Introduction
In total, ~5% of people worldwide suffer from
emotional disorders (1). Among the
negative emotions, anger is one of the most intolerable and the
most closely associated with the occurrence of diseases, such as
cardiovascular events (2). When
anger is triggered, the sympathetic nervous system is activated and
the sympathoadrenomedullary system is stimulated. As a result, the
endocrine system is activated, causing an increase in the levels of
cortisol, angiotensin, thyroxine, hyperglycemic factor and hormones
from the posterior pituitary lobe in the blood. These exert an
effect on the liver and other organs and may thus cause a series of
disorders (3). A previous study
reported that dysfunction of the network consisting of the nervous,
endocrine and immune systems is frequently observed during
senescence and other complex diseases, including cancer, diabetes,
hypertension and asthma (4). Liu
et al (5) demonstrated that
the levels of plasma adrenocorticotropic hormone and serum
serotonin were significantly increased, while the serum level of
interleukin-2 was significantly decreased, in the rat anger groups
compared with the control group. From population, clinical and
animal experimental studies, Qiao et al (6) observed that anger was expressed in
two different manners: ‘Anger-in’ (tendency to suppress anger) and
‘anger-out’ (tendency to express anger through verbal or physical
means); however, the mechanisms underlying this manner of
expression remain incompletely understood (6–8).
Emotion-related brain structures include the
cerebral cortex, hypothalamus and hippocampus. Among these, the
hippocampus plays an important role in context-dependent emotional
regulation as a higher-level regulatory center of stress reactions
(9). Chronic stress may cause
damage to the hippocampal region, leading to changes in its
structure and function. The hippocampus is part of the central
regulatory system of autonomic nervous activities, and hippocampal
damage has been found to be associated with certain cognitive
impairments caused by central nervous system diseases (10).
Jingqianping granule is a novel Chinese medicine
composed of Paeonia lactiflora Pall., Cyperus
rotundus Linn., Fructus Toosendan, Bupleurum
chinensis DC., Rhizoma Chuanxiong, Citrus
aurantium L., Pinellia ternata (Thunb.), Alpinia
katsumadai hayata, Glycyrrhiza uralensis Fisch., and
costus root. The main functional component is saikoside, which has
analgesic, sedative, anticonvulsant and antiepileptic effects. A
second novel Chinese medicine, Jingqianshu granule, is formulated
from Paeonia lactiflora Pall., Angelica sinensis
(Oliv.) Diels., Bupleurum chinensis DC., Atractylodes
lancea (Thunb.) DC., Cortex Moutan and Cyperus rotundus
Linn. Saikoside A from thorowax roots can significantly reverse the
reduction in monoamine neurotransmitters in the brain caused by
depression and thus relieve depressive disorders (11). Yang et al (12) revealed that the antidepressive
effect of thorowax extract may be associated with the strengthening
of oxidation resistance in chronic, unpredictable, mild stress
rats. It is therefore possible to interfere with emotions using
drugs in order to explore potential targets and signaling networks,
and thus provide insights into the molecular mechanisms of
anger.
In the present study, animal models of anger-in and
anger-out emotional reactions were established and the cause and
pathogenesis of the condition were systematically investigated via
multiple perspectives using DNA chip technology. Furthermore, the
effects of the aforementioned Chinese medicines on the anger-in and
anger-out animal models were assessed, and molecular-level dynamics
were investigated with modern biotechnology from systems biology
and bioinformatics.
Materials and methods
Reagents
Jingqianping granules were provided by Sichuan
Hairong Pharmaceutical Co. Ltd. of the Yangtze River Pharmaceutical
Group (Dujiangyan, China). Jingqianshu granules were provided by
the Qinhuangdao Shanhaiguan Pharmaceutical Factory (Qinhuangdao,
China). Other reagents used included the RNase inhibitor (Toyobo,
Osaka, Japan), TRIzol™ (cat no. 15596-026; Invitrogen Life
Technologies, Carlsbad, CA, USA) and Moloney Murine Leukemia Virus
reverse transcriptase (M-MLV RT; cat no. M1701; Promega Corp.,
Madison, WI, USA). Primers were synthesized by Jinan BioAsia
Biotechnology Co. Ltd (Jinan, China).
Animals
A total of 50 male Wistar rats (weighing 220–250 g)
were provided by the Laboratory Animal Center of Shandong
University of Traditional Chinese Medicine (Jinan, China). The
Wistar rats were divided into five groups, with 10 rats in each
group. The five groups were as follows: Normal control (control),
anger-in model (AIM), anger-in Jingqianshu-administered (AIA),
anger-out model (AOM) and anger-out Jingqianping-administered
(AOA). All animal experiments were conducted in accordance with the
ethical guidelines of Shandong University of Traditional Chinese
Medicine. The present study was approved by the Institutional
Committee for Animal Care and Use of Shandong University of
Traditional Chinese Medicine (Approval ID: DWSY200805239).
