Introduction
The minute ventilation/carbon dioxide production
(VE/VCO2) slope reflects the increase in ventilation in
response to CO2 production, and thus shows increased
ventilatory drive (1). Changes in
the VE/VCO2 slope may be induced by increases in the
number of chemoreceptors, the peripheral ergoreceptor response, the
ventilatory dead-space and also by the muscle mass engaged in
exercise (2–5). Arena et al (6) reported that based on the
VE/VCO2 slope, there is a 4-level ventilatory
classification (VC) system (VC-I, ≤29.9 implies negligible risk of
a major cardiac event; VC-II, 30.0–35.9, low risk of major cardiac
event; VC-III, 36.0–44.9, moderate risk of major cardiac event; and
VC-IV, ≥45.0, high risk of major cardiac event) which is currently
used in clinical practice. Other indices, including peak oxygen
consumption rate (VO2), anaerobic threshhold
VO2 and the oxygen uptake efficiency slope, can be used
to indicate the prognosis for cardiac-related events. Studies have
shown that the VE/VCO2 slope exhibits a high prognostic
value for cardiac-related events in patients with chronic heart
failure (CHF) (6–11), and the risk of mortality is
believed to increase when the VE/VCO2 slope is >32.8
(12). The VE/VCO2
slope also provides useful information for the management of CHF.
There is, however, no evidence concerning the VE/VCO2
slope and its prognostic value for cardiac-related events in
Chinese patients, and there are no studies suggesting different
VE/VCO2 slope prognostic values for cardiac-related
events in Chinese patients with CHF versus non-Chinese
subjects.
In China, the estimated prevalence of heart failure
is 0.9%, and there are ~4 million patients with CHF. Furthermore,
the number of patients is increasing annually (13). High morbidity and mortality rates,
recurrent hospitalization and a heavy medical burden are common
concerns in patients with CHF around the world. The aim of the
present study was to investigate ventilatory efficiency by
analyzing the VE/VCO2 slope in Chinese patients with CHF
and to assess the prognostic value of the VE/VCO2 slope
in this population.
Subjects and methods
Subjects
Patients with CHF were recruited from the Department
of Cardiology of the Affiliated Tongji Hospital of Tongji
University (Shanghai, China) between August 2007 and May 2013. The
inclusion criteria consisted of a diagnosis of heart failure
(14) and evidence of left
ventricular systolic dysfunction on two-dimensional
echocardiography obtained within one month of cardiopulmonary
exercise testing (CPET) [patients in the registry with a left
ventricular ejection fraction (LVEF) of ≥49% were excluded from the
analysis]. Patients with a diagnosis of significant pulmonary
disease (maintained on home oxygen therapy for lung disease and/or
inhaled corticosteroids) were excluded from the study. A total of
129 patients (113 males and 16 females) with a mean age of
59.1±11.4 years were enrolled into the CHF group. The mean body
mass index (BMI) of the patients was 24.7±3.7 kg/m2 and
the LVEF was 0.38±0.09%. The cardiac function of the patients was
classified according to the New York Heart Function Assessment
(NYHA) as grades I–III (NYHA I, n=5; NYHA II, n=68; NYHA III, n=56)
(15). Among the 129 patients, 74
were diagnosed with coronary artery disease with indication for
cardiac catheterization, and 55 with dilated cardiomyopathy. During
the study, CHF therapy was allowed to continue with digitalis
(43.0%), β-blocker (89.0%), angiotensin-converting enzyme inhibitor
(ACEI) and angiotensin II receptor blocker (91.0%) and diuretics
(51.0%). One day before the CPET, treatment with digitalis,
β-blocker, ACEI, angiotensin receptor blocker or diuretics was
discontinued, but these treatments were re-initiated following the
CPET.
In addition to the CHF group, healthy volunteers
were screened for inclusion in a control group. Volunteers were
excluded if they had been diagnosed with a chronic illness or were
receiving chronic medication; if they had any current health
complaints, an abnormal physical examination (including a blood
pressure of ≥140/90 mmHg) or abnormal results on the screening
tests [electrocardiogram (ECG), rest and exercise echocardiogram
and spirometry]; or if they were participating in regular exercise.
A total of 129 healthy age-matched volunteers (106 males and 23
females) met these criteria and were enrolled. The gender and body
mass index (BMI) of the volunteers were also matched. Informed
consent was obtained from all patients prior to recruitment in
accordance with the protocol approved by the Ethics Committee of
the Affiliated Tongji Hospital of Tongji University. The study was
registered in the China Clinical Trial Registration Center
(registration no. ChicTR-TRC-00000235).
