Introduction
The study of tissue-engineered cartilage with the
basic goals of predetermined shaping and regeneration has provided
novel ideas and techniques for research into laryngeal cartilage
erosion (1–3); however, due to the special nature of
the morphology, location and function of laryngeal cartilage,
tissue engineering research has not, to date, exhibited its full
advantages in the reconstruction of laryngeal cartilage (4). This is not only due to the large
quantity of seed cells and biomaterials required for the
construction and shaping of the tissue-engineered laryngeal
cartilage, but also due to the difficulty of constructing the ideal
irregular, hollow, three-dimensional structure. Tissue-engineered
laryngeal cartilage with a hollow, semi-flared shape should show
good biocompatibility, biodegradability and structures of the pores
inside the biological materials, as well as sufficient mechanical
strength and flexibility (5). How
to ensure that the cartilage maintains its shape and structure and
how to develop the potential of cartilage reshaping applications
are two problems currently faced in the process of
tissue-engineered, hollow, semi-flared laryngeal cartilage
construction. The exploration of hollow structural biomaterials
that are more suitable for shaping and tissue-engineered cartilage
regeneration, as well as the practical application of the cartilage
tissue engineering regenerative technology, is the only way to
improve laryngeal cartilage repair and reconstruction. In the
present study, on the basis of previously published tissue
engineering findings (6,7), the new biomaterial
poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHH) was used in
the shaping of laryngeal tissue-engineered cartilage tissue into a
hollow, half-flared shape, and the compatibility of the PHBHH with
chondrocytes was investigated. The aim of the study was to
determine the feasibility of constructing laryngeal cartilage
tissue with a hollow, half-flared shape using the muscle wrapping
and filling method and using the fascia lining of a pedicled
myofascial flap, in order to provide an experimental basis for the
tissue engineering of laryngeal cartilage with a hollow,
half-flared shape through the transplantation of myofascial
tissue.
Materials and methods
Chondrocyte harvest
Costal and articular cartilages were obtained under
sterile conditions from young New Zealand rabbits of both genders,
aged between three and seven days, provided by the Center of
Laboratory Animals, Shenyang Military Area Command (Shenyang,
China). The cartilage was cleaned, washed twice with 0.1 mol/l
sterile phosphate-buffered saline (PBS) containing 200 U/ml
penicillin and streptomycin, respectively, and digested with 0.25%
trypsin (Sigma-Aldrich, St. Louis, MO, USA) for 2 min. Following
digestion, the cartilage was washed with PBS a further three times,
cut into pieces (1–2 mm3) and washed again with PBS. The
pieces were then placed in a small 50-ml beaker. Type II
collagenase (0.3%; Sigma-Aldrich) was added and stirred for
digestion at 37°C, and the cells were subsequently collected once
every 0.5 h, starting from 1 h, until all the solids had
disappeared. The cell suspension was obtained and centrifuged at
250 xg for 10 min, the supernatant was discarded and the
precipitate was washed with PBS two times. Dulbecco’s Modified
Eagle’s medium/Ham-F12 (1:1) mixed culture medium (Gibco-BRL;
Invitrogen Life Technologies, Carlsbad, CA, USA), containing 20%
fetal bovine serum (Zhejiang Evergreen Biologicals, Hangzhou,
China), was used to resuspend the cells, and trypan blue staining
was performed. The cells were counted using a cell counting board;
stained cells were considered to be non-viable cells while
unstained cells were considered to be viable). Cells were seeded in
100-ml culture flasks at a density of 2×105 cells/ml and
cultured in a humidified CO2 incubator (Thermo Fisher
Scientific Inc., Waltham, MA, USA). The medium was changed every 48
h, and the cells were passaged when the whole wall of the culture
flask was covered by adhered cells.
Mold preparation
Shaping was performed in reference to the full
morphology of adult laryngeal cartilage, and the plastic models
were prepared by 3:1 shrinking. Polytetrafluoroethylene was taken
as the mold material, and sculpted in the form of the corresponding
female die, which was comprised of an inner core and two outer
plates.
Poration and shaping of the biological
material
The biological materials exhibiting a laryngeal
cartilage-like hollow, semi-flared morphology were prepared by
solvent casting, compression molding and particulate filter
leaching methods (8). In brief,
quantitative PHBHH flocculent material (molecular weight, 600,000;
provided by the Department of Chemical Engineering, Tsinghua
University, Beijing, China) was placed inside a spherical,
heat-resistant glass container, and chloroform solvent (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) was added (at a ratio
of 2:3). The mixture was then heated for reflux under sealed
conditions, stirred with a magnetic stirrer and rapidly poured into
a wide-neck flask containing sodium salt (Sinopharm Chemical
Reagent Co., Ltd.) (screening of 150–200 μm). Once the solution
formed a uniform thin paste, the bottle was sealed and placed at
room temperature overnight (making the material naturally penetrate
the salt layer). The mixture of the material and the salt was
firstly prepared into a sheet, then wrapped around the mold core
portion, closed around the outer mold and fixed by metal fixture;
the liquid was then compressed into the mold. The samples were
placed in the fume cupboard for ≥48 h for full evaporation of the
chloroform and subsequently placed in a vacuum pump suction filter
for 12 h to remove any possible residual chloroform. The obtained
samples were set within an inner metal mesh, suspended in glass
containers containing distilled water, magnetically stirred and
filtered for desalination. The triple-distilled water was replaced
at least three times. The desalinated samples were dried naturally
for porosimetry.
Determination of PHBHH porosity
The liquid displacement method (9) was used in the determination of PHBHH
porosity, Due to the lipophilic nature of the PHBHH, ethanol
(Sinopharm Chemical Reagent Co., Ltd.) was used instead of water.