In the administration period (14 days), the AIA and
AOA groups were subjected to intragastric administration of 2.4
g/kg Jingqianshu and 1.6 g/kg Jingqianping granules, respectively,
at 09:00 AM daily. The dosages were eight-fold greater the clinical
dosages recommended for patients (13). At the same time, the AIM, AOM and
control groups were administered an equal volume of sterilized
drinking water, which served as the control.
Establishment of anger-in and anger-out
rat models
The anger-in and anger-out rat models were
established using the social isolation and resident-intruder
methods. For the control group, 10 rats were maintained in standard
conditions. A total of 40 rats were used to establish the anger-in
and anger-out models. Briefly, for social isolation, 40 rats were
kept in separate cages (one rat/cage) for seven days. Subsequently,
a resident-intruder test was performed on these 40 rats for a
further seven days. Following the resident-intruder test, the
aggressive behavior of the rats was observed. Based on the scores
of aggressive behavior, the 20 rats with higher scores were defined
as the anger-out rat model. The 20 rats with lower scores were
defined as the anger-in rat model. The treatments were administered
once the models had been established. According to the treatments,
the 20 anger-out rats were further divided into the AOA group
(n=10, treated with 1.6 g/kg Jingqianping granules) and the AOM
group (n=10, treated with equal volume of sterilized drinking
water). The 20 anger-in rats were further divided into the AIA
group (n=10, treated with 2.4 g/kg Jingqianshu granules) and the
AIM group (n=10, treated with equal volume of sterilized drinking
water). The 10 rats in the control group were administrated an
equal volume of sterilized drinking water. The administration
lasted for two weeks. The first day of administration was defined
as week 0, the 7th day of administration was defined as week 1, and
the 14th day of administration was defined as week 2. At week 0, 1
and 2, the body weight of the rats was recorded. Open-field and
aggressive behavior tests were performed at week 0, 1 and 2. After
these tests, all rats were sacrificed by cervical dislocation.
Open-field test
The open-field test was performed as described in
our previous study (14).
Behavioral changes of the animals in 5 min were recorded by a
camera system. A horizontal (crossing) score was recorded as the
number of squares that the animal crossed in the time period. A
vertical (rearing) score was recorded as the number of times that
the animal reared in the same period. The sum of the horizontal and
vertical scores was recorded as the total score of each open-field
test. The grooming period, grooming times, time sitting in the
central square and the number of fecal boli were also recorded.
Resident-intruder test
The resident-intruder test was subsequently
performed as described in our previous study (14). Briefly, two randomly selected rats,
which were maintained in separate cages, were placed in the same
cage for 15 min. The rats were then moved back to their individual
cages. The resident-intruder test was conducted at 12:00 PM daily
for seven days.
Aggressive behavior test
Aggressive behaviors were scored in a blinded manner
by three observers who had been identically trained. Each of the
recorded videos was examined and the rats’ behaviors were scored by
the three observers independently. The scored behavior items
included the number of attacks, length of attacking period, number
of biting and climbing episodes, length of climbing periods and
number of instances of piloerection.
Gene expression analyses
RNAs were isolated from the control, AIM, AIA, AOM
and AOA groups using the Qiagen RNeasy Mini kit (Qiagen, Inc.,
Valencia, CA, USA), according to the manufacturer’s instructions.
Global gene expression levels were analyzed using Affymetrix
GeneChip® Expression Analysis (Shanghai Biochip Co.,
Ltd., Shanghai, China). GeneChip Operating Software version 1.4
(Affymetrix, Santa Clara, CA, USA) was used for the statistical
analysis of the data. Probe preparation, microarray hybridization,
scanning and data extraction were performed according to the
Affymetrix GeneChip Expression Analysis manual instructions. For
determination of the differential gene expression, the log ratio
was set as ≥0.8 for the genes with upregulated expression and ≤0.8
for the genes with downregulated expression (P<0.5). GeneSpring
10.0 (Agilent Technologies, Inc., Santa Clara, CA, USA) and public
databases, including BioCarta, Kyoto Encyclopedia of Genes and
Genomes, GenMAPP and National Center for Biotechnology Information
Genbank, were searched to analyze the signaling pathways in which
the regulated genes were likely to be involved.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the hippocampal tissue
of the rats using the Qiagen RNeasy Mini kit, according to the
manufacturer’s instructions. RT was conducted according to the
instructions of the M-MLV Reverse Transcriptase kit (Promega
Corp.). The sequences of the primers are listed in Table I. Following electrophoresis, the
gels were subjected to a densitometric scan with the SmartView
Image System (SmartView, Irvine, CA, USA) in order to determine the
mRNA expression levels of the target genes. The reference gene was
β-actin. For amplification of 5-Htr2C, the following PCR
procedure was used: Pre-denaturation at 94°C for 2 min 30 sec, 30
cycles of denaturation at 94°C for 30 sec, annealing at 57.4°C for
40 sec and extension at 72°C for 1 min, and final extension at 72°C
for 7 min. For amplification of
GABABR2, the following PCR
procedure was used: Pre-denaturation at 94°C for 2 min 30 sec, 30
cycles of denaturation at 94°C for 30 sec, annealing at 57°C for 40
sec and extension at 72°C for 1 min, and final extension at 72°C
for 7 min. For amplification of 5-Htr3B, the following PCR
procedure was used: Pre-denaturation at 94°C for 2 min 30 sec, 35
cycles of denaturation at 94°C for 30 sec, annealing at 53.1°C for
40 sec and extension at 72°C for 1 min, and final extension at 72°C
for 7 min. For amplification of β-actin, the following PCR
procedure was used: Pre-denaturation at 94°C for 2 min 30 sec, 28
cycles of denaturation at 94°C for 30 sec, annealing at 59°C for 40
sec and extension at 72°C for 1 min, and final extension at 72°C
for 7 min. The amplification products were run on agarose gels. The
optical density of the PCR products of each gene was analyzed using
SmartView® software (Fluke, Everett, WA, USA). The ratio
between the optical density of the PCR products of each target gene
and the reference gene β-actin was used to determine the relative
expression level of the target gene.
