Introduction
Peritoneal antiprotease lavage therapy for
pancreatitis has been investigated for a number of years, but the
results of experimental and clinical studies have been conflicted.
Intraperitoneal lavage therapy with added protease inhibitors,
including camostate and glutaryl-trialanin-ethylamide, has been
proven to improve survival rate in several species with
experimentally induced severe acute pancreatitis (SAP) (1–3).
However, certain studies in humans have not shown a significant
improvement in survival rate with peritoneal antiprotease lavage
therapy (4–6). Between 40 and 60% of in-hospital
patient mortalities occurring within one week of admission are
associated with early multi-organ failure in SAP (7). Early-stage intervention, including
peritoneal lavage for SAP, could significantly improve this outcome
(8,9), and the early treatment of SAP, would
play a key role in avoiding high mortalities rates. Peritoneal
antiprotease lavage therapy in the early stages of SAP may achieve
a superior therapeutic effect to late-stage lavage therapy.
Ulinastatin is a purified antiprotease obtained from
the fresh urine of healthy adults has been shown to exert
significant therapeutic effects in several forms of acute
pancreatitis (10,11); however, the effect of peritoneal
ulinastatin lavage in the early stages of SAP has not been studied.
According to our preliminary experiment, peritoneal lavage with
62.5 U/ml ulinastatin added to the lavage fluid exerted the best
therapeutic effect. The aim of the present study was therefore to
use a rat model to investigate the effect of intraperitoneal
ulinastatin lavage in the early stages of SAP on the multi-organ
protection and overall outcome, as well as to provide experimental
and theoretical evidence for the treatment of SAP in the clinical
setting.
Materials and methods
Experimental animals
A total of 80 healthy male Wistar rats (weight,
300±15 g) were obtained from the Experimental Animal Center of the
General Hospital of the PLA (Beijing, China). The experimental
protocol was approved by the Ethics Committee for Animal Research
from the General Hospital of the PLA and all experimental rats
received humane care.
Reagents
The reagents used in the study were purchased from
the following companies: Chloral hydrate (Shanghai Yingxin
Laboratory Equipment Co., Ltd., Shanghai, China); sodium
taurocholate (Shanghai Hufeng Biotechnology Co., Ltd., Shanghai,
China); and ulinastatin (Guangdong Tianpu Biochemical
Pharmaceutical Co., Ltd., Guangzhou, China). The amylase and lipase
assay kit was purchased from Shanghai Shifeng Biotechnology Co.,
Ltd. (Shanghai, China); the aspartate transaminase (AST) and
alanine transaminase (ALT) kit was obtained from Shanghai Hu Ding
Biotechnology Co., Ltd. (Shanghai, China); the creatinine (Cr) and
urea (UR) assay kit was purchased from Beijing Bomaisi Biological
Technology Co., Ltd. (Beijing, China) and the troponin T (TnT)
assay kit was purchased from Shanghai Ji Ning Industrial Co., Ltd.
(Shanghai, China).
Experimental groups
The rats were randomly divided into six groups:
Group C (n=18, control group), sham surgery without the induction
of SAP or peritoneal lavage/intravenous injection, but with
catheter insertion; group M (n=18, SAP model group), induction of
SAP without peritoneal lavage or intravenous injection, but with
catheter insertion; group SL (n=8, saline lavage group), induction
of SAP with saline lavage; group IU (n=8, intravenous ulinastatin
group), intravenous ulinastatin (2,500 U/100 g) immediately
subsequent to the induction of SAP, with catheter insertion but
without peritoneal lavage; group EUL (n=18, early ulinastatin
lavage group), ulinastatin (62.5 U/ml) lavage immediately
subsequent to the induction of SAP; group LUL (n=10, late
ulinastatin lavage group), ulinastatin (62.5 U/ml) lavage 3 h after
the induction of SAP.
Animal model
The rats were fasted for 12 h and had no water for 4
h prior to surgery. The rats were anesthetized with intraperitoneal
injections of 10% chloral hydrate (3 ml/kg). Subsequent to making
an incision in the abdomen and clamping the distal region of the
duodenal bile duct with injury-free metal clips, a syringe needle
was inserted into the opening of the duodenal bile duct and 5%
sodium taurocholate (freshly prepared in saline solution, 0.6 ml)
was retrogradely injected into the duct at constant rate of 0.2
ml/min using an infusion pump. After 5 min, the needle and metal
clips were removed. A consistently high mortality rate (>80%
within 12 h) was obtained. Group C rats underwent sham surgery
without the induction of SAP.
Prior to the closure of the abdomen, a silicon
catheter (catheter A) with five lateral outlets was placed adjacent
to the pancreas and another silicon catheter (catheter B) with five
lateral outlets was placed in the pelvic cavity. All groups
underwent peritoneal catheter insertion.