This facilitated the penetration of the liquid into the material.
The ethanol was added with a volume scale (V1) into a sealable test
tube and the test sample (mass, M) was weighed and then also added
to the tube. The tubes were subsequently sealed, opened after 8 h
(to release the gas) and closed again. After 24 h, the ethanol
penetrated sufficiently into the pores within the sample, and the
volume was recorded as V2. Subsequent to removing the sample
saturated with ethanol, the volume of ethanol in the tube was
recorded as V3. The material density was calculated using the
following formula: ρ=M/(V2−V3). The porosity (%) was calculated
using the formula e=(V1−V3)/(V2−V3)×100.
Assistance for shaping and adhesion
The shaping of the hollow, semi-flared PHBHH objects
was aided by the inner surface of a steel wire with a diameter of
0.4 mm, which was uninterruptedly woven into the form of two
approximate rings and three longitudinal supports. Compared with
the PHBHH with the hollow semi-flared shape, the larger ring
fluctuated correspondingly up and down along the semiflared shape
of the corners and edges of the stent, mainly helped the shaping of
the edges and rear parts with less material of the PHBHH with
hollow semi-flared shape. The smaller ring was nearly round, which
assisted in the shaping of the small end of the hollow, semi-flared
PHBHH, and the three longitudinal wire supports were located in the
sides of the PHBHH shape and in the middle of the front. The steel
support frame was placed tightly inside the PHBHH model with no
special fixation. The hollow, semi-flared PHBHH constructions and
the steel wire support frame were disinfected by immersion into 75%
alcohol and then rinsed three times with PBS, prior to being dried
under sterile conditions. Adhesion was promoted by immersing the
PHBHH and the frame into sterile poly-L-lysine aid (relative
molecular mass, 1.89 million; Sigma-Aldrich) for 1 h, prior to
drying.
Compounding of chondrocytes with PHBHH
porous materials and culturing in vitro
Following the adjustment of the cell suspension to a
density of 5×107/ml, the chondrocytes were inoculated in
the pre-dried shaping materials by culture medium containing ~30%
wet culture medium, a small amount of cell suspension each time
when inoculated, was scattered and multicasted to make the cells
distribute as evenly as possible, the inoculated cells of the inner
surface of the shaping hollow material were pipetted using a bend
head. The cells were placed in a CO2 saturated humidity
incubator (Thermo Fisher Scientific, Inc.) at 37°C and 5%
CO2 for 0.5 h, and then removed and agitated. The cell
suspension around the bottom of the material was sucked out and
inoculation was performed from top to bottom twice, prior to
further incubation for 1 h. Fresh culture medium was then added
slowly along the sidewall so that the composite was infiltrated by
2–3 cm. The medium was changed once every 48 h. Cell growth and
adhesion in the composite edge were observed under an inverted
microscope (Huxing XSP-20CD; Shanghai Huxing Optical Instrument
Co., Shanghai, China). The shaped material loaded with chondrocytes
was used in the experimental group, while a control group was
established by shaping the PHBHH without the chondrocytes. All
other steps in the two groups were identical. Each 5×5-mm
sheet-like chondrocyte-PHBHH composite was fixed with 2.5%
glutaraldehyde, dehydrated progressively with alcohol, dried,
mounted and observed using light-emitting plasma spraying with
scanning electron microscopy (SEM) (S570; Hitachi, Tokyo,
Japan).
Surgical procedure
Experimental grouping
Twelve male New Zealand white rabbits (age, ~6
months; weight, 2.5±0.5 kg) were randomly divided into an
experimental (n=9) and a control (n=3) group. This study was
carried out in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health. The animal use protocol was reviewed and
approved by the Institutional Animal Care and Use Committee of the
General Hospital of Shenyang Military Area Command (Shenyang,
China; permit no. 20060828001).
Design of the pedicled myofascial
flap
Intravenous anesthesia was induced with 3%
barbiturate. The side of the back skin of the rabbit was
disinfected and shaved, and the middle portion of the sacral spine
muscle and fascia was isolated from the distal side of the rabbit’s
back to form the myofascial tissue flap with the pedicle for the
proximal and distal sides of the flap. The filled myofascial flap
with a conical hollow portion was designed according to the size of
the chondrocyte-PHBHH composite. The fascial layer of the
myofascial flap, similar to a conical shape, was then stabbed into
the form of a net with a scalpel blade for filling in the prepared
hollow part of the composite. During flap preparation, the sacral
spinal fascia in the sides of the depression was freed, simply
sutured, connected and placed in the recessed slots, and the fascia
in the depressed trench was subjected to poration for the in
situ implantation of the PHBHH-chondrocyte complex. Following
implantation, the remaining sacral spine muscle and fascia were
separated to enclose the chondrocyte-PHBHH complex. Autologous
fascia tissues were used for each implanted chondrocyte-PHBHH
composite.
Implantation in vivo
The chondrocyte-PHBHH composites were co-cultured
in vitro for 7–10 days, until abundant extracellular matrix
was observed using inverted microscopy. The composites were removed
subsequent to reaching a certain degree of support (hardness). The
in situ implantation was designed, filled and wrapped in the
body according to the design of myofascial tissue, and the distal
portion of the filled myofascial flap was fixed. The subcutaneous
skin was sutured. Each batch of surgical procedures was performed
by the same group of technicians, and the surgical procedures and
technology used were consistent. The rabbits were administered an
intramuscular injection of 800,000 units penicillin, and the
surgery was then repeated the next day for a total of four
times.
Experimental observation and
evaluation
Poration, shaping and cellular
compatibility of PHBHH
The PHBHH composites were observed and assessed for
shapes similar to the hollow, semi-flared laryngeal cartilage
morphology. The composite pore continuity and chondrocyte adhesion,
distribution and growth were observed using SEM.