 | Table IPrimers used in the present study. |
Table I
Primers used in the present study.
| Genes | Sequences | Product size
(bp) |
|---|
| β-actin | F:
5′-TGGTGGGTATGGGTCAGAAGGACTC-3′
R: 5′-CATGGCTGGGGTGTTGAAGGTCTCA-3′ | 265 |
| 5-Htr2C | F:
5′-TTCGTTCTCATCGGGTCCTT-3′
R: 5′-CACATAGCCAATCCACACAA-3′ | 441 |
|
GABABR2 | F:
5′-CCATCTGGCTTGGCATTGTC-3′
R: 5′-CTGTGCTCTCTGTGAAGTTGC-3′ | 370 |
| 5-Htr3B | F: 5′-TCTCTCCCT
CTCAGTGCCAT-3′
R: 5′-CAAGAGGCTCACAACATAGGC-3′ | 582 |
Western blotting
Total proteins were harvested from rat hippocampal
tissue, separated on 10% SDS/PAGE gel and subjected to immunoblot
analysis. The following primary antibodies were used: Goat
anti-rabbit polyclonal γ-aminobutyric acid B receptor 2
(GABABR2; cat. no. G9920; 1:200), which was purchased
from Sigma-Aldrich (St. Louis, MO, USA); and goat anti-sheep
polyclonal 5-hydroxytryptamine receptor 2C (5-Htr2C; cat. no.
sc-1464; 1:200), goat anti-sheep polyclonal 5-Htr3B (cat. no.
sc-51198; 1:200) and mouse anti-chicken monoclonal β-actin (cat.
no. sc-47778; 1:10,000), which were all obtained from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). The secondary antibodies
used were donkey anti-goat immunoglobulin G (IgG)-horseradish
peroxidase (HRP) (cat. no. sc-2020; 1:5,000; Santa Cruz
Biotechnology, Inc.) and goat anti-mouse IgG-HRP (cat. no. sc-2005;
1:10,000; Santa Cruz Biotechnology, Inc.). Bound antibodies were
detected using an ECL system (Pierce Biotechnology; Thermo Fisher
Scientific Inc., Rockford, IL USA). The immunoblot experiments were
repeated ≥3 times. The mean normalized optical density of the
target protein band relative to that of the β-actin band from the
same individual was calculated.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Data were analyzed with SPSS 16.0 statistical analysis
software (SPSS., Inc., Chicago, IL, USA). Two-way analysis of
variance was used to compare the mean values from multiple groups
of samples. Fisher’s Least Significant Difference t-test was used
for comparisons between two groups. The significance level α was
set at 0.05. P<0.05 was considered to indicate a statistically
significant difference.