Peritoneal lavage
Intraperitoneal lavage was performed immediately
subsequent to the establishment of the SAP model in groups SL and
EUL and 3 h later in group LUL. Warmed (37°C) lavage fluid was
injected from catheter A at 80 ml/h for 15 min and catheter B was
blocked. Following this, catheter A was blocked and fluid was
allowed to flow out for 15 min from catheter B. Each lavage
procedure thus lasted 30 min, and the lavage was performed six
times (for 3 h in total) (1). The
volume input and output were monitored. The lavage fluid consisted
of saline solution with or without the addition of 62.5 U/ml
ulinastatin. This concentration of ulinastatin was shown to exert
the best therapeutic effect in our preliminary studies. Following
lavage, catheters A and B were blocked and the rats were kept in
single cages with free access to water but no solid food.
Intravenous ulinastatin
To compare the effect of peritoneal lavage with that
of intravenous ulinastatin administration, group IU rats were
administered intravenous ulinastatin at 2,500 U/100 g (freshly
prepared in 0.15 ml saline solution, approximately equivalent to
the total dose applied in groups EUL and LUL) to the caudal vein
immediately subsequent to SAP induction. These rats did not undergo
lavage.
Assays and calculations
The survival times of the rats in groups C, M, EUL
and LUL (n=10 per group) were recorded over a 12-h period and the
median survival time was calculated. Animals surviving to 12 h were
anesthetized and sacrificed. Rats in groups C, M, SL, IU and EUL
(n=8 per group) were sacrificed for histopathological analyses and
biochemical parameter (amylase, lipase, AST, ALT, CR, UR and TnT)
measurements 3 h after the establishment of each model. As the rats
in group LUL did not receive the intervention (ulinastatin lavage)
until 3 h following the induction of the SAP model, histological
analysis was not performed as the results would be incomparable to
the other groups due to the difference in time. Arterial blood was
additionally collected into heparinized syringes from the abdominal
aorta following general anesthesia and a second laparotomy. The
biochemical parameter measurements were conducted using an
automatic biochemical analyzer (Beckman Coulter-AU5800; Beckman
Coulter, Brea, CA, USA).
The organs selected for histological examination
(pancreas, liver, kidney and lung) were fixed in formalin,
subjected to conventional dehydration and embedded in paraffin. The
samples were then cut into 5-μm sections and stained with
hematoxylin and eosin. Examination by light microscopy was
performed by two professional pathologists using a double-blind
method as to whether the section was from the control or one of the
treatment groups. Three slices were randomly selected for each
group; for each slice, 10 high-power fields of vision were again
randomly selected. The pathological score was calculated according
to the methods described by Zhang et al (12–14).
Statistical analysis
Data are expressed as the mean (standard deviation)
for normally distributed variables or as the median (interquartile
range) for highly skewed variables. Statistical analyses were
performed using the SPSS 19.0 software package (IBM-SPSS, Armonk,
NY, USA).
In the survival experiments, analysis of the median
survival time at the end of the 12-h observation period was
conducted by the Kaplan-Meier or Kruskal-Wallis H tests. Analysis
of variance was used for the comparison of normally distributed
data. Multiple comparisons were subjected to Kruskal-Wallis H and
Bonferroni correction tests. The χ2 test was used to
evaluate the equality of frequencies for discrete variables.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of early peritoneal lavage with
ulinastatin on the survival time
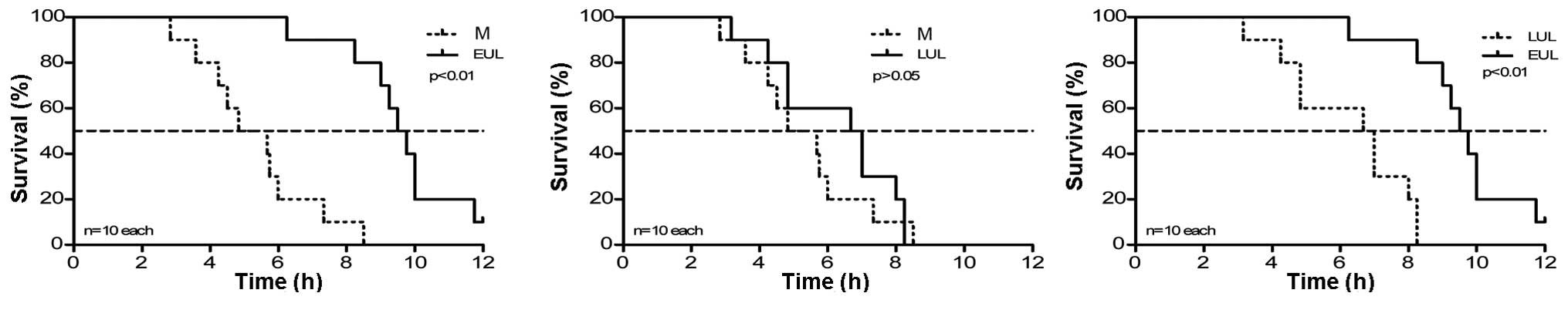
All rats in group C were alive at 12 h. The median
survival time of the group M rats was 4.83 h. The survival time of
rats in group EUL (ulinastatin lavage was performed immediately
subsequent to the induction of SAP) was significantly longer than
that of the group M rats (9.50 vs. 4.83 h). Group LUL rats
(ulinastatin lavage was performed at 3 h after induction of SAP)
also exhibited an increased median survival time compared with the
group M rats (6.67 vs. 4.83 h), but the difference was not
significant (P>0.05). Early ulinastatin lavage therefore
improved the prognosis of the SAP rats to a greater extent than the
late lavage (9.50 vs. 6.67 h). The results are summarized in
Table I and Fig. 1.