Construction of tissue-engineered,
hollow, semi-flared laryngeal cartilage
The animals were anesthetized and the implants were
removed: Three rabbits from the experimental group and one from the
control group were selected at each time-point, six, 12 and 18
weeks after surgery, respectively. The gross morphology of the
tissue-engineered laryngeal cartilage was observed, and hematoxylin
and eosin (HE), Masson’s trichrome and Alcian blue/periodic acid
Schiff reaction (AB/PAS) staining were performed, as well as
collagen type II immunohistochemical detection for the evaluation
of cartilage formation.
Results
Fabrication of PHBHH porous models
The porous PHBHH composite was prepared by solvent
casting, compression molding and particulate filtering, and had a
hollow, semi-flared shape, which was substantially similar to
laryngeal cartilage morphology (Fig.
1). Following the filtering and demineralization, the whole
structure was porous and spongy. The porosity was measured using
the ethanol static volumetric measurement method (92±2%), and the
pore size and thickness were found to be 100–150 μm and ~1.5 mm,
respectively.
Experimental observation in vitro
At 24 h after the inoculation of chondrocytes into
the hollow, semi-flared PHBHH biomaterials, the chondrocytes were
observed to attach to the surface of the lower edge of the material
under an inverted microscope. Following co-culture of the
chondrocytes and PHBHH for one week, a jelly-like matrix, secreted
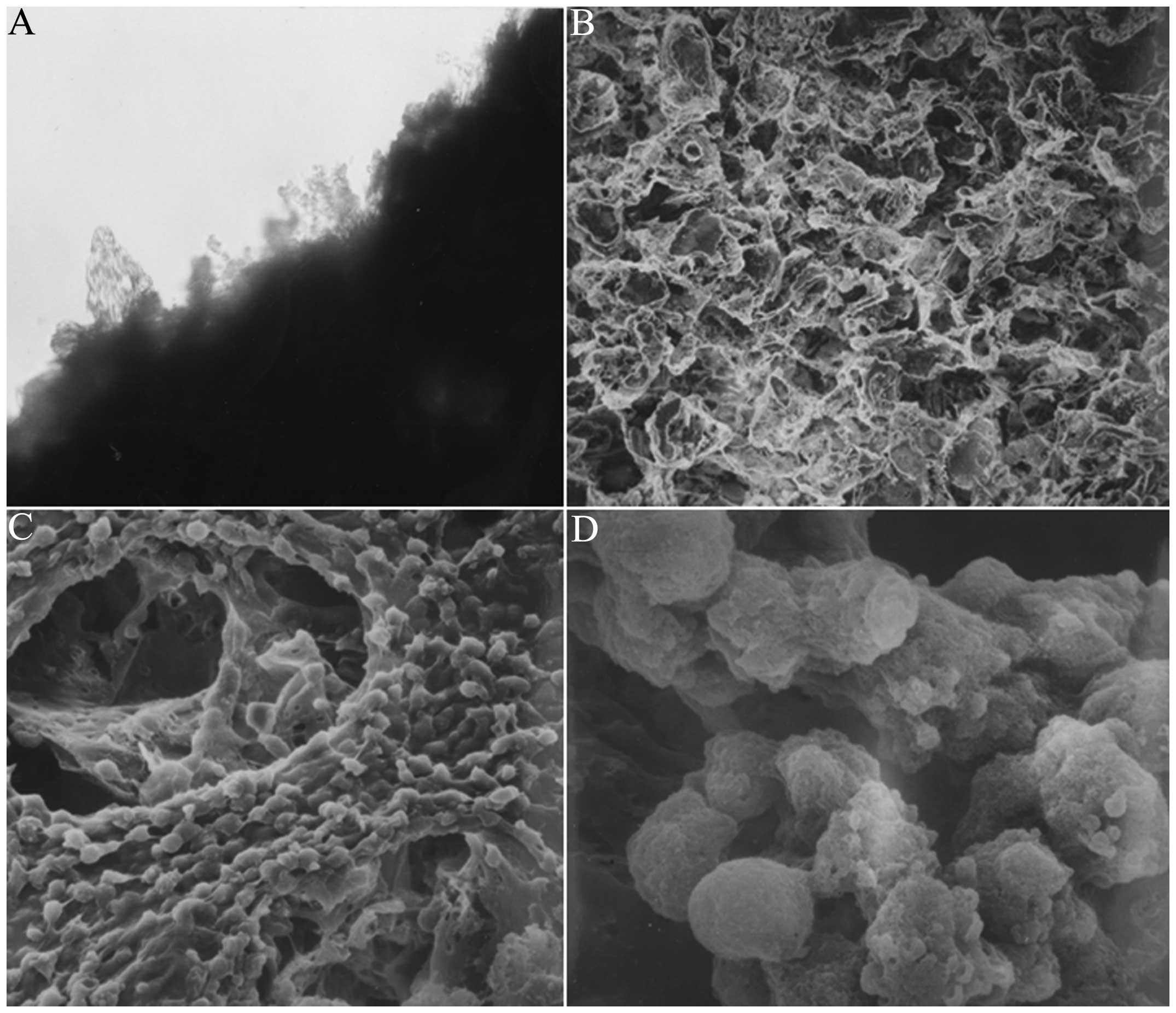
by the chondrocytes, was visible at edge of the material (Fig. 2A).
SEM
The simple PHBHH material was porous and spongy,
exhibiting a pore size of 100–150 μm (Fig. 2B). SEM showed that the cells
(single, string or cluster) were distributed at the surface and in
the cavernous, spongy hollow of the chondrocyte-PHBHH complex.
