Slow transit constipation (STC) has attracted
increasing attention from medical researchers due to its serious
impact on the physical and mental health and quality of life of
patients. A questionnaire survey in the United States revealed that
~3% of the participants suffered from constipation (1). Among these, 15–30% of the
constipation cases were STC (2).
Frattini et al (3) defined
STC as a severe functional constipation that excludes pelvic floor
dysfunction, lacks physiological activity of the colon and exhibits
no response to medication. Knowles et al (4) proposed that the causes of STC include
primary intestinal neuronal or smooth muscle dysfunction,
degeneration of the interstitial cells of Cajal (ICC), an
autoimmune response, infectious agents, exogenous neurotoxic drugs,
psychological factors, intestinal absorption dysfunction and
endogenous morphine peptide and opioid receptor abnormalities. The
causes of STC are not isolated, but are closely associated with
each other. The existing treatment methods for STC primarily
include drugs, biofeedbacks and surgical treatments. Due to the
complex causes, the efficacy of STC treatment requires
improvement.
A cathartic colon is an important manifestation of
STC. In 1943, Heilbrun (5)
proposed the concept of a cathartic colon based on X-ray findings
of the colons of STC patients who had chronically used laxatives.
The author reported the shrinkage or disappearance of haustra and
the non-transit expansion and contraction of the colon diameter,
which were similar to pathological stenosis. These observations are
commonly present in the right half of the colon and often involve
the terminal ileum, leading to ileocecal valve opening and ileal
fold disappearance. Urso et al (6) pathologically examined surgical
specimens of cathartic colons and identified mucosal atrophy,
surface punctiform ulceration and chronic inflammation-induced
reactive thickening of the muscularis mucosa, as well as submucosal
fat-like infiltration and fibrosis.
STC can significantly affect patient quality of
life; however, only a subset of cases ultimately require surgical
treatment. Thus, human specimens for study are difficult to obtain.
For this reason, Zhang et al (7) established a rat model of a cathartic
colon by simulating the pathological changes in STC patients with
chronic use of irritant laxatives. Li et al (8) confirmed the usefulness of the rat
cathartic colon model for the study of STC.
Opiates inhibit gastrointestinal motility and
secretive functions through the activation of G protein-coupled
opioid receptors (9). Opioid
receptors can be divided into three subtypes, including μ (MOR), δ
(DOR) and κ (KOR), which are all highly expressed in the enteric
nervous system (ENS) (10,11). Opiates are widely administered for
the treatment of moderate to severe pain and have attracted
considerable attention from medical researchers for their effects
on gastrointestinal functions. A previous study reported that among
patients who chronically used opiates to treat non-cancerous pain,
~40% of patients experienced constipation, compared with 7.6% in
the control group. In the patients who used laxatives to treat
constipation, satisfactory results were achieved in only 46% of
cases. (12) STC is characterized
by severe gastrointestinal dysfunction and is to a certain degree
similar to opiate-induced constipation. Therefore, changes in
opioid receptor expression, location and/or activity in the colon
may play an important role in the course of STC.
In the present study, the involvement of opioid
receptors in the course of STC was investigated by quantitative
analysis of the expression levels of the opioid receptors, MOR, DOR
and KOR, as well as those of regulator of G protein signaling 4
(RGS-4) and β-arrestin-2, in a cathartic colon rat model. The aim
of the study was to provide a foundation for further investigation
into opioid receptor function and signaling in STC patients.
Materials and methods
Animals
In total, 20 Wister rats (age, 7–8 weeks; male, 10;
female, 10; body weight, 200±20 g) were provided by the
Experimental Animal Center of the Third Military Medical University
(Chongqing, China). The rats were housed in individual cages with
conditions of 18–28°C and 40–80% relative humidity, access to food
and water ad libitum and a 12-h light/dark cycle. All
procedures and animal experiments were approved by the Animal
Ethical Committee of the Third Military Medical University.
Cathartic colon rat model
Animals were randomly divided into normal control
and cathartic colon groups (n=10 each). Ordinary soft feed was
provided for the control group, while experimental feed containing
phenolphthalein (analytical reagent; Fangzheng Chemistry, Tianjin,
China) was provided for the cathartic colon group. The two types of
feed were provided by the Experimental Animal Center of the Third
Military Medical University.