 | Table IComparison of the effect of early and
late peritoneal ulinastatin lavage on survival time. |
Table I
Comparison of the effect of early and
late peritoneal ulinastatin lavage on survival time.
| | | | P-value |
|---|
| | | |
|
|---|
| Group | n | Median survival time
(h) | Interquartile
range | Compared with group
M | Compared with group
LUL |
|---|
| C | 10 | 12.00 | | | |
| M | 10 | 4.83 | 2.83–8.50 | | |
| LUL | 10 | 6.67 | 3.17–8.25 | 0.45 | |
| EUL | 10 | 9.50 | 6.25–12.00 | <0.01 | <0.01 |
Effect of early peritoneal lavage with
ulinastatin on multi-organ functional protection
The analysis of the pancreatic enzyme activity
showed that the activity of amylase and lipase in the plasma of
rats in group M was significantly higher than that in the plasma of
rats in group C. Furthermore, the amylase activity in groups SL, IU
and EUL was significantly reduced compared with that in group M,
with the greatest reduction observed in group EUL. The lipase
activity in groups SL, IU and EUL was also significantly reduced
compared with that in group M, and the reduction was similarly most
marked in group EUL (Table II and
Fig. 2).
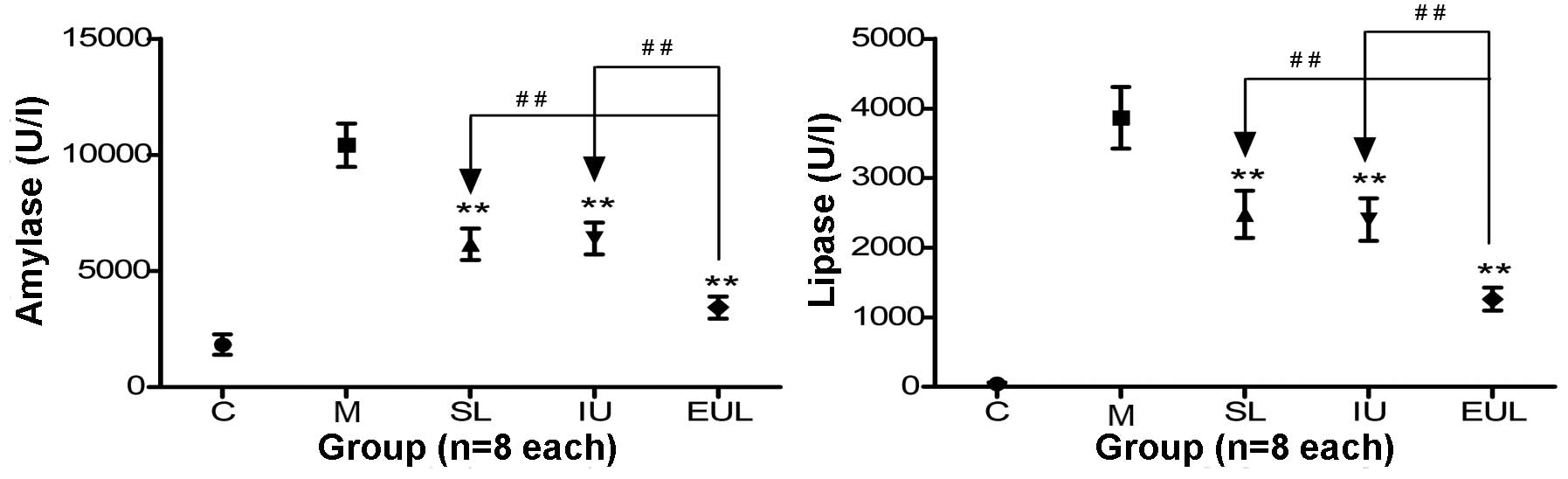 | Figure 2Effect of early peritoneal ulinastatin
lavage on the activity of pancreatic enzymes (amylase and lipase).