Mucus-like stromal substances around the cells exhibited
interlinking adhesions (Fig. 2C),
and numerous small projections were visible under
high-magnification microscopy, which may have been fused matrix
components secreted by the chondrocytes (Fig. 2D).
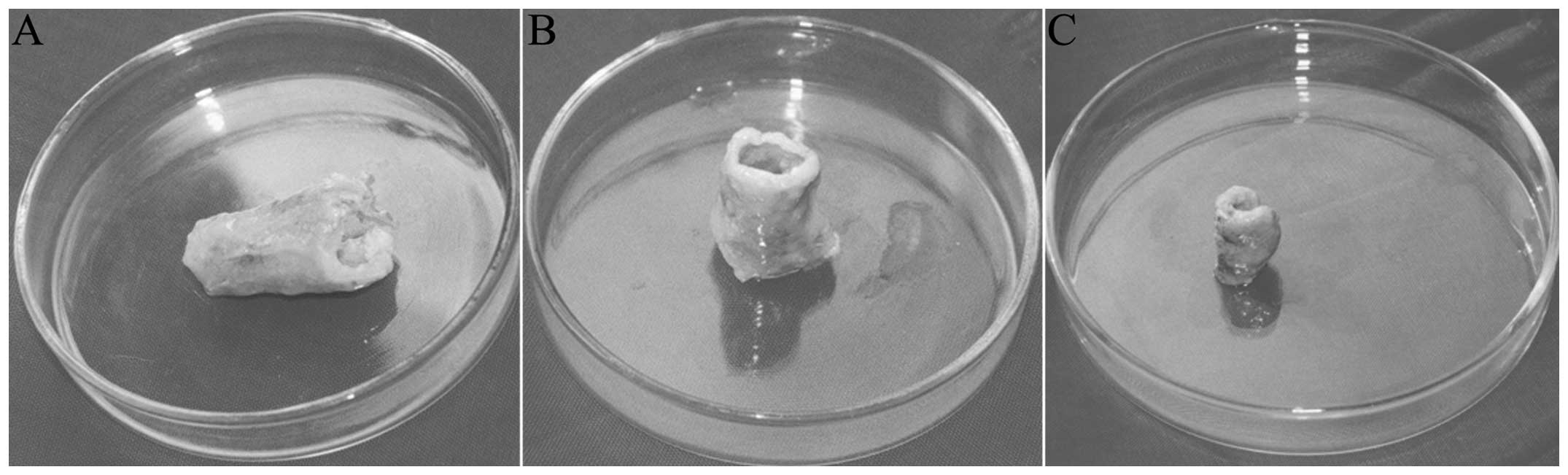
General morphology
The materials were harvested six weeks after
implantation, and the implants and connective tissue myofascial
adhesions were wrapped together. Dense, tiny blood vessels were
distributed on the connective tissue. Subsequent to stripping away
part of the wrapped organization it was observed that the filled
myofascial tissue was closely in contact with the implants. The
steel wire support frame, filled packages and fascia tissues were
removed, and it was found that the general form of the implants in
the two cases was consistent with the morphology prior to
implantation (Fig. 3A). The
specimens possessed a certain hardness with smooth inner and outer
surfaces. In one case the implant had collapsed, showing angular
release and a loss of the predetermined hollow, semi-flared shape
(Fig. 3B). At 12 weeks, only two
complete specimens of the three experimental group implants
remained for harvesting, as the third collapsed; the general form
showed a hollow, semi-flared, tissue-engineered laryngeal
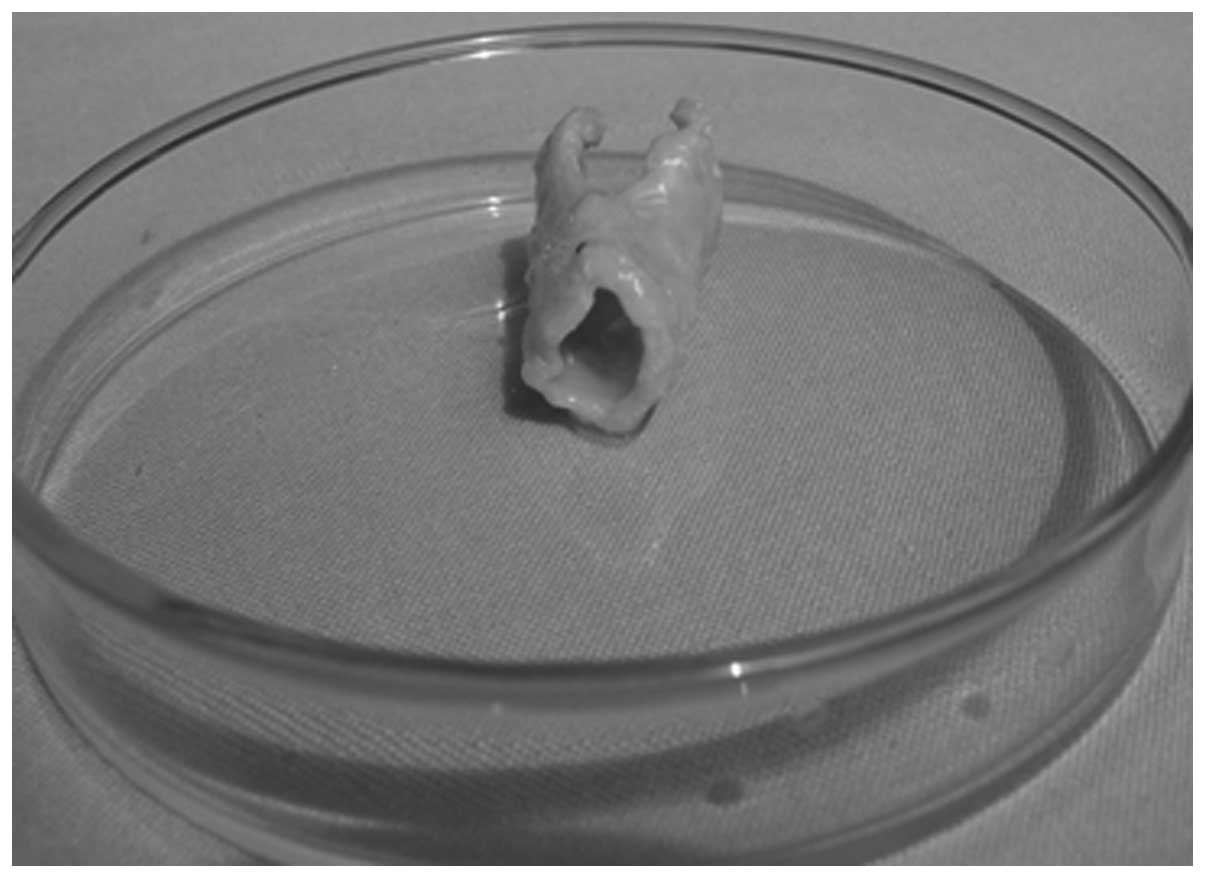
cartilage. When harvested at 18 weeks, the implant shape of the
three cases was consistent, and the milky white, hollow,
semi-flared, tissue-engineered laryngeal cartilage exhibited a
realistic shape (Fig. 4). In the
control group, one implant was removed at each corresponding
time-point. The retained hollow, semi-flared shape of the gross
specimens in the control group at six weeks (Fig. 3C) was significantly less than that
of the experimental group at the same time-point (Fig. 3A). At 12 and 18 weeks the gross