The animals were administered with an initial dose
of phenolphthalein at 200 mg/kg, which was increased by 200 mg/kg
daily until half of the animals had loose stools. Phenolpthalein is
a type of laxative, which is taken by patients with STC to loosen
stools. These patients have tolerance and dependence on the
laxative and taking phenolpthalein for long periods causes stools
to become less loose, requiring larger doses to have the same
loosening effect (13). This dose
was maintained until 80% of the animals had no loose stools.
Thereafter, the animals continuously received the drug until half
of the animals had loose stools. This drug protocol was repeated
three times. During the final protocol, drug administration was
stopped one week after loose stools had disappeared in 80% of the
animals. The animals were subsequently provided with ordinary soft
feed prior to further tests. The dose of the drug at the onset of
diarrhea was 1,200 mg/kg, and the final dose was 3,400 mg/kg
(8).
After eating a normal diet for one week, the animals
were fasted for 24 h and then sacrificed by cervical dislocation.
Complete colonic tissue samples were collected at ~3-cm distance
from the ileocecal junction. Sub-samples of colonic tissues were
stored at −70°C, and the remaining sections were fixed in 10%
formalin prior to further use.
Hematoxylin and eosin (H&E)
staining
Histopathological characteristics of 10%
formalin-fixed colonic tissue samples were examined by H&E
staining to confirm the successful establishment of the cathartic
colon rat model. The tissue samples were embedded in paraffin, cut
into 4-μm sections, dewaxed with xylene and then dehydrated in a
series of graded ethanol. Following washing with distilled water,
the sections were stained with hematoxylin for 5 min, followed by
rinsing in distilled water. Next, the sections were differentiated
in hydrochloric acid-ethanol for 30 sec, immersed in warm distilled
water (50°C) for 5 min and stained with eosin for 2 min. Following
dehydration and clarification, the sections were mounted and fixed
with neutral resin prior to examination under an XDS-500D inverted
microscope (Shanghai Caikang Optical Instrument Co., Ltd.,
Shanghai, China).
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
Colonic tissue samples were ground in liquid
nitrogen, and total RNA extraction was conducted with TRIzol
reagent (Tiangen Biotech Co., Ltd., Beijing, China), according to
the manufacturer’s instructions. The RNA purity and concentration
were determined using a UV spectrophotometer (Beckman Coulter,
Brea, CA, USA). Reverse transcription of the extracted RNA (2 μg)
was performed using a ReverTra Ace® reverse
transcription kit (Toyobo Co., Ltd., Osaka, Japan), according to
the manufacturer’s instructions. Primers were designed using Primer
Premier 5 software (PREMIER Biosoft International, Palo Alto, CA,
USA) and are shown in Table I. The
20-μl PCR system contained 2 μl cDNA, 0.5 μl forward primer, 0.5 μl
reverse primer, 10 μl Taq 2X PCR Master Mix (Tiangan Biotech Co.,
Ltd.), containing Taq DNA polymerase, PCR buffer, Mg2+, dNTPs, PCR
stabilizer and PCR reinforing agent, and 7 μl double-distilled
water. The PCR conditions were as follows: Predenaturation at 94°C
for 5 min, followed by 30 cycles of denaturation at 94°C for 30
sec, annealing at 57°C for 30 sec and extension at 72°C for 30 sec,
with a final extension step at 72°C for 10 min. The PCR products
were subjected to 1% agarose gel electrophoresis and the relative
mRNA expression levels were normalized against that of β-actin
using Quantity One software (Bio-Rad, Hercules, CA, USA).
 | Table IRT-PCR primers. |
Table I
RT-PCR primers.
| Gene | Primer sequences
(5′-3′) | Product length
(bp) |
|---|
| β-actin |
| Forward |
ACCCCGTGCTGCTGACCGAG | 249 |
| Reverse |
TCCCGGCCAGCCAGGTCCA | |
| KOR |
| Forward |
TCCCTGTTATCATCACCGCTGTC | 210 |
| Reverse |
CTCCAAAAGGCCAAGAATTCATCA | |
| DOR |
| Forward |
CCGTTCGGAGAGCTGCTGTG | 267 |
| Reverse |
GGGGAACTGGAGCGTGCATAC | |
| MOR |
| Forward |
ACCCCCCGAAATGCCAAAAT | 196 |
| Reverse |
CCGGCATGATGAAAGCGAAGA | |
| RGS-4 |
| Forward |
TTGGATCCATGTGCAAAGGACTCGACTAGGGAAG | 198 |
| Reverse |
ATACTCGAGTTAGGCACACTGAGGGACTAGGGAAG | |
| β-arrestin-2 |
| Forward |
GGGCAACTCAAGCACGAA | 205 |
| Reverse |
CCTCGCAAAGTCCTCAAAC | |
Western blot analysis
Colonic tissue samples were ground to powder in
liquid nitrogen and harvested in Eppendorf tubes (50–80 mg each).