Rats in groups C, M, SL, IU and EUL (n=8 per group) were sacrificed
for amylase and lipase measurements 3 h after the establishment of
each model. The plasma amylase and lipase activity in group M was
significantly higher than that in group C. The amylase and lipase
activity in groups SL, IU and EUL was significantly reduced
compared with that in group M, with the biggest reduction observed
in group EUL. Results are presented as the mean ± standard
deviation. **P<0.01 vs. group M;
##P<0.01. Group C, control (sham-operated) group;
group M, severe acute pancreatitis model group; group SL, saline
lavage group; group IU, intravenous ulinastatin group; group EUL,
early ulinastatin lavage group. |
 | Table IIEffect of different treatments on
multi-organ protection. |
Table II
Effect of different treatments on
multi-organ protection.
| Parameter | C | M | SL | IU | EUL |
|---|
| Pancreas |
| Amylase, U/l | 1831.1
(437.60)a | 10422.11
(937.18) | 6151.84
(681.31)a | 6400.05
(678.65)a | 3426.76
(484.43)a |
| Lipase, U/l | 42.8 (21.40)a | 3864.43 (443.13) | 2476.95
(336.20)a | 2403.51
(304.51)a | 1261.34
(166.34)a |
| Liver |
| ALT, U/l | 20.00 (4.83)a | 214.04 (17.58) | 84.85 (12.15)a | 98.61 (18.50)a | 79.86 (21.39)a |
| AST, U/l | 20.33 (3.35)a | 783.99 (86.99) | 259.26
(56.53)a | 277.33
(69.77)a | 229.15
(45.78)a |
| Kidney |
| CR, μmol/l | 25.09 (3.42)a | 57.73 (7.06) | 31.70 (7.60)a | 55.39 (7.23) | 30.85 (5.82)a |
| UR, mmol/l | 6.07 (1.30)a | 10.52 (1.34) | 8.37 (1.06)a | 9.37 (1.30) | 6.83 (1.39)a |
| Heart |
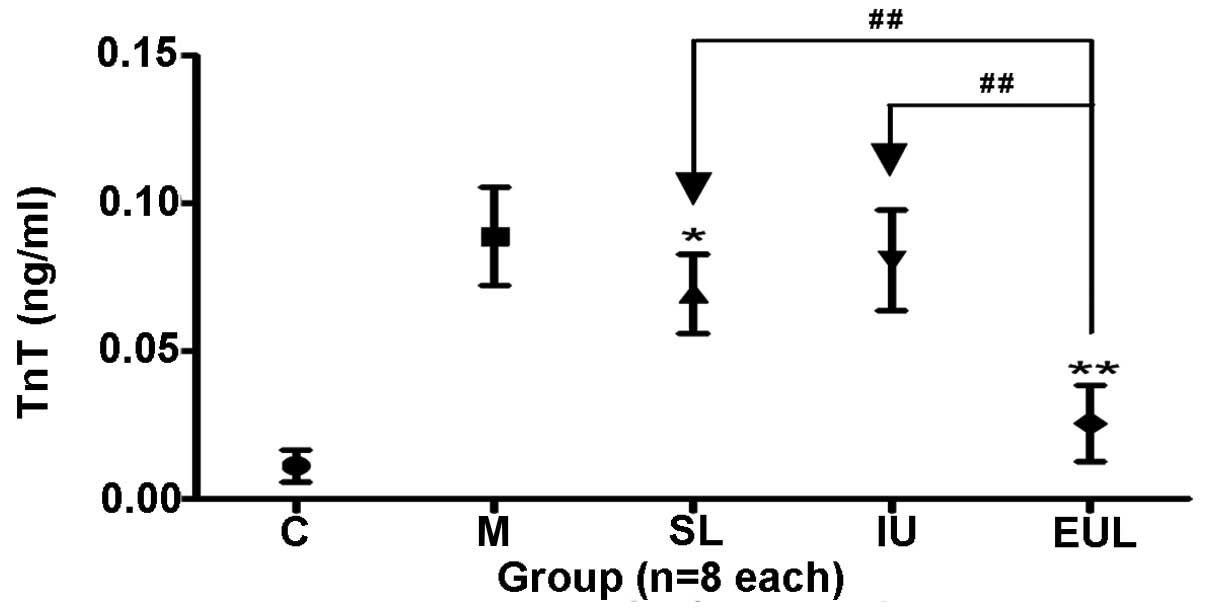
| TnT, ng/ml | 0.011 (0.005)a | 0.089 (0.017) | 0.070 (0.014)b | 0.081 (0.017) | 0.026 (0.013)a |
The analysis of liver enzyme activity showed that
the plasma ALT and AST activity in Group M was significantly
increased compared with that in group C. Compared with group M,
groups SL, IU and EUL exhibited significantly reduced ALT activity.
Furthermore, the ALT activity in group EUL was significantly lower
than that in group IU. The AST activity in groups SL, IU and EUL
was also significantly reduced compared with that in group M, but
no significant difference was found among the results for groups
SL, IU and EUL (Table II and
Fig. 3).