morphology of the implants in the control group had almost
disappeared; only the built-in medical steel support frame was
visible in the implant area.
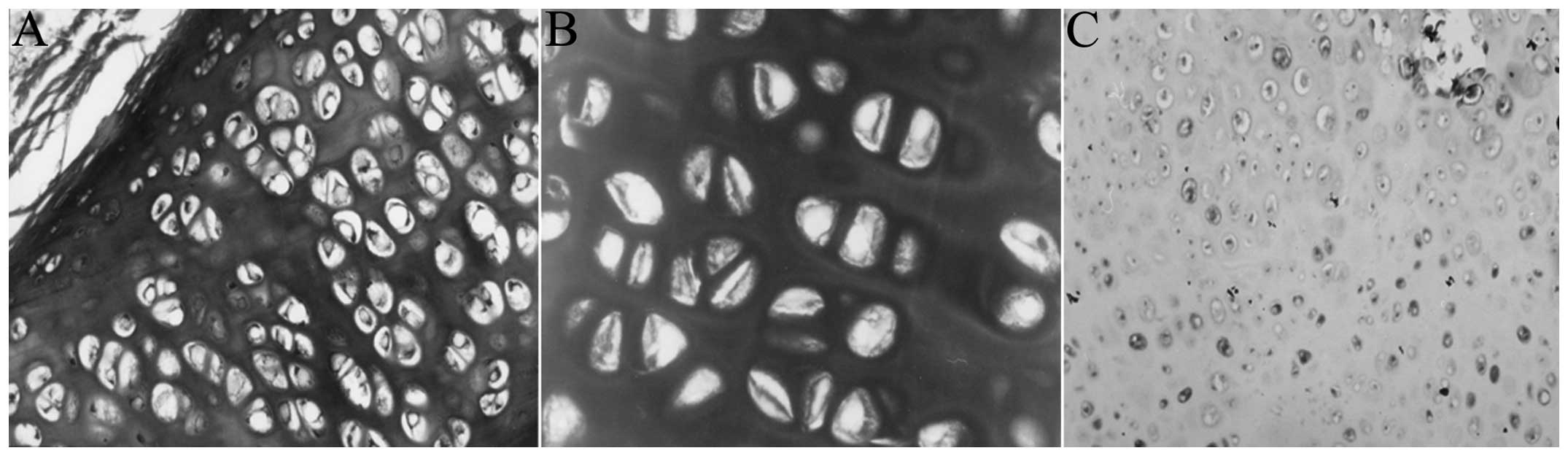
Histological observation
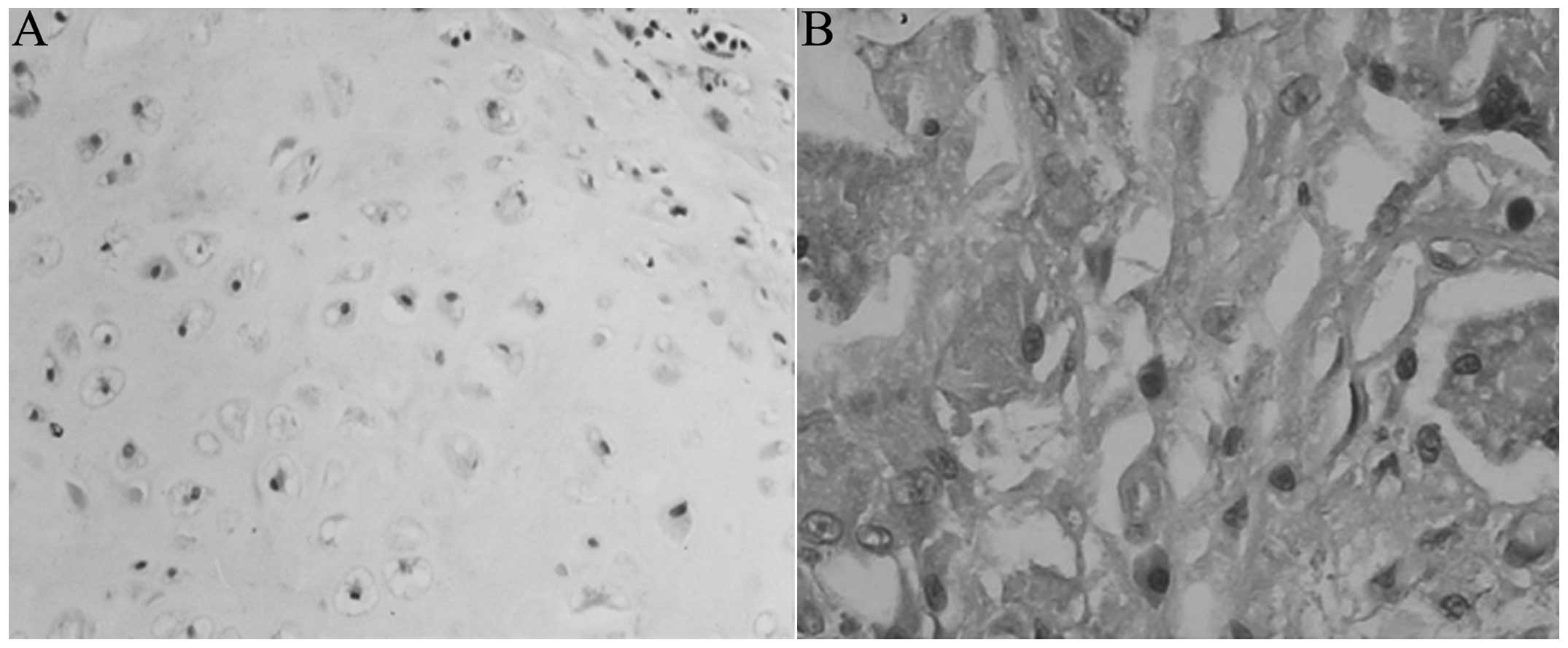
HE staining
At the six-week time-point the cells of the
experimental group exhibited a circular, oval or polygonal
morphology, and single cells were scattered. Incomplete degradation
‘impurities’, such as material around the PHBHH, were visible among
the cells, and inflammatory cell infiltration was present around
the cartilage (Fig. 5A). At 12
weeks, the majority of the cells exhibited an oval morphology; two
to three cells were commonly clustered together and showed a
uniform stromal staining. The morphology of the cells at 18 weeks
was similar to that at 12 weeks; the ‘impurities’ had disappeared,
but a small number of inflammatory cells remained in the
surroundings. Cartilage-like cells in the control group were
visible amongst the collapse of the pores and degradation of the
material at six weeks, and few other tissue cells were scattered on
the surface (Fig. 5B).
Masson’s trichrome staining
Pale green objects were observed in the chondrocytes
and cartilage matrix following cytoplasmic staining of the
experimental group at the sixth week, and the presence of the green
pollutants increased significantly at the 12th week, with deeper
staining (Fig. 6A). The cell
morphology, distribution and coloring at 18 weeks were not
different from the observations at 12 weeks.
AB/PAS
The matrix was purple at the six-, 12- and 18-week
time-points (Fig. 6B).
Immunohistochemistry for type II
collagen
At six weeks, immunohistochemistry for type II
collagen in the experimental group (Boster Biotechnology Co., Ltd.,
Wuhan, China) revealed weakly positive staining, which was mainly
distributed in the cytoplasm, with very small amounts in the