Echocardiography
Echocardiography was performed on each subject using
the GE Vivid™ 7 EchoPAC™ system (GE Healthcare, Pittsburgh, PA,
USA) with a high-definition 3.2-MHz transducer. All data were
measured by the same qualified physician. Standard M-mode and
two-dimensional echocardiography, as well as Doppler blood flow
measurements, were performed in accordance with the American
Society of Echocardiography guidelines (16). Interventricular septum thickness
(IVST) and left ventricular posterior wall thickness (LVPWT) were
obtained from the parasternal long axis view. The left ventricular
end systolic diameter (LVESD) and left ventricular end diastolic
diameter (LVEDD) were obtained from two-dimensional apical images.
The LVEF was calculated from two-dimensional apical images
according to the Simpson method (17), and the left ventricular mass (LVM)
was calculated according to the formula proposed by Devereux et
al (18): LVM (g) = 0.8 ×
[1.04 × (IVST + LVEDD + LVPWT)3 - LVEDD3] +
0.6. The LVM was subsequently adjusted for body surface area (BSA)
to obtain the LVM index (LVMI): LVMI (g/m2) =
LVM/BSA.
CPET
The modified Ramp 10 protocol (19) was used to conduct the
symptom-limited CPET with Variobike 500 exercise apparatus (GE
Healthcare). Immediately prior to exercise testing the subjects
were encouraged to produce a maximal effort. Analysis of the
ventilatory expired gas was conducted using a metabolic cart
(Innocur™ version 5.00, Breath-by-Breath; Innovision A/S, Odense,
Denmark). Prior to each test, reference and calibration gases were
used to calibrate the equipment according to the manufacturer’s
instructions. Standard 12-lead ECGs were obtained at rest and
during the exercise and recovery phases; blood pressure was
measured using a standard cuff sphygmomanometer. Prior to
exercising, the patients were asked to rest for 3 min. The exercise
began with a 3-min warm-up at 0 W and 60–70 rpm, and thereafter a
10-W increment in load was administered every minute subsequent to
exercising at 20 W for 2 min. Continuous ECG, manual blood pressure
measurement and heart rate recording were performed every minute,
and the Borg scale was used to rate the perceived exertion at each
stage (20). Exercise was
discontinued when the patients were physically exhausted, or
developed severe dyspnea or dizziness. Several parameters were
obtained through the metabolic systems during exercise testing,
including oxygen consumption (VO2), VCO2 and
VE. The anaerobic threshold was determined by the V slope (21). The VE/VCO2 slope was
determined using linear regression analysis of VE and
VCO2 obtained throughout the exercise period (22).
End-points
The subjects were followed-up for a median of 33.7
months (maximum follow-up period, six years) for assessments of
cardiac-related mortality and hospitalization following the CPET
via medical chart review. Any mortality or hospitalization that
occurred as a result of cardiac dysfunction, as per the hospital
discharge diagnosis, was regarded as an event. The most common
causes of mortality noted were cardiac arrest, myocardial
infarction and end-stage heart failure. The most common causes of
hospitalization were decompensated heart failure and coronary
artery disease.
Statistical analysis
All continuous data are presented as the mean ±
standard deviation. Categorical variables are reported as
percentages. The Student’s t-test and χ2 analysis were
utilized to compare differences in the continuous and categorical
variables, respectively. The NYHA grades were compared with a
nonparametric rank-sum test. Receiver operating characteristic
(ROC) and Cox regression analyses were employed to evaluate the
predictive value of the VE/VCO2 slope for
cardiac-related mortality and hospitalization. SPSS version 18.0
(SPSS Inc., Chicago, IL, USA) was used for the statistical
analysis. A two-tailed P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
General patient data
Table I shows the
characteristics of the patients with CHF and controls at baseline.
No significant differences were found in the age, gender or BMI
between the two groups; however, the LVMI was significantly higher
and the LVEF was markedly lower in the patients with CHF when
compared with those in the controls (P<0.01). The peak
VO2 was significantly lower in patients with CHF
compared with controls (P<0.01). The VE/VCO2 slope
was significantly higher for the patients with CHF as compared with
that for the controls (P<0.01). The patients with CHF (n=129)
were followed-up for a median period of 33.7 months (maximum, six
years), and 19 cardiac-related mortalities and 198 cardiac-related
hospitalizations were recorded. The control group was followed up
for a median period of 37.8 months (maximum, six years) and no
cardiac-related mortalities or hospitalizations were recorded.
 | Table IBaseline characteristics for each
group of participants. |
Table I
Baseline characteristics for each
group of participants.