Results
Result of the rat behavioral tests
To investigate the genes associated with the
anger-in and anger-out emotions in humans, a rat model was
established. A total of 50 male Wistar rats were divided into five
groups (control, AIM, AIA, AOM and AOA), with 10 rats in each
group. Following the establishment of the rat models, the weights
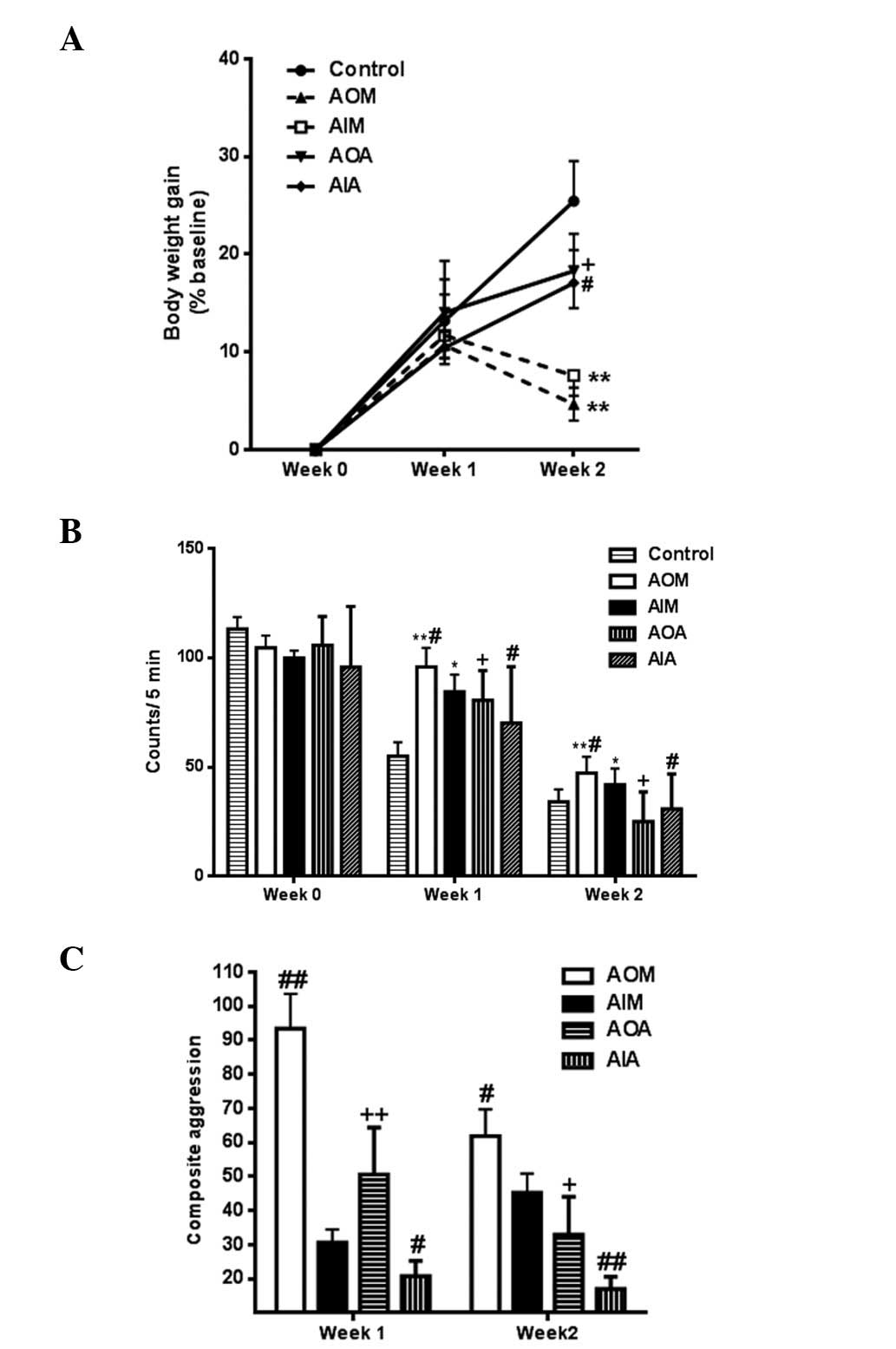
of the rats were measured over the first 2 weeks. As shown in
Fig. 1A, no significant
differences in body weight were observed among the rat groups in
the first week; however, after 2 weeks, the body weights of the
rats in the AIM and AOM groups were significantly decreased
compared with those of the control group rats. The body weight of
the rats in the AIA group at week 2 was significantly higher, as
compared with the AIM group (P<0.05). In addition, at week 2,
the body weight of the rats in the AOA group was also significantly
higher, as compared with the AOM group (P<0.05).
Open-field tests were performed to assess the
behavioral changes in the rats. As indicated in Fig. 1B, the scores of the rats in the AIM
and AOM groups were significantly higher (P<0.05) than those in
the control group in the first and second weeks. In the first and
second weeks, the scores of the AIM group were significantly lower,
as compared with the AOM group (P<0.05), whereas the scores were
significantly higher, as compared with the AIA group (P<0.05).
Furthermore, the AOA group had significantly lower scores in the
first and second weeks, as compared with the AOM group
(P<0.05).
As shown in Fig.
1C, the aggression scores of the rats in the AOM group were
significantly higher than those of the AIM group rats in the first
and second weeks. In addition, the AIA group had significantly
lower aggression scores in the first and second weeks, as compared
with the AIM group (P<0.05). Furthermore, in the first and
second weeks the AOA groups had significantly lower aggression
scores, as compared with the AOM group (P<0.05).
Differentially expressed genes in the rat
groups
To investigate the gene expression profiles in the
control, AIM, AIA, AOM and AOA groups, RNA was extracted from each
animal and subjected to global gene expression analysis. For the
determination of differential gene expression, the log ratio was
set as ≥0.8 for the genes with upregulated expression and ≤0.8 for
the genes with downregulated expression (P<0.5).