An appropriate volume (100 μl/10 mg) of radioimmunoprecipitation
assay protein lysis buffer (Jinmai Biotechnology, Chongqing, China)
was added to each tube. The tissue samples were lysed on ice for 30
min and then centrifuged at 13,000 xg at 4°C for 15 min. The
supernatants were collected to determine the protein concentration
using the bicinchoninic acid method (Beyotime Institute of
Biotechnology, Haimen, China). The protein samples (80 μg each)
were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gels for
electrophoresis. Following separation, the protein products were
transferred to polyvinylidene membranes (Millipore Corporation,
Billerica, MA, USA) and blocked with 5% milk powder diluted in
Tris-buffered saline with 0.05% Tween 20 (TBST) for 2 h. The
membranes were washed and incubated sequentially with primary
antibodies (1:500) targeted against MOR (sc-27072), KOR (sc-7493),
DOR (sc-7492), RGS-4 (sc-6203), β-arrestin-2 (sc-13140) and β-actin
(sc-47778; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
4°C overnight. Next, the membranes were washed with TBST three
times for 10 min each time. Appropriate secondary antibodies (Santa
Cruz Biotechnology, Inc.) were added to the membranes, and the
reaction system was shaken at room temperature for 2 h. Thereafter,
the membrane was washed with TBST three times for 10 min each time.
Luminescence of the protein was achieved using an enhanced
chemiluminescence method (Thermo Fisher Scientific, Waltham, MA,
USA). Gel images obtained from the western blot analysis assays
were processed using Quantity One software. Relative protein
expression levels were normalized against those of β-actin.
Statistical analysis
All statistical analyses were conducted using SPSS
version 21.0 (IBM, Armonk, NY, USA). Data are presented as the mean
± standard deviation. Differences between groups were analyzed
using the independent samples t-test, where P<0.05 was
considered to indicate a statistically significant difference.
Results
Histopathological changes in the rat
cathartic colon
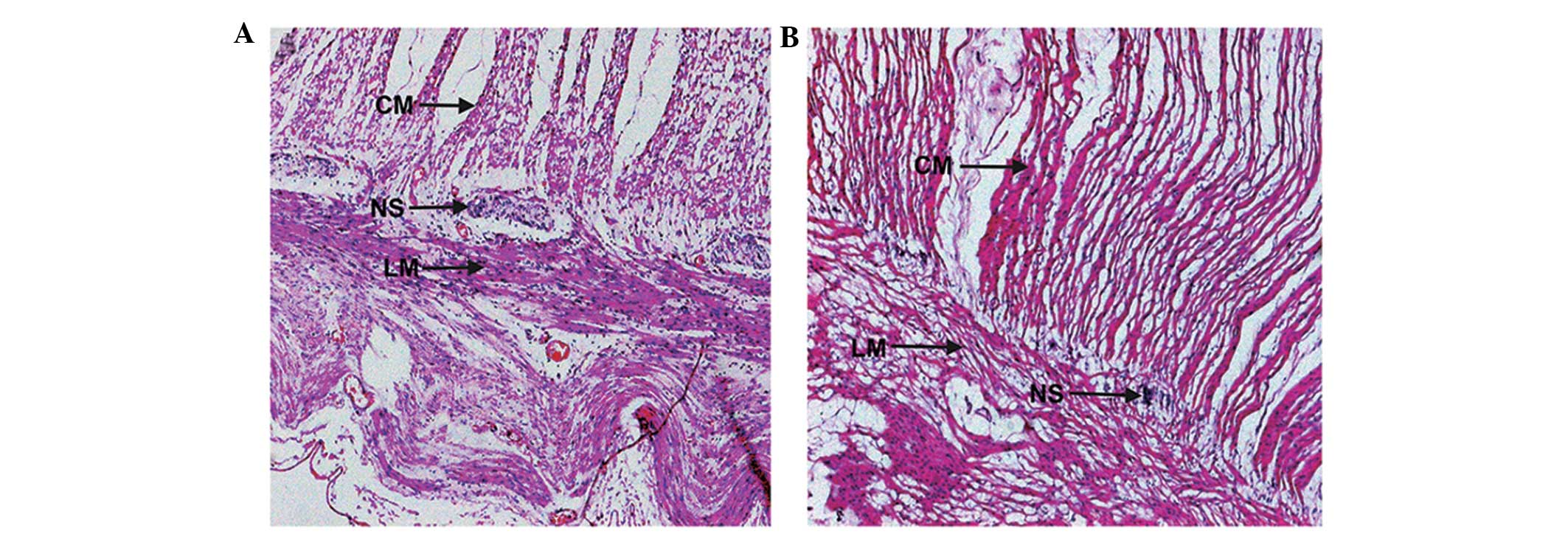
H&E staining revealed that in the normal control
rat colon, myenteric neurons were regular and plump with uniformly
stained cytoplasm. In addition, dense muscle fibers were present in
the intestinal wall (Fig. 1A). In
the cathartic colon group, the myenteric neurons were visibly
shrunken with a reduced volume, reduced cytoplasmic staining and
loose muscle fibers in the intestinal wall (Fig. 1B). The histopathological changes in
cathartic colon confirmed that the rat model was successfully
established in all the treated animals.