| Characteristic | CHF group | Control group | P-value |
|---|
| n | 129 | 129 | |
| Age, years | 59.1±11.4 | 56.8±8.8 | 0.068 |
| Gender, M/F | 113/16 | 106/23 | 0.297 |
| BMI,
kg/m2 | 24.7±3.7 | 24.5±3.0 | 0.807 |
| LVMI,
g/m2 | 138.8±46.5 | 100.7±15.8 | 0.001 |
| LVEF, % | 38±9 | 69±4 | <0.001 |
| NYHA grade,
I/II/III | 5/68/56 | 129/0/0 | <0.001 |
| β-blockers, n
(%) | 115 (89.0) | 0 | <0.001 |
| ACEI or ARB, n
(%) | 117 (91.0) | 0 | <0.001 |
| Diuretics, n
(%) | 66 (51.0) | 0 | <0.001 |
| Digoxin, n (%) | 55 (43.0) | 0 | <0.001 |
| Peak
VO2, ml/kg/min | 14.0±3.9 | 22.2±4.2 | <0.001 |
| VE/VCO2
slope | 38.9±8.7 | 28.6±3.9 | <0.001 |
Predictive value of the
VE/VCO2 slope
ROC analysis was conducted to evaluate the
predictive value of the VE/VCO2 slope for
cardiac-related mortalities and hospitalizations in patients with
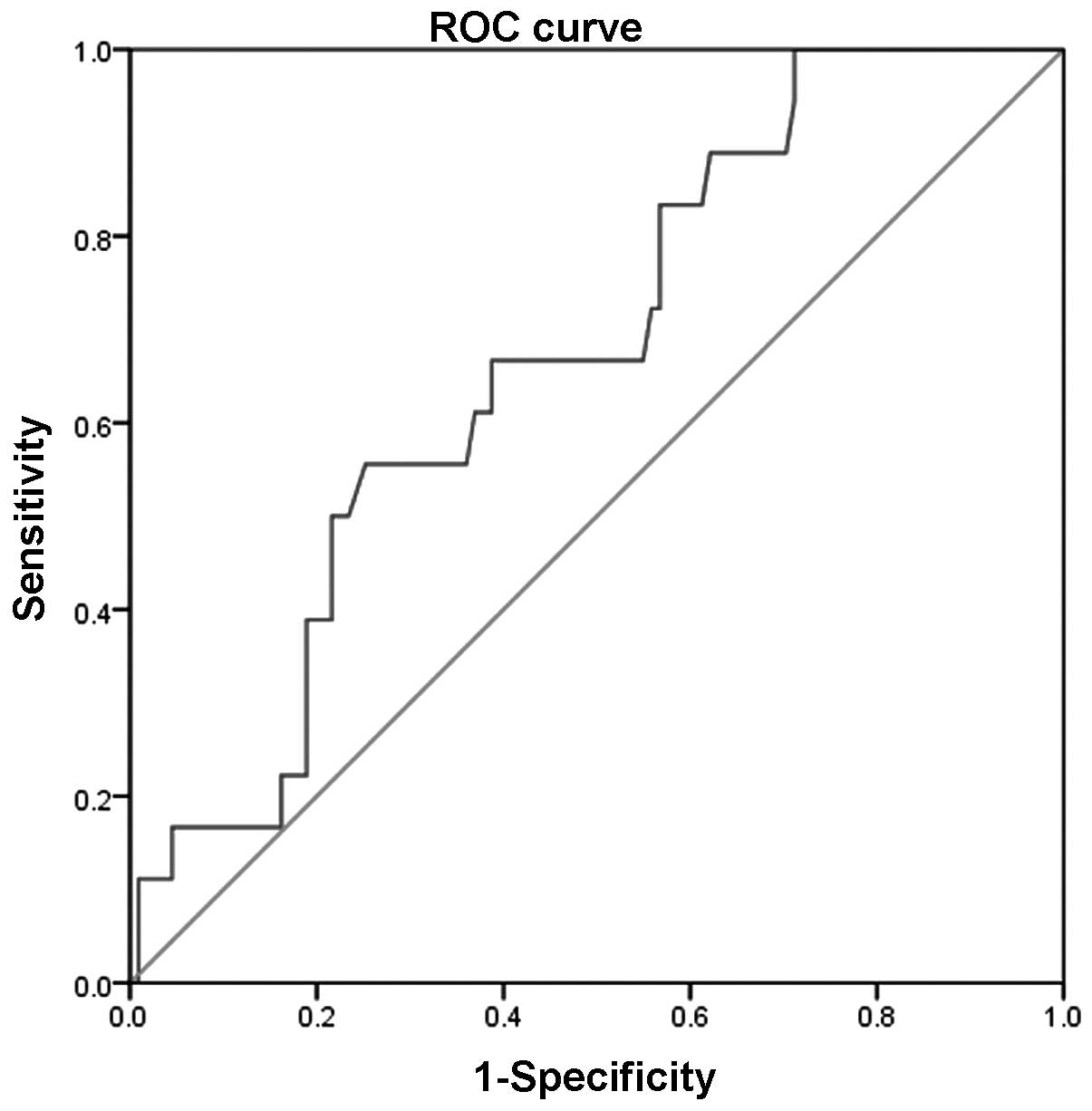
CHF. The area under the curve (AUC) of the VE/VCO2 slope
for predicting cardiac-related mortalities was 0.670 (P<0.05),
and the sensitivity and specificity were 0.667 and 0.620,
respectively. The optimal threshold of the VE/VCO2 slope
for predicting cardiac-related mortalities was ≥39.3 in the