The number of regulated genes in each group is shown
in Table II. The numbers in the
control group were set as 0. The expression of 63 genes was
upregulated and that of 153 genes was downregulated in the AIM
group. In the AIA group, the expression of 23 genes was upregulated
and that of 196 genes was downregulated. In the AOM group, the
expression of 246 genes was upregulated and that of 256 genes was
downregulated. In the AOA group, the expression of 174 genes was
upregulated and that of 165 genes was downregulated. The variation
in gene regulation may have resulted from the differential levels
of stimulation in these groups. The drug-administered groups (AIA
and AOA) showed decreased numbers of up- and downregulated genes as
compared with the model groups (AIM and AOM), respectively,
suggesting that Jingqianshu and Jingqianping granules may attenuate
the behavioral changes of the rats.
 | Table IIScreening results of differentially
expressed genes among the groups. |
Table II
Screening results of differentially
expressed genes among the groups.
| Groups | Upregulated genes
(n) | Downregulated genes
(n) |
|---|
| Control | 0 | 0 |
| AIM | 63 | 153 |
| AIA | 23 | 196 |
| AOM | 246 | 256 |
| AOA | 174 | 165 |
Analysis of the 5-Htr2C,
GABABR2 and 5-Htr3B mRNA levels
Previous studies have reported that the genes
5-Htr2C and GABABR2
are associated with irritability and that 5-Htr3B is
associated with depression (15–17).
DNA chip analysis in the present study revealed that the three
genes were expressed differentially in the AIM and AOM rat groups.
The three genes were then subject to RT-qPCR analysis; the RT-qPCR
results (Fig. 2) were consistent
with the findings of the DNA chip analysis (Table II). As shown in Fig. 2A, the relative level of
5-Htr2C mRNA in the hippocampal tissue of the rats in
the AIM and AOM groups was higher than that in the control group
(P<0.01). The drugs attenuated the increase in the level of
5-Htr2C mRNA, as indicated by comparisons of the
levels of mRNA in the AIA and AOA groups versus those in the AIM
and AOM groups. As shown in Fig.
2B, the relative mRNA level of
GABABR2 was upregulated in the AIM
group but downregulated in the AOM group (P<0.01). The drugs
attenuated the alterations in the