matrix. At 12 weeks, the positive staining in the cytoplasm was
significantly enhanced, and increased type II collagen-positive
staining was detected in the matrix (Fig. 6C). Similar results were observed at
18 weeks.
Discussion
Different from the regeneration of sheet cartilage
tissue by tissue engineering technology, the construction of
hollow, semi-flared, tissue-engineered laryngeal cartilage has a
higher demand for biocompatibility, biodegradability, pore
structure, mechanical strength and the shaping of extracellular
matrix materials (10).
Biomaterials that are currently used in cartilage tissue
engineering include natural biomaterials, such as collagen, fibrin
and chitosan; synthetic biomaterials, such as polyglycolic acid;
microbial biosynthetic material, such as types of biological
polyhydroxyalkanoates and copolymers; and composite materials, such
as polylactic acid-collagen and fibrin-polyurethane (11–14).
The biomaterials each have individual advantages and disadvantages
in terms of biocompatibility, degradation suitability, cell
adhesion, metabolic microenvironment, mechanical strength, shaping
and flexibility. Natural biological materials lack sufficient
mechanical strength, while artificial and compound biomaterials are
expensive and involve complex processing, but are easy to shape
(15–19).
Polyhydroxyalkanoate is a natural substance produced
by microorganisms and exhibits characteristics such as good
biocompatibility, no induction of inflammation, no degradation and
reduced rejection (20,21). As more types of polymers become
available, and as the compatibility among the polymers is enhanced,
tissue engineering scaffolds with improved strength, toughness,
biocompatibility and controlled degradation are likely to be able
to be obtained through the advantages resulting from the
combination of different polymers (6).