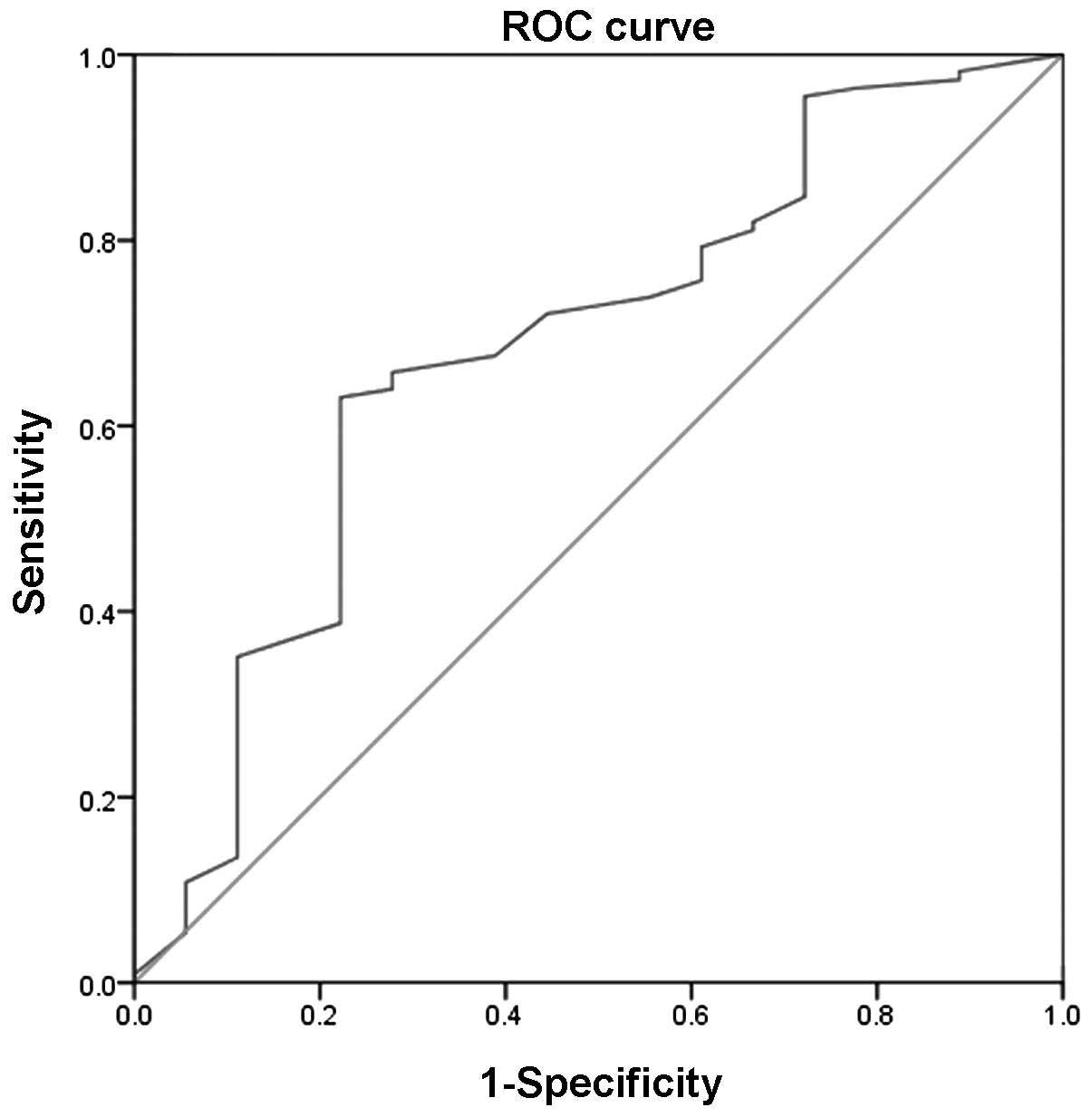
patients with CHF. The AUC of the VE/VCO2 slope for
predicting cardiac-related hospitalizations was 0.682 (P<0.05),
and the sensitivity and specificity were 0.631 and 0.778,
respectively. The optimal threshold of the VE/VCO2 slope
for predicting cardiac-related hospitalizations was ≥32.9 in the
patients with CHF (Table II,
Figs. 1 and 2). The univariate Cox regression analysis
showed that the optimal threshold of the VE/VCO2 slope
was significantly correlated with the number of cardiac-related
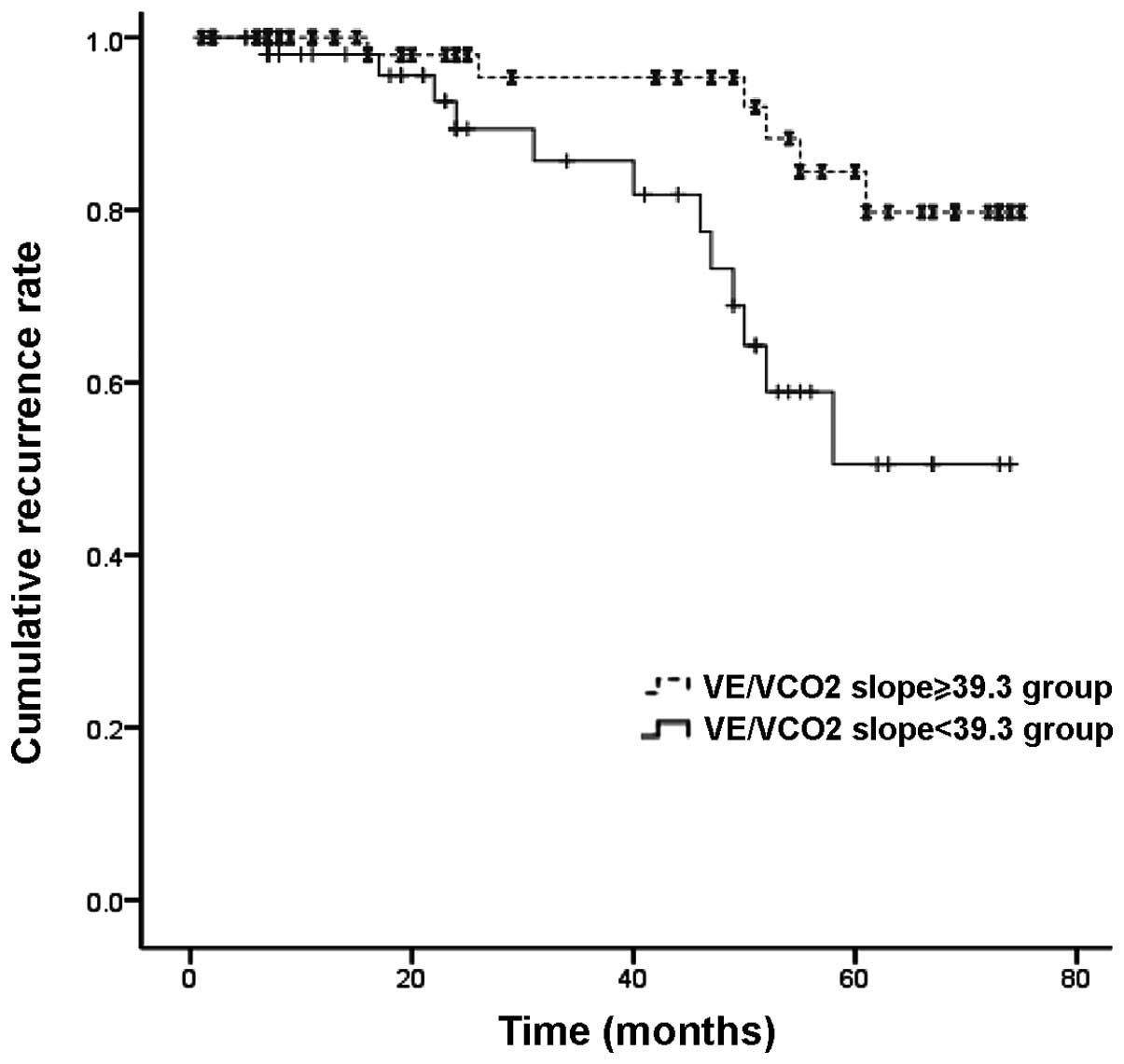
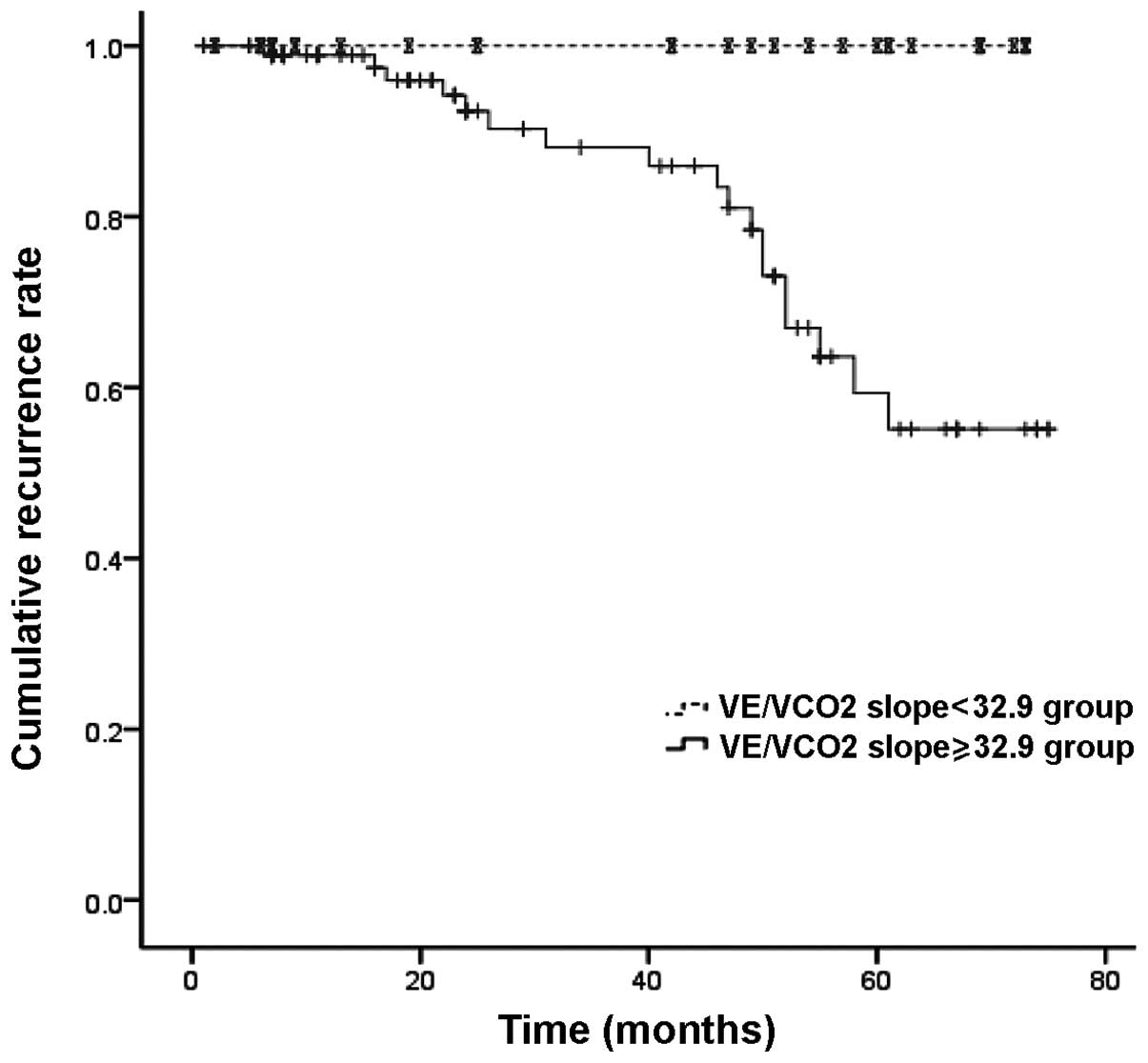
mortalities and hospitalizations (P<0.05) (Table III). The survival model showed
the efficacy of the VE/VCO2 slope in predicting
cardiac-related mortalities (Log-rank, 7.2; P=0.007; Fig. 3) and cardiac-related
hospitalizations (Log-rank, 8.5; P=0.004; Fig. 4) in patients with CHF.
 | Table IIReceiver operating characteristic
curve analysis of the VE/VCO2 slope for cardiac-related
events in patients with chronic heart failure. |
Table II
Receiver operating characteristic
curve analysis of the VE/VCO2 slope for cardiac-related
events in patients with chronic heart failure.