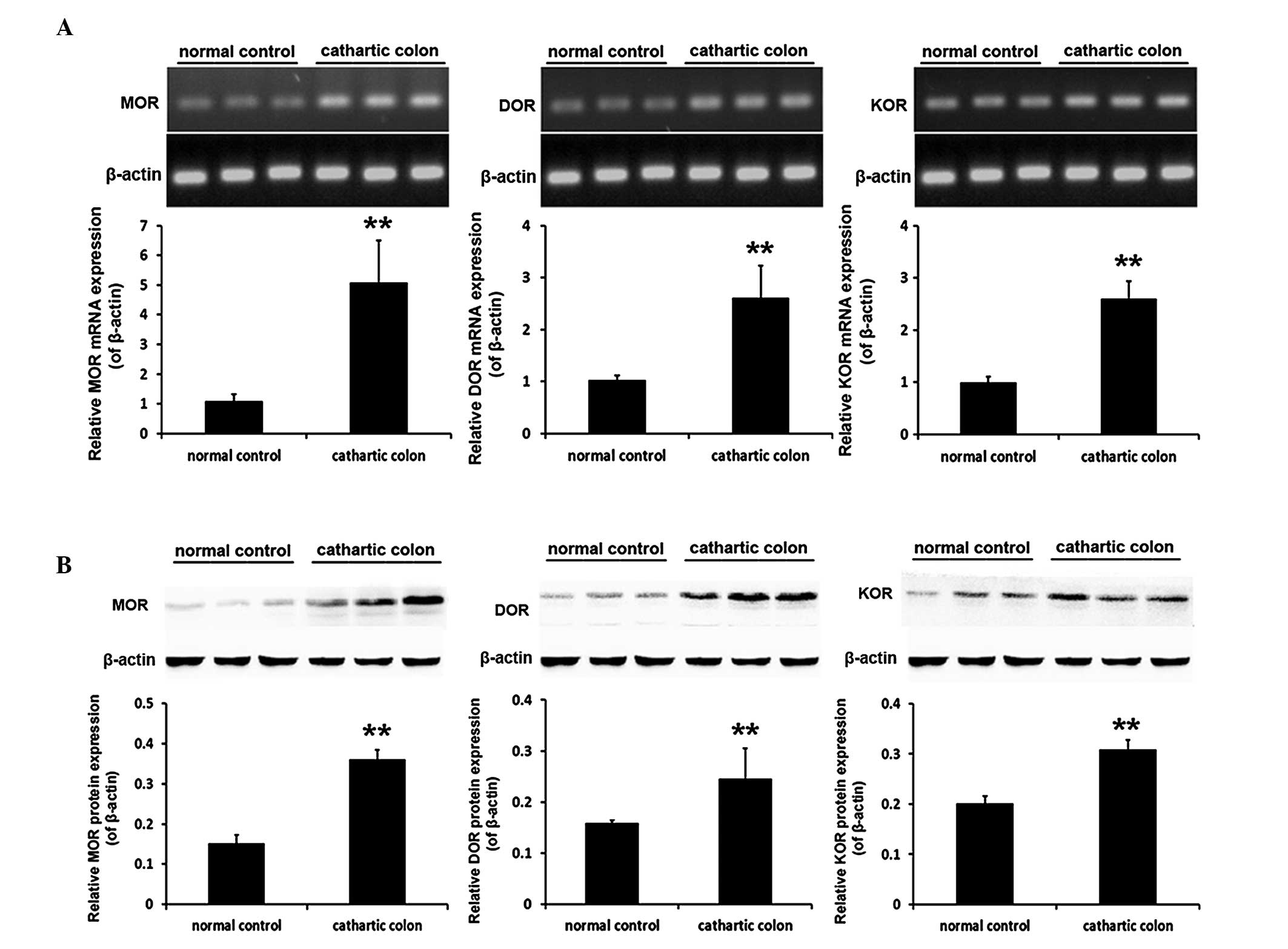
mRNA and protein expression levels of the
opioid receptors in the rat cathartic colon
Following the successful establishment of a
cathartic colon rat model using a phenolphthalein stimulus, the
mRNA and protein expression levels of the three opioid receptor
subtypes in the rat colon were found to be significantly higher in
the cathartic colon group compared with the levels in the normal
control group (all P<0.001). The mRNA expression levels of MOR
were 4.7-fold greater, while DOR expression was 2.5-fold greater
and KOR protein levels had increased by 2.6 fold. With regard to
the protein expression levels, MOR protein was 2.4-fold greater,
DOR expression had increased by 1.5 fold and KOR protein was
1.5-fold greater (Fig. 2).