In the present study, the selected material PHBHH, a
copolymer of poly-3-hydroxybutyrate and poly-3-hydroxyhexanoate
ester, not only had the advantages common to biological materials
belonging to the polyhydroxyalkanoate class, but could also be
found at low prices and from abundant sources. PHBHH was made into
a hollow, semi-flared, tissue-engineered, larynx-shape model by
solvent casting and compression molding methods; the material
maintained a good shape subsequent to desalting by filtering and
exhibited considerable mechanical strength. The material was porous
and spongy under the electron microscope, the pore size was
consistent with the screening of the salt, the porosity was high,
which was in line with the basic requirements for a porous
structure, and the model showed a certain degree of mechanical
strength. Following the co-culture of the cartilage cells with the
PHBHH composite material in vitro, the model showed good
cell adhesion, distribution and vigorous growth; however, the
mechanical strength was still insufficient to maintain the
predetermined shape upon implantation, which highlighted the
necessity of the auxiliary, in-built support frame. The
implantation of simple PHBHH laryngeal cartilage scaffolds, without
cells, was taken as the control group; after six weeks an increased
loss of general shape was observed in the control group compared
with the experimental group, in which a chondrocyte-PHBHH composite
had been implanted. Under the microscope, no cartilage-like
structure was observed in the control group, and more incomplete
degradation impurities of PHBHH were apparent in the cartilage
tissue. The shape of the control group implants was lost at the
12th and 18th weeks, suggesting that implantation of the material
for six weeks resulted in significant, but not complete,
degradation and absorption. Implantation of PHBHH alone, without
seed cells, led to an ineffective generation of cartilage tissue.
By contrast, after 12 weeks of in vivo implantation in the
experimental group, mature, hollow, semi-flared, tissue-engineered
laryngeal cartilage could be obtained.
Although basic research in cartilage tissue
engineering has achieved encouraging results (22,23),
the functional repair and reconstruction of tissue-engineered
laryngeal cartilage has not yet been implemented in a practical
sense (24,25). The factors impeding this
implementation are the blood supply and mucosal and supportive
defects of the regenerated cartilage. The limitations in the
regeneration of cartilage could be overcome by combining the
advantages of tissue engineering technology and the
predetermination of cartilage morphology with the advantages of the
use of a tissue flap to provide a blood supply. Through this
strategy, and using the characteristics of epithelization (26), an affiliation could be achieved
between the fibrous tissues and the tissue flap. The tissue flap
could be prepared using the design principle of filling the lining
of the cavity fascia and wrapping the outer cavity with muscle to
adhere to the tissue-engineered cartilage. The complex could then
be used to reconstruct the laryngeal cartilage with scaffold
functions according to the common nearest transfer or graft method
(27–31). This technique is likely to overcome
the problems in vascularization, mucosalization and support in
cartilage repair and reconstruction for laryngeal cartilage.
Based on the above idea, the present study attempted
to take a fascia tissue lining and use myofascial tissue flap
filling and wrapping technology to build a predetermined, hollow,
semi-flared, tissue-engineered laryngeal cartilage. In the
experimental procedure, the myofascial tissue of the fascial
portion was placed on the surface of the proposed cartilage
construction for contact. Fascia poration not only enhanced blood
circulation, but was also conducive to adhesion between the
structures. Experimentally designed muscle flap was filled with a
rich blood supply, while the seperated flap, which remained in the
recessed part, also formed an equally rich blood supply. The
cultured chondrocyte-hollow, semi-flared PHBHH composite was placed
off the depression of the tissue flap and cultured for a certain
period of in vitro. The cavity was filled with fascia and
muscle lining, and the periphery was supported by the tissue around
depression or recessed slots. The upper part of the complex was
covered and wrapped with myofascial tissues. The results of the
study showed that the application of myofascial tissue with a flap
blood supply with functional filling and wrapping technology could
successfully lead to the generation of hollow, semi-flared,
tissue-engineered laryngeal cartilage. Difficulties may arise,
however, in avoiding the collapse of the samples, considering the
multiple steps in the construction of the hollow, semi-flared,
tissue-engineered laryngeal cartilage.
This study has raised the potential for a common
transfer transplantation with complex of the tissue flap and
hollow, semi-flared, tissue-engineered cartilage, and has indicated
the success of the myofascial flap filling and wrapping method in
generating hollow, semi-flared, tissue-engineered laryngeal
cartilage for functional repair and reconstruction. The study has
also demonstrated the potential of the mucosal epithelial
transformation of an ideal hollow cartilage surface; however, the
results of this study are preliminary, and the design of laryngeal
cartilage scaffolds based on this design concept, and the
construction, transplantation and practical applications of the
composite tissues, should be the direction of future studies.
References
|
1
|
Sterodimas A and de Faria J: Human
auricular tissue engineering in an immunocompetent animal model.
Aesthet Surg J. 33:283–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng NC, Estes BT, Young TH and Guilak F:
Engineered cartilage using primary chondrocytes cultured in a
porous cartilage-derived matrix. Regen Med. 6:81–93. 2011.