| End-point | AUC of the
VE/VCO2 slope | Optimal
threshold | Sensitivity | Specificity | P-value | AUC 95% CI |
|---|
| Cardiac-related
mortalities | 0.670 | ≥39.3 | 0.667 | 0.620 | 0.021 | 0.549–0.790 |
| Cardiac-related
hospitalizations | 0.682 | ≥32.9 | 0.631 | 0.778 | 0.045 | 0.485–0.798 |
 | Table IIIUnivariate Cox regression analysis
for the VE/VCO2 slope in patients with chronic heart
failure. |
Table III
Univariate Cox regression analysis
for the VE/VCO2 slope in patients with chronic heart
failure.
| Variable | Optimal
threshold | HR | P-value | HR 95% CI |
|---|
| VE/VCO2
slope | ≥39.3 | 1.38 | 0.017 | 1.14–2.28 |
| VE/VCO2
slope | ≥32.9 | 0.71 | 0.012 | 0.41–0.97 |
Discussion
CHF is a common condition with high rates of
morbidity and mortality and a high incidence of recurrent
hospitalization around the world (23,24).
With the increase in the prevalence of CHF in China (13), it is imperative to develop a simple
and flexible measurement to provide more beneficial information for
the management of CHF. CPET remains an important and objective
means of assessing the functional capacity of patients with CHF.
During CPET, peak VO2 and the VE/VCO2 slope
during exercise are traditionally considered to be the major
indices influencing the functional impairment and prognostic value.
Exercise capacity, as reflected by peak VO2, has been
consistently shown to be one of the most powerful prognostic
markers in patients with CHF (11,25,26);
however, the peak VO2 has important limitations and may
not be accurately obtained, as it is susceptible to influence by
the movement such that a plateau is not always achieved at the peak
exercise. By contrast, the VE/VCO2 slope is easier to
determine objectively than the maximal exercise capacity, and the
VE/VCO2 slope is elevated in the majority of patients
with CHF. Numerous studies have confirmed that the
VE/VCO2 slope has equivalent or even superior prognostic
value to the measurement of peak VO2 in patients with
CHF (8,27–31).
The present results confirmed previous findings that
the exercise ventilatory response was increased in the patients
with CHF, as demonstrated by a higher VE/VCO2 slope.
Patients who exhibit an increased ventilatory response to exercise
also demonstrate a poorer exercise tolerance, as indicated by a
reduction in the peak VO2 (32). Excluding the influence of matched
factors, such as age, gender and BMI, the difference in the
VE/VCO2 slope between the two groups was significant. By
comparing the results of the two groups it was shown that the LVEF
was lower, the LVMI and NYHA grade of cardiac function were higher
and the peak VO2 was lower in patients with CHF as
compared with the controls.
The present study demonstrated that the
VE/VCO2 slope was a significant predictor of
cardiac-related events in Chinese patients with CHF. The optimal
threshold of the VE/VCO2 slope for predicting
cardiac-related mortalities was ≥39.3 in the patients with CHF,
which was consistent with the results in another study, in which
the end-points were mortality, transplantation or left ventricular
assist device implantation (optimal threshold of the
VE/VCO2 slope, ≥45) (21). Furthermore, the optimal threshold
of the VE/VCO2 slope for predicting cardiac-related
hospitalizations was ≥32.9 in the patients with CHF. The present
findings were also consistent with those in the study by Arena
et al (33), which utilized
the same end-points (cardiac-related hospitalization), and the
optimal threshold of the VE/VCO2 slope was ≥32.9.
Although β-blockers have been shown to improve the outcomes of
patients with CHF, the results of the study by Arena et al
(34) indicated that β-blockers
have no influence on the prognostic value/characteristics of the
VE/VCO2 slope. In the present study, the influence of
β-blockers should be taken into account.
In conclusion, the present results indicate that
ventilatory efficiency decreases in Chinese patients with CHF and
support the proposal that the VE/VCO2 slope is a
valuable prognostic indicator in Chinese patients with CHF.
Acknowledgements
This study was supported by grants from the United
Brainstorm Project of Shanghai New Technology among the top
hospitals (no. SHDC12010117) and from the Shanghai Health and
Family Planning Committee (no. WSJ1324).
Abbreviations:
|
CHF
|
chronic heart failure
|
|
CPET
|
cardiopulmonary exercise testing
|
|
peak VO2
|
peak oxygen consumption
|
|
VE/VCO2
|
minute ventilation/carbon dioxide
production
|
|
BMI
|
body mass index
|
|
LVMI
|
left ventricular mass index
|
|
LVEF
|
left ventricular ejection fraction
|
|
NYHA
|
New York Heart Function Assessment
|
|
ACEI
|
angiotensin-converting enzyme
inhibitor
|
References
|
1
|
Johnson RL Jr: Gas exchange efficiency in
congestive heart failure. Circulation. 101:2774–2776. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ponikowski P, Chua TP, Anker SD, et al:
Peripheral chemoreceptor hypersensitivity: an ominous sign in
patients with chronic heart failure. Circulation. 104:544–549.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Metra M, Dei Cas L, Panina G and Visioli
O: Exercise hyperventilation chronic congestive heart failure, and
its relation to functional capacity and hemodynamics. Am J Cardiol.