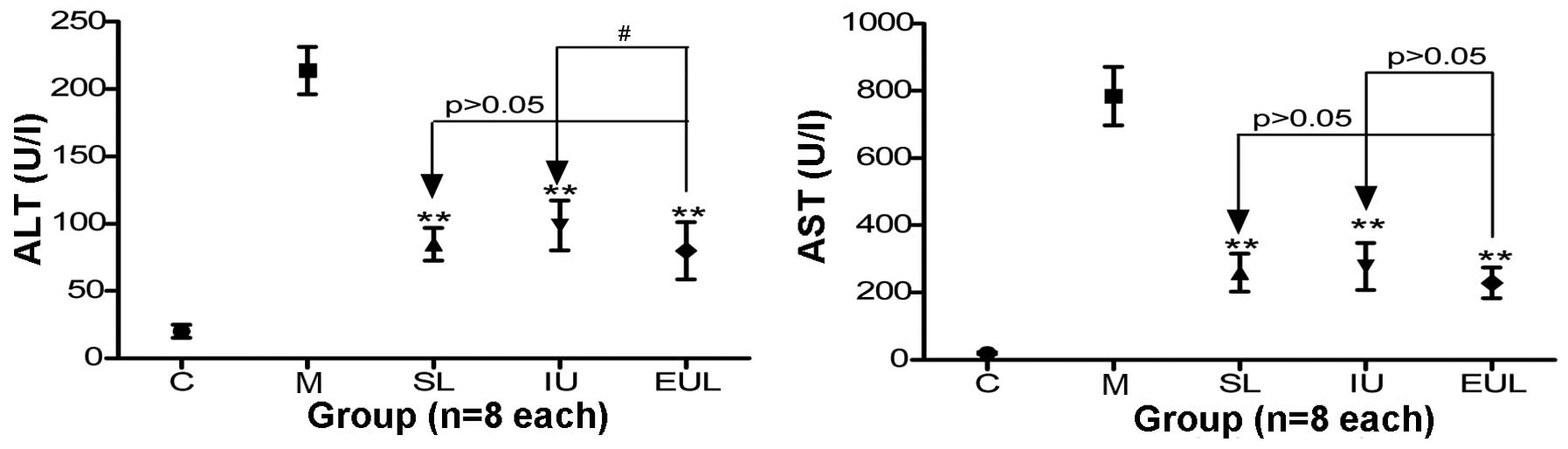 | Figure 3Effect of early peritoneal ulinastatin
lavage on the activity of liver enzymes (ALT and AST). Rats in
groups C, M, SL, IU and EUL (n=8 per group) were sacrificed for ALT
and AST measurements 3 h after the establishment of each model. The
plasma ALT and AST activity in group M was significantly increased
compared with that in group C. The ALT and AST activity in groups
SL, IU and EUL was significantly reduced compared with that in
group M. The activity of ALT in group EUL was significantly lower
than that in group IU, but the AST activity in group EUL was not
significantly different from that in groups IU or SL. Results are
presented as the mean ± standard deviation. **P<0.01
vs. group M; #P<0.05. Group C, control
(sham-operated) group; group M, severe acute pancreatitis model
group; group SL, saline lavage group; group IU, intravenous
ulinastatin group; group EUL, early ulinastatin lavage group; ALT,
alanine transaminase; AST, aspartate transaminase. |
The plasma CR and UR levels in the kidney in group M
were significantly increased compared with those in group C.
Compared with the CR level in group M, the CR level in groups SL
and EUL was reduced to a greater extent than that in group IU; no
significant difference was found between the results for groups SL
and EUL. The UR level in groups SL and EUL was significantly
reduced compared with that in group M and was lower than that in
group IU (Table II and Fig. 4).
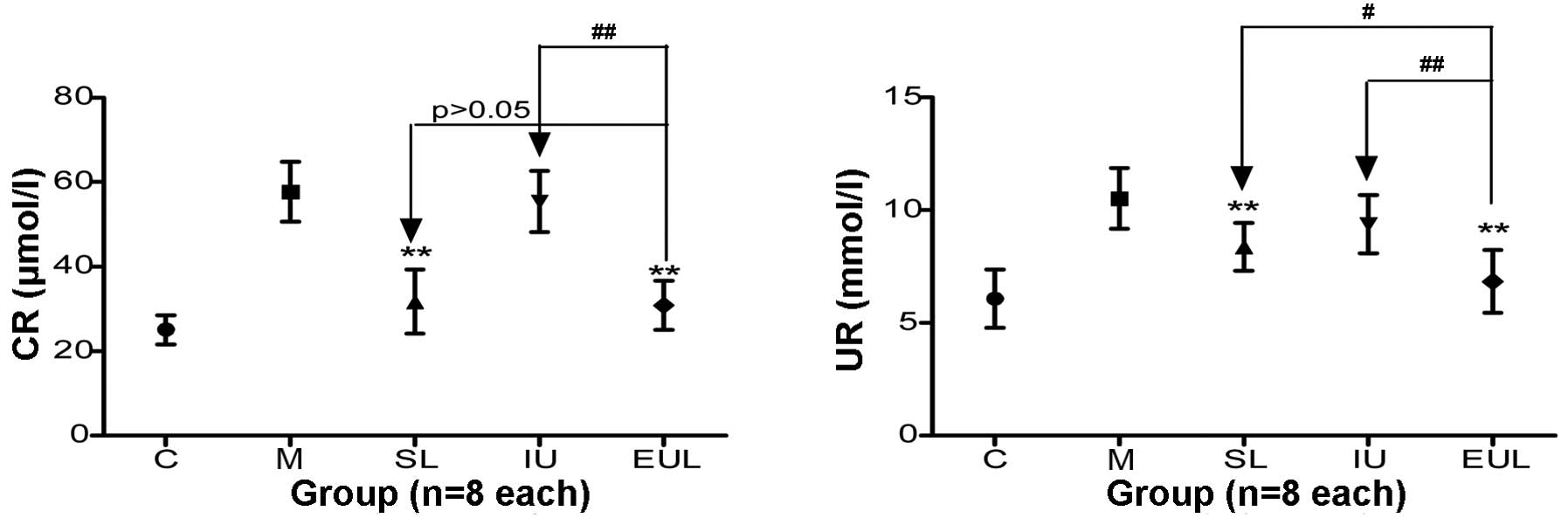 | Figure 4Effect of early peritoneal ulinastatin
lavage on the CR and UR levels in the kidney. Rats in groups C, M,
SL, IU and EUL (n=8 per group) were sacrificed for CR and UR
measurements 3 h after the establishment of each model. The plasma
CR and UR levels in group M were significantly increased compared
with those in group C. The CR and UR levels in groups SL and EUL
were significantly lower than those in group M and better than
those in group IU. The effect in group EUL was superior to that in
other groups. Results are presented as the mean ± standard
deviation. **P<0.01 vs. group M;
#P<0.05 and ##P<0.01. Group C, control
(sham-operated) group; group M, severe acute pancreatitis model
group; group SL, saline lavage group; group IU, intravenous
ulinastatin group; group EUL, early ulinastatin lavage group; CR,
creatinine; UR, urea. |
The plasma TnT level in the heart in group M was
significantly increased compared with that in group C. The
intervention in groups EUL and SL effectively inhibited the
increase in TnT observed in group M, but the intervention in group
IU did not. (Table II and
Fig. 5).
Effect of early peritoneal lavage with
ulinastatin on the pathological severity scores of multiple
organs
The pathological severity scores of the pancreas,
liver, kidney and lung in group M were significantly higher than
those in group C; however, the scores in group EUL were