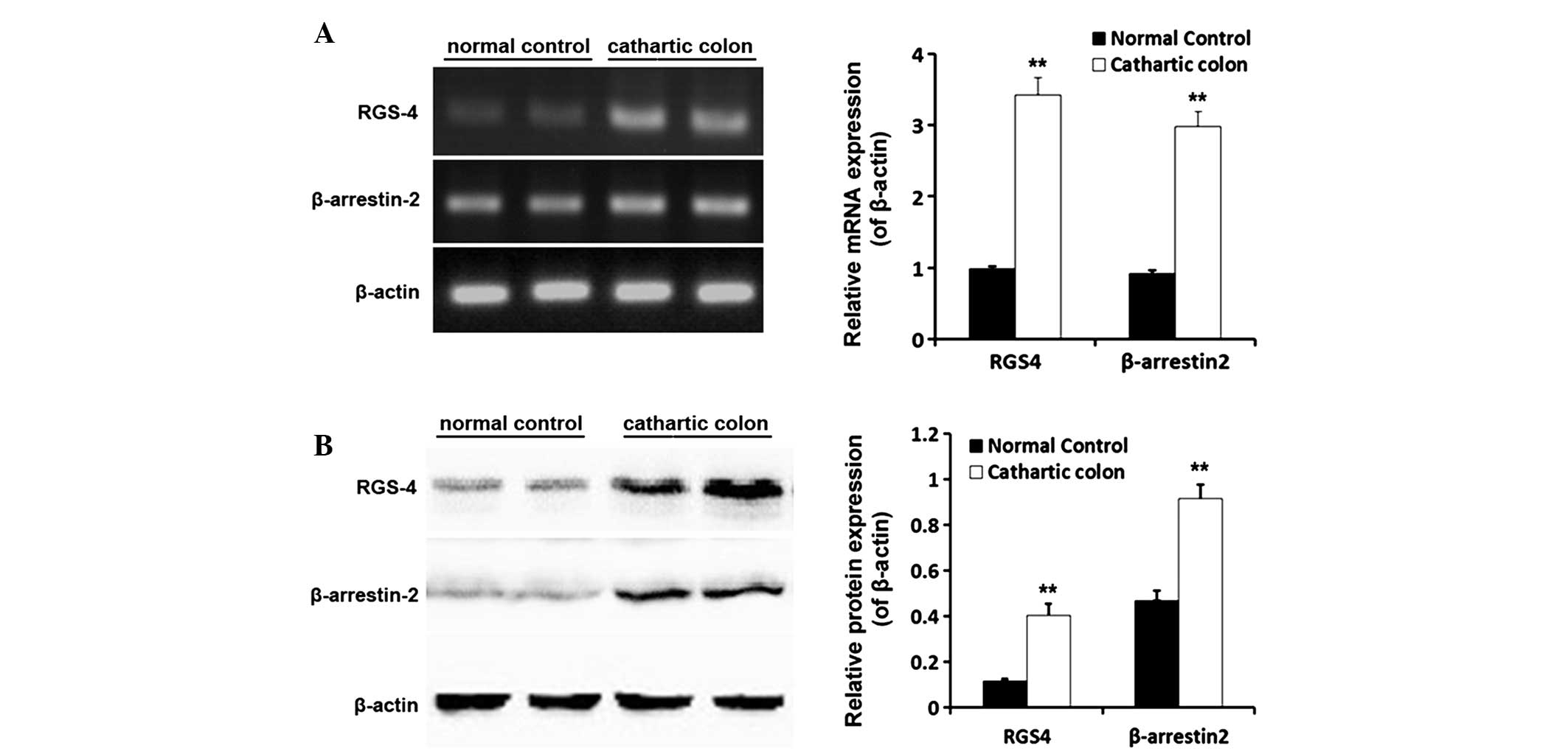
mRNA and protein expression levels of
RGS-4 and β-arrestin-2 in the rat cathartic colon
Following successful establishment of the cathartic
colon rat model using a phenolphthalein stimulus, the mRNA and
protein expression levels of RGS-4 and β-arrestin-2 in the rat
colon were shown to be significantly higher in the cathartic colon
group when compared with those in the normal control group (all
P<0.01). The mRNA expression levels of RGS-4 increased 3.4 fold,
while β-arrestin-2 mRNA expression was 3.2-fold greater. With
regard to the protein expression levels, RGS-4 levels were 3.5-fold
greater and β-arrestin-2 expression had increased by 2.0 fold
(Fig. 3).
Discussion
The ENS is the largest and most complex nervous
system outside of the central nervous system, consisting of
ganglionated plexuses, the myenteric plexus between the
longitudinal and circular muscles, the deep muscular plexus and the
submucosal plexus, all of which are interconnected by nerve fibers
to form a network system (14).
The regulation of gastrointestinal physiological functions by the
ENS involves multiple processes, including motility patterns,
gastric acid secretion, fluid flow through epithelial cells, local
blood flow changes, digestion and absorption of nutrients and
interactions with the gastrointestinal immune and endocrine systems
(15).
Previous studies have shown that MOR, DOR and KOR
are highly expressed in the myenteric and submucosal plexuses. In
addition, the receptors are distributed in nerve fibers throughout
the muscle, mucosa, intestinal blood vessels, lymphatic nodes and
the adjacent ICC (10,16).
In the present study, a cathartic colon rat model
with typical STC was successfully established by feeding the
animals with gradually increasing doses of phenolphthalein,