GABABR2 mRNA expression, as
indicated by comparisons of the levels of mRNA in the AIA and AOA
groups versus those in the AIM and AOM groups. As shown in Fig. 2C, the relative levels of
5-Htr3B in the AIM and AOM groups were higher than those in
the control group (P<0.01). The drugs attenuated the increase in
5-Htr3B mRNA, as indicated by comparisons of the
levels of mRNA in the AIA and AOA groups versus those in the AIM
and AOM groups. These results therefore suggest that Jingqianshu
and Jingqianping granules attenuated the alterations in the levels
of mRNA induced by anger-in and anger-out emotions.
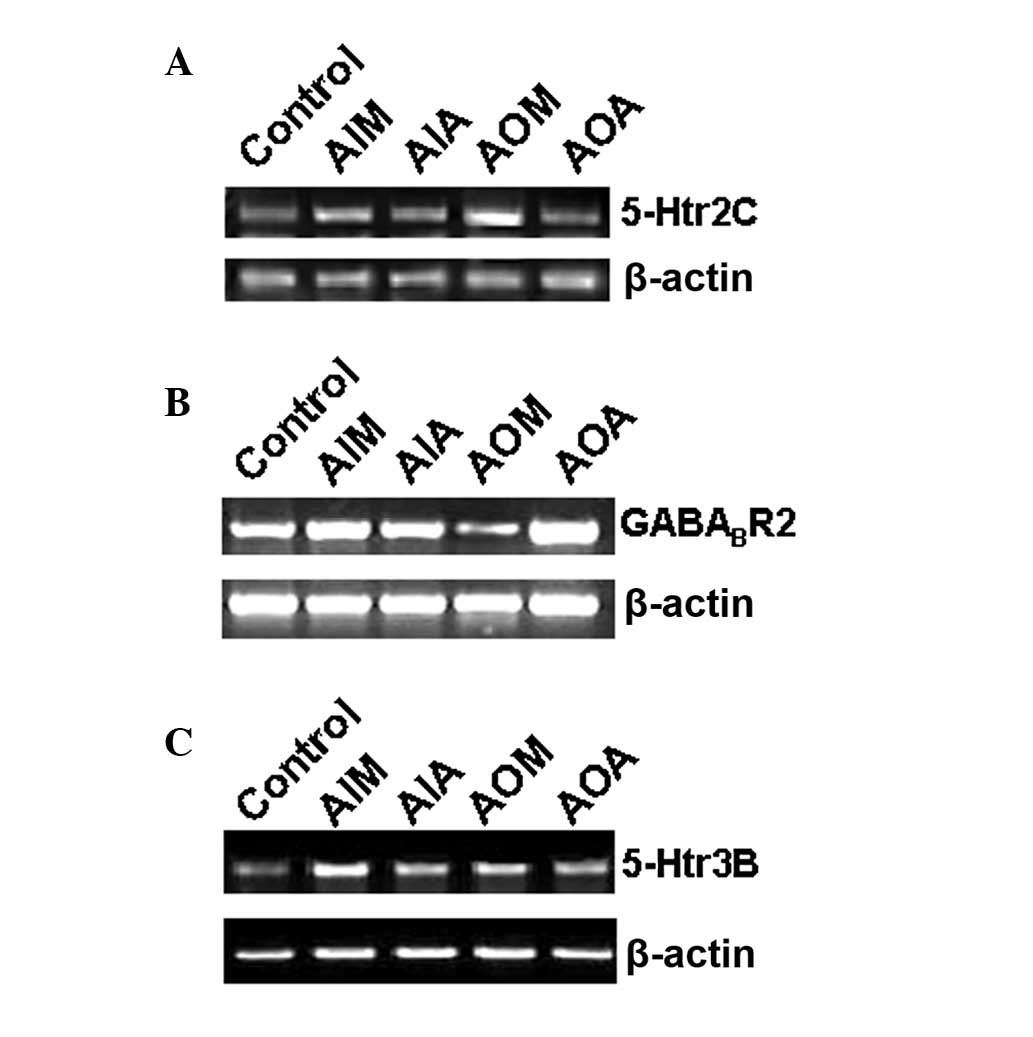 | Figure 2Reverse transcription-quantitative
polymerase chain reaction results for the (A)
5-Htr2C, (B) GABABR2,
and (C) 5-Htr3B. genes Total RNA was extracted from
the hippocampal tissue of the rats and reverse transcription was
performed. Following electrophoresis, the gels were subjected to a
densitometric scan with a SmartView Image System (Smartview,
Irvine, CA, USA) in order to determine the mRNA expression levels
of the target genes and the reference gene, β-actin. AIM, anger-in
model; AIA, anger-in Jingqianshu-administered; AOM, anger-out
model; AOA, anger-out Jingqianping-administered. |
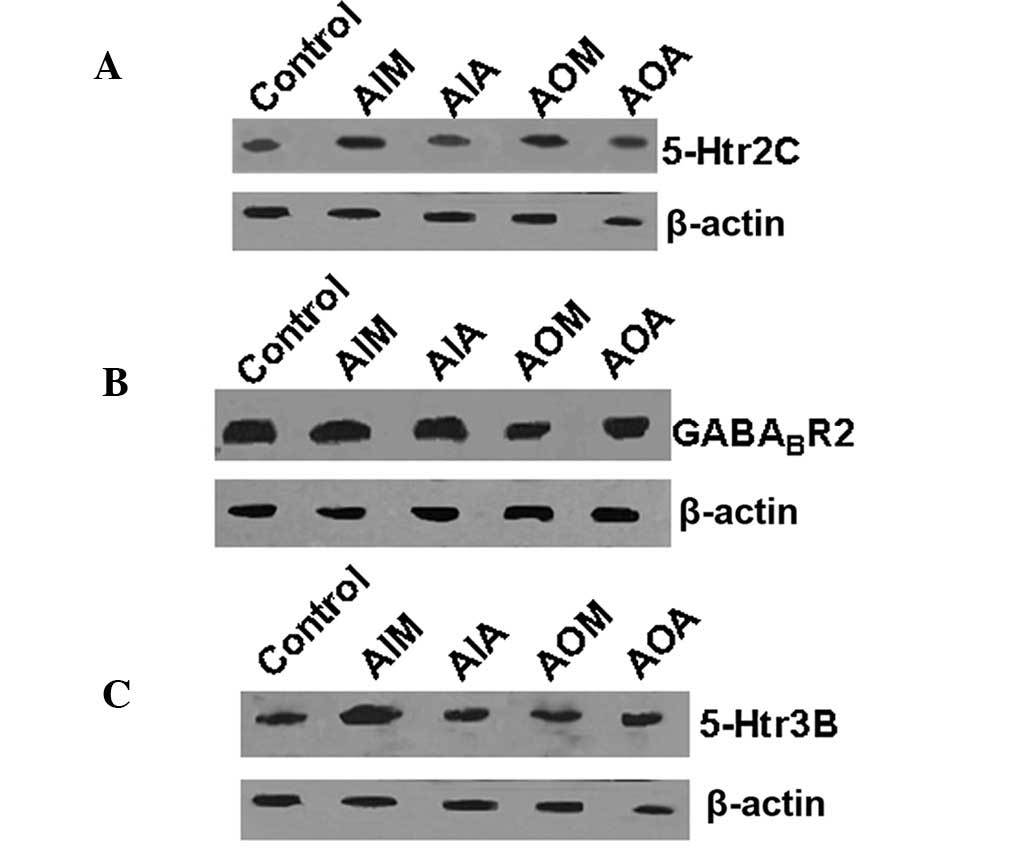
Protein levels of target genes revealed
by western blot analysis
To further determine the expression profiles of the
5-Htr2C, GABABR2, and
5-Htr3B genes, the levels of the proteins encoded by
the three genes were examined by western blotting. As shown in
Fig. 3A, the relative levels of
the 5-Htr2C protein in the hippocampal tissue of rats in the AIM
and AOM groups were higher than those in the control group rats
(P<0.01). The drugs attenuated the increase in 5-Htr2C protein
expression, as indicated by comparisons of the levels of protein in
the AIA and AOA groups versus those in the AIM and AOM groups.
As shown in Fig.
3B, the relative level of GABABR2 protein was
upregulated in the AIM group but downregulated in the AOM group
(P<0.01). The drugs attenuated the alterations in
GABABR2 protein expression, as indicated by comparisons
of the levels of protein in the AIA and AOA groups versus those in
the AIM and AOM groups.