significantly lower than those in group M. The pathological
severity scores of the pancreas, liver, kidney and lung in the
group EUL showed a greater improvement than those in groups SL and
IU. The results are summarized in Table III.
 | Table IIIComparison of the pathological
severity scores in multiple organs. |
Table III
Comparison of the pathological
severity scores in multiple organs.
| Organ | C | M | SL | IU | EUL |
|---|
| Pancreas | 0.0 (0.0)a | 12.0 (2.0) | 9.0 (2.0)a | 8.0 (2.0)a | 7.0 (2.0)a |
| Liver | 0.0 (0.0)a | 1.0 (1.0) | 0.5 (1.0) | 0.5 (1.0) | 0.0 (1.0) |
| Kidney | 0.0 (0.0)a | 2.0 (1.0) | 1.0 (1.0) | 2.0 (1.0) | 1.0 (0.3)b |
| Lung | 0.0 (0.0)a | 2.0 (1.0) | 2.0 (1.0) | 1.0 (0.5)b | 1.0 (1.0)b |
Discussion
Peritoneal antiprotease lavage therapy has been the
focus of experimental and clinical research in pancreatitis for
decades. It has been well established that the release of
pancreatic enzymes into the peritoneal exudate is extremely toxic
and can lead to multi-organ damage and death; at such times,
peritoneal antiprotease lavage becomes necessary and should be
directed into the peritoneal cavity for the best therapeutic effect
(4). Based on this theory,
numerous experimental and clinical studies concerning peritoneal
antiprotease lavage therapy have been performed. Experimental
studies have predominantly shown that peritoneal antiprotease
lavage improves the SAP-related mortality rate and outcome
(1,15), whereas clinical studies have
yielded more conflicting results for unknown reasons (16,17).
It has been found that 40–60% of in-hospital patient
mortalities occurring within one week of admission are associated
with early multi-organ failure in SAP (7). Early intervention, including
peritoneal lavage for SAP, could significantly improve the early
prognosis (8,9). The stage at which peritoneal
antiprotease lavage therapy is performed during the course of SAP
may therefore be an important factor in achieving the optimal
therapeutic effect.
Ulinastatin is a purified glycoprotein obtained from
the fresh urine of healthy adult males. Ulinastatin has been
suggested to have a considerable therapeutic effect in SAP, as it
can suppress the pathogenesis and development of pancreatitis by
inhibiting pancreatic enzyme activation. Furthermore, the
therapeutic effects of ulinastatin are believed to be superior to
those of aprotinin (10). The
administration of ulinastatin through an arterial infusion catheter
route has been suggested to result in enhanced therapeutic efficacy
compared with intravenous administration, since only a small
proportion of the administered ulinastatin reaches the pancreas
with intravenous application (18,19).
To date, there have been no experimental studies reporting the
effect of early peritoneal ulinastatin lavage for SAP, which is a
significant obstacle for a commitment to a clinical study.