designed to simulate the long-term use of irritant laxatives in STC
patients. The results of the semi-quantitative RT-PCR and western
blot analysis assays demonstrated that all three subtypes of opioid
receptor (MOR, DOR and KOR) were expressed at significantly higher
levels (mRNA and protein) in the cathartic colon group when
compared with the control group (P<0.001). Similarly, a previous
study reported that the activity of opioid receptors in the
cathartic colon of rats was significantly higher compared with the
control group (17) Opioid
receptors are G protein-coupled metabotropic membrane receptors.
Once activated, opioid receptors immediately enter cells via a
concentration-dependent endocytosis mechanism, and exert biological
effects through the activation of K+ channels, membrane
hyperpolarization, Ca2+ channel inhibition and cyclic
adenosine monophosphate generation (16). Opioid receptor agonists can
simultaneously inhibit excitatory and inhibitory neurons of the
ENS. Blocking the excitatory pathway can inhibit the release of
excitatory neurotransmitters, including acetylcholine, thereby
preventing intestinal smooth muscle tension-dependent peristaltic
contractions. By contrast, blocking the inhibitory pathway can
reduce the release of nitric oxide, subsequently increasing the
resting tension and non-propulsive peristalsis of the smooth muscle
(17). Previous studies have also
demonstrated that morphine, a non-selective opioid receptor
agonist, can inhibit the Na+ pathway in ENS neurons,
which increases the action potential threshold, but reduces the
amplitude, with a net effect of a lower neuronal excitability
(18). Bell et al (19) found that 45% of patients who
chronically used opioids reported less than three bowel movements
per week.