As shown in Fig.
3C, the relative level of 5-Htr3B protein in the AIM and AOM
groups was higher than that in the control group (P<0.01). The
drugs attenuated the increase in 5-Htr3B protein expression, as
indicated by comparisons of the levels of protein in the AIA and
AOA groups versus those in the AIM and AOM groups. In combination,
these results suggest that Jingqianshu and Jingqianping attenuated
the protein expression changes induced by anger-in and anger-out
emotions.
Discussion
In the present study, anger emotion rat models were
established through social isolation and resident-intruder methods.
Aggressive behavior and open-field tests were carried out to assess
the emotional status of the rats. It was found that the scores of
the model rats were significantly higher than those of the rats in
the control group (P<0.05). The results suggested that the
models were well established and that the combination of open-field
and composite aggressive behavioral scores may be used to
distinguish anger-in and anger-out model rats from one other and
from normal rats at the initial stages of anger presentation. The
behavioral changes in the anger-in and anger-out model rats may
resemble those in human patients, which may aid with clinical
application and treatment options.
In the current study, a number of differentially
expressed genes were revealed to be representative of anger-in
and/or anger-out emotions. Preliminarily comparisons at the
molecular level have revealed the genetic mechanisms that may
trigger anger-in and anger-out emotions. Furthermore, the results
of the present study have demonstrated the main target genes and
signals involved in the regulatory pathways of Jingqianshu and
Jingqianping granules during their intervention against anger-in
and anger-out emotions.
In the treatment of the anger-out emotion,
Jingqianping can modulate the expression of important regulatory
factors, such as adenylate cyclase (AC) and JNK/SAPK-inhibitory
kinase, and thus affect upstream pathways, such as ligand-receptor
interaction in neuron activation and the AC/cyclic adenosine
monophosphate-dependent protein kinase pathways (18). The effect of Jingqianping can then
be transmitted via downstream signaling pathways, including
mitogen-activated protein kinase (MAPK), Ca2+ signal
transduction and gonadotropin-releasing hormone, to be activated or
inhibited. As a result, the overexcitation of the
hypothalamic-pituitary-adrenal axis and hypofunction of the
hypothalamic-pituitary-thyroid and hypothalamic-pituitary-gonadal
axes are counterbalanced, and the dysfunction in the network
comprising the nervous, endocrine and immune systems is relieved
(19–21).
The main targets of Jingqianshu in its effect on
anger-in emotion include PRLR, GRM8, FGFR2,
HCRT, PLA2G5, BACE1 and NCR3 (22). The biological functions of these
targets are associated with the protection of neurons, inhibition
of apoptosis, protection of signal transduction through synaptic
transmission, stabilization of the Ca2+ pathway and
antagonism of anoxia. Compared with the regulatory function of
Jingqianping granules, fewer target genes are regulated by
Jingqianshu (22). With regard to
the functional mechanisms of Jingqianshu, the relevant
ligand-receptor interactions involved in neuron activation and
other signaling pathways, such as glycerophospholipid metabolism,
MAPK and natural killer cell-mediated cytotoxicity, require further
study.
Compound prescriptions in Traditional Chinese
Medicine have the advantage of integrated regulatory functions
through multiple ingredients, links and targets in the treatment of
chronic, complex and multifactorial disorders. The chemical
complexity of the compound prescriptions and the diversity of their
formulae and functions have, however, made them difficult to be
determined, which has hindered the international application of
Chinese medicine. With modern biological technology, it has been
revealed that Realgar-Indigo naturalis formula can be used for the
treatment of acute promyelocytic leukemia through multiple-target
and synergistic mechanisms (23).
This is, to the best of our knowledge, the first time that a
Chinese compound prescription mechanism has been elucidated at the
level of the molecular networks.
In conclusion, the present study has investigated
how anger emotions cause disease and evaluated the efficacy of
Chinese medicines for the treatment of anger emotions. Rat models
of anger emotions were established, and human genomic and proteomic
databases were searched. Using the rat models, the preliminary
application of Jingqianshu and Jingqianping and the functional
mechanisms underlying their effect on anger emotions were
investigated at the level of regulatory molecular networks.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81173151, 81202616,
81102537 and 81273619) and the Postdoctoral Science Foundation Key
Program of China (no. 20090451343).
References
|
1
|
Kessler RC, Aguilar-Gaxiola S, Alonso J,
et al: The global burden of mental disorders: an update from the
WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc.
18:23–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mostofsky E, Penner EA and Mittleman MA:
Outbursts of anger as a trigger of acute cardiovascular events: a
systematic review and meta-analysis. Eur Heart J. 35:1404–1410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tao HY, Qiao MQ and Wang WY: The
dependability of anger pathopoiesis and psychological stress.
Zhejiang Zhong Yi Yao Da Xue Xue Bao. 33:140–141. 2009.(In
Chinese).
|
|
4
|
Wang CX, Zheng HX, Wang JW, et al: A
probing into the imbalance of immunity system, endocrine, nerves
and the liver affected by anger. Liaoning Zhong Yi Za Zhi.