On the basis of our preliminary experiment,
peritoneal lavage with 62.5 U/ml ulinastatin added to the lavage
fluid was shown to be most beneficial on the outcome of SAP. To the
best of our knowledge, the present study is the first to evaluate
the effect of early versus late peritoneal ulinastatin lavage on
the median survival time in an SAP model. The effect of the early
peritoneal ulinastatin lavage on multi-organ protection was also
compared with the effect of intravenous ulinastatin
administration.
In the present study, a retrograde injection of 5%
sodium taurocholate (freshly prepared in saline solution, 0.6 ml)
was applied into the pancreatic duct at a rate of 0.2 ml/min to
produce an SAP experimental model with a high mortality rate
(>80% within 12 h) (12). This
high-mortality SAP model was selected for the study as it reflects
life-threatening pancreatitis in humans. The results showed that
early peritoneal ulinastatin lavage significantly improved the
median survival time of the rats and was superior to late lavage.
This indicates that peritoneal ulinastatin lavage could show
efficacy at improving outcomes when performed in the early stages
of SAP, but not in the late stages of the condition. A relevant
clinical study showed that early fluid resuscitation was associated
with a reduced incidence of systemic inflammatory response syndrome
and organ failure (20).
Peritoneal lavage may therefore replicate the role of fluid
resuscitation, which is important for the prognosis in the early
stage of the condition, and may enable ulinastatin to exert its
optimal therapeutic effect directly and act locally on the pancreas
and its associated enzymes in the peritoneal cavity. This theory
was supported by the present results, which showed that the
activity of amylase and lipase was reduced to a greater extent in
group EUL than that in the group with intravenous administration;
however, further studies are required to verify this theory.
The present study showed that, in the early stages
of SAP, saline lavage, intravenous ulinastatin administration and
peritoneal ulinastatin lavage could all reduce the activity of the
liver enzymes AST and ALT. The results indicated that peritoneal
lavage with or without ulinastatin may have been more effective
than the intravenous route, but no significant difference was
observed. Intravenous ulinastatin administration achieved higher
therapeutic efficacy in the liver than in the pancreas; this may
have been because intravenous administration led to the
accumulation of the compound in the liver, but did not deliver
sufficient quantities to the pancreas (18). The results suggested that
ulinastatin could protect liver function and exert a superior
therapeutic effect when administered via a peritoneal lavage
route.
Plasma UR and CR levels in the kidney were reduced
more effectively with peritoneal lavage than with intravenous
administration, irrespective of whether the lavage fluid contained
ulinastatin. Although ulinastatin can accumulate in the kidney
through intravenous injection, the fluid resuscitation provided by
the peritoneal lavage may play a more vital role in organ
protection in the early stages of SAP. It is therefore possible
that the significant improvement in the circulation induced by
intraperitoneal lavage may be responsible for the reduction in the
levels of UR and CR.
The results of the present study additionally showed
that ulinastatin administration via peritoneal lavage was more
effective at reducing the plasma TnT level in the heart than
peritoneal lavage without ulinastatin or intravenous ulinastatin
administration. Comparison of the TnT level in groups EUL
(peritoneal ulinastatin lavage) and SL (peritoneal saline lavage)
indicated that ulinastatin could have a protective effect on the
heart, while comparison of groups EUL and IU showed that the
protective effect of ulinastatin was enhanced with peritoneal
administration. Peritoneal ulinastatin lavage in the early stages
of SAP is therefore likely to have a significant beneficial effect
on the prognosis.
The survival rate of the rats in group EUL at 12 h
(one out of 10) was not significantly improved compared that in
group M (zero out of 10), which may have been due to the short-term
nature of the peritoneal lavage and insufficient fluid
resuscitation. It has been reported that early and extended
peritoneal lavage may be a useful therapy in the management of SAP
(21). Early and target-oriented
fluid resuscitation remains crucial in the early stages of SAP,
although the peritoneal lavage may exert a similar effect (22,23).
Peritoneal ulinastatin lavage in the early stages of SAP is
therefore a promising strategy, as it not only replicates fluid
resuscitation but also enhances the antiprotease effect. The early
administration of ulinastatin by peritoneal lavage may be worthy of
a clinical trial to ascertain whether pancreatitis-associated
multiple organ dysfunction can be prevented or reduced in order to
improve the outcome of patients with SAP.
Acknowledgements
The authors would like to thank Professor Chong-hui
Li (Department of Hepatobiliary Surgery, General Hospital of the
PLA, Beijing, China) for her recommendations for the study.
References
|
1
|
Leonhardt U, Seidensticker F, Fussek M,
Stöckmann F and Creutzfeldt W: Camostate (FOY-305) improves the
therapeutic effect of peritoneal lavage on taurocholate induced
pancreatitis. Gut. 31:934–937. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson C and Imrie CW: Effective
intraperitoneal antiprotease therapy for taurocholate-induced
pancreatitis in rats. Br J Surg. 77:1252–1255. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fric P, Slabý J, Kasafírek E, Kocna P and
Marek J: Effective peritoneal therapy of acute pancreatitis in the
rat with glutaryl-trialanin-ethylamide: a novel inhibitor of
pancreatic elastase. Gut. 33:701–706. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balldin G, Borgström A, Genell S and
Ohlsson K: The effect of peritoneal lavage and aprotinin in the
treatment of severe acute pancreatitis. Res Exp Med (Berl).