In addition, opioid receptor agonists can
significantly affect the movement and secretive functions of the
gastrointestinal tract (20). Pol
et al (9) reported that the
upregulation of MOR expression in the intestinal tract led to
intestinal dysfunction (9). Liu
et al (21) found that MOR
and KOR play an important role in regulating intestinal tract
movement in a cathartic colon rat model. Furthermore, De Luca and
Coupar (22) indicated that
morphine and other opiates can reduce the rate of peristalsis,
cause the excessive absorption of water and electrolytes from the
intestinal contents and reduce intestinal fluid secretion,
resulting in constipation. Wood (17) demonstrated that morphine can
hyperpolarize secretomotor neurons and inhibit intestinal mucosa
secretory activity. Previous studies investigating the effect of
endogenous opioid peptides on the colons of STC patients revealed
that met-enkephalin (23,24) and dynorphin (22) expression levels exhibited no
evident abnormalities, while leu-enkephalin expression was
decreased compared with the control colon (24). Ross et al (26) studied changes in morphine tolerance
in the intestinal tract of mice and concluded that the reduced
tolerance of the colon to opiates is one of the fundamental causes
of gastrointestinal dysfunction. Regardless of whether the opiates
are endogenous or exogenous, opioid receptors must be activated to
exert their biological effects. Therefore, increased expression and
enhanced activity levels of opioid receptors in the colon may play
an important role in the course of STC characterized by movement
disorders.
In the present study, the expression levels of RGS-4
and β-arrestin-2 were also investigated, and it was found that the
expression levels were significantly higher in the cathartic colon
group rats. RGS-4 is involved in the regulation of smooth muscle
contraction (27), and increased
levels are associated with decreased contraction (28). β-arrestins are a group of
scaffolding proteins that modulate inflammatory pathways, and
previous studies have indicated that β-arrestin-2 is involved in
opioid-induced bowel dysfunction and opioid tolerance (29,30).
The results of the present study provide evidence that these
signaling pathways are likely to be involved in the pathogenesis of
STC and may be potential novel therapeutic targets.
In conclusion, the experimental results demonstrated
that the expression levels of MOR, DOR and KOR were significantly
increased in the cathartic colons of rats. The increase in MOR
expression was more evident compared with that observed for KOR and
DOR expression, indicating that MOR potentially plays a more
important role than the other two subtypes of opioid receptors in
the course of STC. However, this hypothesis requires further
investigation and more in-depth research on the three receptors.
Due to the complex etiology of STC, the enhanced expression levels
of opioid receptors, RGS-4 and β-arrestin-2 in the cathartic colons
of the rats can be regarded as a result of the pathophysiological
changes in the colon during the course of STC; however, this change
cannot be considered as an initiating factor for STC based on the
data presented in the current study. A large-scale epidemiological
survey on STC, combined with a summary of predisposing factors and
an investigation into the regulatory mechanisms of opioid receptor
expression, may significantly contribute to and further the
understanding into the causes of STC.
Acknowledgements
The study was supported by grants from the
International S&T Cooperation Program of Chongqing (no.
CSTC201110010), the National Natural Science Foundation of China
(no. 81100259) and the Natural Science Foundation of Chongqing (no.
CSTC2011jjA10061).
References
|
1
|
Johanson JF: Definitions and Epidemiology
of Constipation. Constipation. Wexner SD and Duthie GS: 2nd
Edition. Springer Publishing; London: pp. 1–8. 2008
|
|
2
|
Bassotti G, Roberto GD, Sediari L and
Morelli A: Toward a definition of colonic inertia. World J
Gastroenterol. 10:2465–2467. 2004.PubMed/NCBI
|
|
3
|
Frattini JC and Nogueras JJ: Slow transit
constipation: a review of a colonic functional disorder. Clin Colon
Rectal Surg. 21:146–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Knowles CH and Martin JE: Slow transit
constipation: a model of human gut dysmotility. Review of possible
aetiologies. Neurogastroenterol Motil. 12:181–196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heilbrun N: Roentgen evidence suggesting
enterocolitis associated with prolonged cathartic abuse. Radiology.
41:486–491. 1943. View
Article : Google Scholar
|
|
6
|
Urso FP, Urso MJ and Lee CH: The cathartic
colon: pathological findings and radiological/pathological
correlation. Radiology. 116:557–559. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Ferguson SS, Barak LS, et al:
Role for G protein-coupled receptor kinase in agonist-specific
regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci
USA. 95:7157–7162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li HY, Yan X, Xue QL, et al: Effects of
nociceptin/orphanin FQ on rats with cathartic colon. World J
Gastroenterol. 13:141–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pol O, Alameda F and Puig MM: Inflammation
enhances mu-opioid receptor transcription and expression in mice
intestine. Mol Pharmacol. 60:894–899. 2001.PubMed/NCBI
|
|
10
|
Bagnol D, Mansour A, Akil H and Watson SJ:
Cellular localization and distribution of the cloned mu and kappa
opioid receptors in rat gastrointestinal tract. Neuroscience.