View Article : Google Scholar :
|
|
3
|
Fishman JM, De Coppi P, Elliott MJ, Atala
A, Birchall MA and Macchiarini P: Airway tissue engineering. Expert
Opin Biol Ther. 11:1623–1635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fisher MB and Mauck RL: Tissue engineering
and regenerative medicine: recent innovations and the transition to
translation. Tissue Eng Part B Rev. 19:1–13. 2013. View Article : Google Scholar :
|
|
5
|
Lu T, Li Y and Chen T: Techniques for
fabrication and construction of three-dimensional scaffolds for
tissue engineering. Int J Nanomedicine. 8:337–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng Y, Lin XS, Zheng Z, Deng JG, Chen JC,
Ma H and Chen GQ: Poly(hydroxybutyrate-co-hydroxyhexanoate)
promoted production of extracellular matrix of articular cartilage
chondrocytes in vitro. Biomaterials. 24:4273–4281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Veleirinho B, Coelho DS, Dias PF,
Maraschin M, Ribeiro-do-Valle RM and Lopes-da-Silva JA: Nanofibrous
poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/chitosan scaffolds
for skin regeneration. Int J Biol Macromol. 51:343–350. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mikos AG, Sarakinos G, Leite SM, Vacanti
JP and Langer R: Laminated three-dimensional biodegradable foams
for use in tissue engineering. Biomaterials. 14:323–330. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang R and Ma PX:
Poly(alpha-hydroxylacids)/hydroxyaptite porous composites for
bone-tissue engineering. I Preparation and morphology. J Biomed
Mater Res. 44:446–455. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gilpin DA, Weidenbecher MS and Dennis JE:
Scaffold-free tissue-engineered cartilage implants for
laryngotracheal reconstruction. Laryngoscope. 120:612–617. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shahin K and Doran PM: Improved seeding of
chondrocytes into polyglycolic acid scaffolds using semi-static and
alginate loading methods. Biotechnol Prog. 27:191–200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El Sayed K, Marzahn U, John T, et al:
PGA-associated heterotopic chondrocyte cocultures: implications of
nasoseptal and auricular chondrocytes in articular cartilage
repair. J Tissue Eng Regen Med. 7:61–72. 2013. View Article : Google Scholar
|
|
13
|
Chaim IA, Sabino MA, Mendt M, Müller AJ
and Ajami D: Evaluation of the potential of novel PCL-PPDX
biodegradable scaffolds as support materials for cartilage tissue
engineering. J Tissue Eng Regen Med. 6:272–279. 2012. View Article : Google Scholar
|
|
14
|
Xue J, Feng B, Zheng R, et al: Engineering
ear-shaped cartilage using electrospun fibrous membranes of
gelatin/polycaprolactone. Biomaterials. 34:2624–2631. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vacanti CA and Upton J: Tissue-engineered
morphogenesis of cartilage and bone by means of cell
transplantation using synthetic biodegradable polymer matrices.
Clin Plast Surg. 21:445–462. 1994.PubMed/NCBI
|
|
16
|
Cohen SB, Meirisch CM, Wilson HA and
Diduch DR: The use of absorbable co-polymer pads with alginate and
cells for articular cartilage repair in rabbits. Biomaterials.
24:2653–2660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baek CH and Ko YJ: Characteristics of
tissue-engineered cartilage on macroporous biodegradable PLGA
scaffold. Laryngoscope. 116:1829–1834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhijun L and Wanming W: Latest progress in
biological scaffold materials for tissue engineered cartilage.
Zhongguo zu zhi gong cheng yan jiu. 11:3625–3628. 2007.
|
|
19
|
Asti A and Gioglio L: Natural and
synthetic biodegradable polymers: different scaffolds for cell
expansion and tissue formation. Int J Artif Organs. 37:187–205.
2014.PubMed/NCBI
|
|
20
|
Zhao K, Deng Y, Chun Chen J and Chen GQ:
Polyhydroxyalkanoate (PHA) scaffolds with good mechanical
properties and biocompatibility. Biomaterials. 24:1041–1045. 2003.
View Article : Google Scholar
|
|
21
|
Ye C, Hu P, Ma MX, Xiang Y, Liu RG and
Shang XW: PHB/PHBHHX scaffolds and human adipose-derived stem cells
for cartilage tissue engineering. Biomaterials. 30:4401–4406. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun AK, Li WT, Meng QY, Liu SB, Chen W and
Tang WW: Fabrication of larynx-shape tissue engineered cartilage by
means of filling together with wrapping with pedicle myofascial
flap. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 46:1019–1023.
2011.(In Chinese).
|
|
23
|
Nomoto Y, Okano W, Imaizumi M, Tani A,
Nomoto M and Omori K: Bioengineered prosthesis with allogenic
heterotopic fibroblasts for cricoid regeneration. Laryngoscope.
122:805–809. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lange P, Fishman JM, Elliott MJ, De Coppi
P and Birchall MA: What can regenerative medicine offer for infants
with laryngotracheal agenesis? Otolaryngol Head Neck Surg.
145:544–550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanemaru S, Hirano S, Umeda H, et al: A
tissue-engineering approach for stenosis of the trachea and/or
cricoid. Acta Otolaryngol Suppl. 563:79–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Glatz F, Neumeister M, Suchy H, Lyons S,
Damikas D and Mowlavi A: A tissue-engineering technique for
vascularized laryngotracheal reconstruction. Arch Otolaryngol Head
Neck Surg. 129:201–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aslim EJ, Rasheed MZ, Lin F, Ong YS and
Tan BK: Use of the anterolateral thigh and vertical rectus
abdominis musculocutaneous flaps as utility flaps in reconstructing
large groin defects. Arch Plast Surg. 41:556–561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang SL, Wang YY, Chen WL and Zhang DM:
Use of extended vertical lower trapezius island myocutaneous flaps
to cover exposed reconstructive plates. J Oral Maxillofac Surg.
72:209.e1–7. 2014. View Article : Google Scholar
|
|
29
|
Zhi L, Wenli W, Pengfei G, et al:
Laryngotracheal reconstruction with autogenous rib cartilage graft
for complex laryngotracheal stenosis and/or anterior neck defect.
Eur Arch Otorhinolaryngol. 271:317–322. 2014. View Article : Google Scholar
|
|
30
|
Chanowski EJ, Haxer MJ and Chepeha DB:
Microvascular cricoid cartilage reconstruction with the
thoracodorsal artery scapular tip autogenous transplant.
Laryngoscope. 122:282–285. 2012. View Article : Google Scholar
|
|
31
|
Zhao Y, Zhang W, Zhao J, Li Z and He S:
Reconstruction of soft tissue defects in oral and maxillofacial
regions after tumors surgery using cervical pedicle tissue flaps.
Zhogguo Xiu Fu Chong Jian Wai Ke Za Zhi. 19:780–783. 2005.
|