183:203–213. 1983. View Article : Google Scholar
|
|
5
|
Berling R, Borgström A and Ohlsson K:
Peritoneal lavage with aprotinin in patients with severe acute
pancreatitis. Effects on plasma and peritoneal levels of trypsin
and leukocyte proteases and their major inhibitors. Int J
Pancreatol. 24:9–17. 1998.PubMed/NCBI
|
|
6
|
Dong Z, Petrov MS, Xu J, Shanbhag S,
Windsor JA and Pang S: Peritoneal lavage for severe acute
pancreatitis: a systematic review of randomised trials. World J
Surg. 34:2103–2108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McKay CJ, Evans S, Sinclair M, Carter CR
and Imrie CW: High early mortality rate from acute pancreatitis in
Scotland, 1984–1995. Br J Surg. 86:1302–1305. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirata K: Essential therapeutic strategies
for acute pancreatitis - guidelines for initial treatment and their
significance. Nihon Rinsho. 62:2049–2056. 2004.(In Japanese).
PubMed/NCBI
|
|
9
|
Botoi G and Andercou A: Early and
prolonged peritoneal lavage with laparoscopy in severe acute
pancreatitis. Chirurgia (Bucur). 104:49–53. 2009.(In Romanian).
|
|
10
|
Inoue K, Takano H, Shimada A, et al:
Urinary trypsin inhibitor protects against systemic inflammation
induced by lipopolysaccharide. Mol Pharmacol. 67:673–680. 2005.
View Article : Google Scholar
|
|
11
|
Ohnishi H, Kosuzume H, Ashida Y, Kato K
and Honjo I: Effects of urinary trypsin inhibitor on pancreatic
enzymes and experimental acute pancreatitis. Dig Dis Sci. 29:26–32.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang XP, Zhang L, Wang Y, et al: Study of
the protective effects of dexamethasone on multiple organ injury in
rats with severe acute pancreatitis. JOP. 8:400–412.
2007.PubMed/NCBI
|
|
13
|
Zhang XP, Tian H, Lu B, et al: Tissue
microarrays in pathological examination of apoptotic acinar cells
induced by dexamethasone in the pancreas of rats with severe acute
pancreatitis. Hepatobiliary Pancreat Dis Int. 6:527–536.
2007.PubMed/NCBI
|
|
14
|
Zhang XP, Tian H, Lai YH, et al:
Protective effects and mechanisms of Baicalin and octreotide on
renal injury of rats with severe acute pancreatitis. World J
Gastroenterol. 13:5079–5089. 2007.PubMed/NCBI
|
|
15
|
Niederau C, Crass RA, Silver G, Ferrell LD
and Grendell JH: Therapeutic regimens in acute experimental
hemorrhagic pancreatitis. Effects of hydration, oxygenation,
peritoneal lavage and a potent protease inhibitor.
Gastroenterology. 95:1648–1657. 1988.PubMed/NCBI
|
|
16
|
Platell C, Cooper D and Hall JC: A
meta-analysis of peritoneal lavage for acute pancreatitis. J
Gastroenterol Hepatol. 16:689–693. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeyama Y: Indications for and efficiency
of peritoneal lavage in severe acute pancreatitis. Nihon Rinsho.
62:2087–2093. 2004.(In Japanese). PubMed/NCBI
|
|
18
|
Keck T, Balcom JH, Antoniu BA,
Lewandrowski K, Warshaw AL and Fernández-del Castillo CF: Regional
effects of nafamostat, a novel potent protease and complement
inhibitor, on severe necrotizing pancreatitis. Surgery.
130:175–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsukawa H, Hara A, Ito T, et al:
Continuous arterial infusion of protease inhibitor with
supplementary therapy for the patients with severe acute
pancreatitis - clinical effect of arterial injection of
ulinastatin. Nihon Shokakibyo Gakkai Zasshi. 95:1229–1234. 1998.(In
Japanese). PubMed/NCBI
|
|
20
|
Warndorf MG, Kurtzman JT, Bartel MJ, et
al: Early fluid resuscitation reduces morbidity among patients with
acute pancreatitis. Clin Gastroenterol Hepatol. 9:705–709. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schröder T: The effect of early
pancreatectomy and peritoneal lavage on the development of
experimental haemorrhagic pancreatitis in pigs. Scand J
Gastroenterol. 17:167–171. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trepte CJ, Bachmann KA, Stork JH, et al:
The impact of early goal-directed fluid management on survival in
an experimental model of severe acute pancreatitis. Intensive Care
Med. 39:717–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuwabara K, Matsuda S, Fushimi K, Ishikawa
KB, Horiguchi H and Fujimori K: Early crystalloid fluid volume
management in acute pancreatitis: association with mortality and
organ failure. Pancreatology. 11:351–361. 2011. View Article : Google Scholar : PubMed/NCBI
|