81:579–591. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poonyachoti S, Kulkarni-Narla A and Brown
DR: Chemical coding of neurons expressing delta- and kappa-opioid
receptor and type I vanilloid receptor immunoreactivities in the
porcine ileum. Cell Tissue Res. 307:23–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pappagallo M: Incidence, prevalence, and
management of opioid bowel dysfunction. Am J Surg. 182(5A Suppl):
11S–18S. 2001. View Article : Google Scholar
|
|
13
|
Bin Lu, Mei Wang, Fan YH, et al: The study
of nerve growth factor and its receptor in the rat cathartic colon.
Chinese Journal of Digestion. 11:684–687. 2004.
|
|
14
|
Furness JB: The enteric nervous system and
neurogastroenterology. Nat Rev Gastroenterol Hepatol. 9:286–294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Furness JB: The Enteric Nervous System.
1st Edition. Blackwell Publishing; Oxford: pp. 132–198. 2006
|
|
16
|
Poole DP, Pelayo JC, Scherrer G, Evans CJ,
Kieffer BL and Bunnett NW: Localization and regulation of
fluorescently labeled delta opioid receptor, expressed in enteric
neurons of mice. Gastroenterology. 141:982–991. e1–e8. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wood JD and Galligan JJ: Function of
opioids in the enteric nervous system. Neurogastroenterol Motil.
16(Suppl 2): 17–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith TH, Grider JR, Dewey WL and Akbarali
HI: Morphine decreases enteric neuron excitability via inhibition
of sodium channels. PloS One. 7:e452512012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bell TJ, Panchal SJ, Miaskowski C, Bolge
SC, Milanova T and Williamson R: The prevalence, severity, and
impact of opioid-induced bowel dysfunction: results of a US and
European Patient Survey (PROBE 1). Pain Med. 10:35–42. 2009.
View Article : Google Scholar
|
|
20
|
Sanger GJ and Tuladhar BR: The role of
endogenous opioids in the control of gastrointestinal motility:
predictions from in vitro modelling. Neurogastroenterol Motil.
16(Suppl 2): 38–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu BH, Mo P and Zhang SB: Effects of mu
and kappa opioid receptor agonists and antagonists on contraction
of isolated colon strips of rats with cathartic colon. World J
Gastroenterol. 10:1672–1674. 2004.PubMed/NCBI
|
|
22
|
De Luca A and Coupar IM: Insights into
opioid action in the intestinal tract. Pharmacol Ther. 69:103–115.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dolk A, Brodén G, Holmström B, Johansson C
and Schultzberg M: Slow transit chronic constipation (Arbuthnot
Lane’s disease). An immunohistochemical study of
neuropeptide-containing nerves in resected specimens from the large
bowel. Int J Colorectal Dis. 5:181–187. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sjölund K, Fasth S, Ekman R, et al:
Neuropeptides in idiopathic chronic constipation (slow transit
constipation). Neurogastroenterol Motil. 9:143–150. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Porter AJ, Wattchow DA, Hunter A and Costa
M: Abnormalities of nerve fibers in the circular muscle of patients
with slow transit constipation. Int J Colorectal Dis. 13:208–216.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ross GR, Gabra BH, Dewey WL and Akbarali
HI: Morphine tolerance in the mouse ileum and colon. J Pharmacol
Exp Ther. 327:561–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Li F, Liu S, et al:
MEKK1-MKK4-JNK-AP1 pathway negatively regulates Rgs4 expression in
colonic smooth muscle cells. PloS One. 7:e356462012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu W, Mahavadi S, Li F and Murthy KS:
Upregulation of RGS4 and downregulation of CPI-17 mediate
inhibition of colonic muscle contraction by interleukin-1beta. Am J
Physiol Cell Physiol. 293:C1991–C2000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maguma HT, Dewey WL and Akbarali HI:
Differences in the characteristics of tolerance to μ-opioid
receptor agonists in the colon from wild type and β-arrestin2
knockout mice. Eur J Pharmacol. 685:133–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang M, Maguma HT, Smith TH, Ross GR,
Dewey WL and Akbarali HI: The role of β-arrestin2 in the mechanism
of morphine tolerance in the mouse and guinea pig gastrointestinal
tract. J harmacol Exp Ther. 340:567–576. 2012. View Article : Google Scholar
|