24:205–206. 1997.(In Chinese).
|
|
5
|
Liu XW, Qu HD, Zhang HM, et al: Content
change of ATCH/CORT/IL-2/IL-8 in blood from rats with Qi impaired
by anger. Zhong Guo Quan Ke Yi Xue. 11:1653–1654. 2004.(In
Chinese).
|
|
6
|
Qiao MQ, Wang WY and Zhang HY:
Epidemiological survey on etiology of Gan-qi inversion syndrome and
Gan-qi stagnation syndrome and study on the evocative mode of
emotional diseases. Zhongguo Zhong Xi Yi Jie He Za Zhi. 27:117–119.
2007.(In Chinese). PubMed/NCBI
|
|
7
|
Chao YB, Wei S, Qiao MQ, Wang JQ and Zhang
HY: Analysis of monoamine neurotrasmitter content in serum and
different encephalic regions of PMS liver-qi invasion, depression
rat models. Yi Xue Yan Jiu Za Zhi. 39:19–21. 2010.(In Chinese).
|
|
8
|
Qiao MQ, Zhang HY and Wang HJ:
Relationship between anger-in and anger-out and premenstrual
syndrome, Gan-qi inversion syndrome and Gan-qi stagnation syndrome.
Shanxi Yi Yao Za Zhi. 27:1359–1361. 2006.(In Chinese).
|
|
9
|
Czéh B, Michaelis T, Watanabe T, et al:
Stress-induced changes in cerebral metabolites, hippocampal volume,
and cell proliferation are prevented by antidepressant treatment
with tianeptine. Proc Natl Acad Sci USA. 98:12796–12801. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zuo HY and Wang DW: Advances of
hippocampus proteomics. Zhong Guo Lin Chuang Jie Pou Xue Za Zhi.
23:333–336. 2007.(In Chinese).
|
|
11
|
Ge HY, Chen B, Xu D, et al: Influence of
Saikosaponina A on monoamine neurotransmitters and the
corresponding metabolin compositions in depressed rats’ brain.
Zhong Guo Gao Deng Xue Xiao Hua Xue Xue Bao. 29:1535–1538. 2008.(In
Chinese).
|
|
12
|
Yang XY, Ma SP and Qu R: Effects of
Bupleurum chinense extracts on lipid peroxidation in chronic
unpredictable mild stress model of depression in rats and
lymphocyte proliferation in mice. Zhong Guo Yao Ke Da Xue.
38:442–445. 2007.(In Chinese).
|
|
13
|
Xu SY, Bian RL and Chen X: Methodology of
Pharmacological Experiment. 3rd edition. People’s Medical
Publishing House; Beijing: pp. 202–203. 2002, (In Chinese).
|
|
14
|
Wei S, Zhang H, Gao J, et al: Impact of
social isolation and resident intruder stress on aggressive
behavior in the male rat. Neural Regen Res. 5:1175–1179. 2010.
|
|
15
|
Rauser L, Savage JE, Meltzer HY and Roth
BL: Inverse agonist actions of typical and atypical antipsychotic
drugs at the human 5-hydroxytryptamine(2C) receptor. J Pharmacol
Exp Ther. 299:83–89. 2001.PubMed/NCBI
|
|
16
|
Ulrich D and Bettler B: GABA(B) receptors:
synaptic functions and mechanisms of diversity. Curr Opin
Neurobiol. 17:298–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blier P: The pharmacology of putative
early-onset antidepressant strategies. Eur Neuropsychopharmacol.
13:57–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo YH, Sheng Wei, Gao J, Song CH and Qiao
MQ: Effect of Jingqianping granule on gene expression profile in
the hippocampus of the anger-out rat model. Zhong Guo Yao Li Xue
Tong Bao. 28:150–154. 2012.
|
|
19
|
Páez-Pereda M: New drug targets in the
signaling pathways activated by antidepressants. Prog
Neuropsychopharmacol Biol Psychiatry. 29:1010–1016. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H and Zhang M: The role of
Ca2+-stimulated adenylyl cyclases in bidirectional
synaptic plasticity and brain function. Rev Neurosci. 23:67–78.
2012. View Article : Google Scholar
|
|
21
|
Ganea D, Rodriguez R and Delgado M:
Vasoactive intestinal peptide and pituitary adenylate
cyclase-activating polypeptide: players in innate and adaptive
immunity. Cell Mol Biol (Noisy-le-grand). 49:127–142. 2003.
|
|
22
|
Guo YH, Gao J, Xu KY, Song CH and Qiao MQ:
Effect of Jingqianshu granule on gene expression profile in the
hippocampal of the anger-in rat model. Zhong Guo Yao Li Xue Tong
Bao. 27:1317–1321. 2011.
|
|
23
|
Wang L, Zhou GB, Liu P, et al: Dissection
of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis
as an effective treatment for promyelocytic leukemia. Proc Natl
Acad Sci USA. 105:4826–4831. 2008. View Article : Google Scholar : PubMed/NCBI
|