70:622–628. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clark AL, Volterrani M, Swan JW and Coats
AJ: The increased ventilatory response to exercise in chronic heart
failure: relation to pulmonary pathology. Heart. 77:138–146. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cicoira M, Zanolla L, Franceschini L, et
al: Skeletal muscle mass independently predicts peak oxygen
consumption and ventilatory response during exercise in
non-cachectic patients with chronic heart failure. J Am Coll
Cardiol. 37:2080–2085. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arena R, Myers J, Abella J, Peberdy MA,
Bensimhon D, Chase P and Guazzi M: The ventilatory classification
system effectively predicts hospitalization in patients with heart
failure. J Cardiopulm Rehabil Prev. 28:195–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Francis DP, Shamim W, Davies LC, Piepoli
MF, Ponikowski P, Anker SD and Coats AJ: Cardiopulmonary exercise
testing for prognosis in chronic heart failure: continuous and
independent prognostic value from VE/VCO(2) slope and peak VO(2).
Eur Heart J. 21:154–161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arena R, Myers J, Aslam SS, Varughese EB
and Peberdy MA: Peak VO2 and VE/VCO2 slope in
patients with heart failure: a prognostic comparison. Am Heart J.
147:354–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Corrà U, Mezzani A, Bosimini E, Scapellato
F, Imparato A and Giaanuzzi P: Ventilatory response to exercise
improves risk stratification in patients with chronic heart failure
and intermediate functional capacity. Am Heart J. 143:418–426.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bard RL, Gillespie BW, Clarke NS, Egan TG
and Nicklas JM: Determining the best ventilatory efficiency measure
to predict mortality in patients with heart failure. J Heart Lung
Transplant. 25:589–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arena R, Guazzi M, Myers J, et al: The
prognostic utility of cardiopulmonary exercise testing stands the
test of time in patients with heart failure. J Cardiopulm Rehabil
Prev. 32:198–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poggio R, Arazi HC, Giorgi M and Miriuka
SG: Prediction of severe cardiovascular events by
VE/VCO2 slope versus peak VO2 in systolic
heart failure: a meta-analysis of the published literature. Am
Heart J. 160:1004–1014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chinese Society of Cardiology of Chinese
Medical Association; Editorial Board of Chinese Journal of
Cardiology. Guidelines for the diagnosis and management of chronic
heart failure. Zhonghua Xin Xue Guan Bing Za Zhi. 35:1076–1095.
2007.(In Chinese).
|
|
14
|
Radford MJ, Arnold JM, Bennett SJ, et al;
American College of Cardiology; American Heart Association Task
Force on Clinical Data Standards; American College of Chest
Physicians; International Society for Heart and Lung
Transplantation; Heart Failure Society of America. ACC/AHA key data
elements and definitions for measuring the clinical management and
outcomes of patients with chronic heart failure: a report of the
American College of Cardiology/American Heart Association Task
Force on Clinical Data Standards (Writing Committee to Develop
Heart Failure Clinical Data Standards): developed in collaboration
with the American College of Chest Physicians and the International
Society for Heart and Lung Transplantation: endorsed by the Heart
Failure Society of America. Circulation. 112:1888–1916. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fisher JD: New York Heart Association
Classification. Arch Intern Med. 129:8361972. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheitlin MD, Armstrong WF, Aurigemma GP,
et al; ACC; AHA; ASE. ACC/AHA/ASE 2003 Guideline Update for the
Clinical Application of Echocardiography: summary article. A report
of the American College of Cardiology/American Heart Association
Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update
the 1997 Guidelines for the Clinical Application of
Echocardiography). J Am Soc Echocardiogr. 16:1091–1110.
2003.PubMed/NCBI
|
|
17
|
Lebeau R, Di Lorenzo M, Amyot R, et al: A
new tool for estimating left ventricular ejection fraction derived
from wall motion score index. Can J Cardiol. 19:397–404.
2003.PubMed/NCBI
|
|
18
|
Devereux RB, Alonso DR, Lutas EM, Gottlieb
GJ, Campo E, Sachs I and Reichek N: Echocardiographic assessment of
left ventricular hypertrophy: comparison to necropsy findings. Am J
Cardiol. 57:450–458. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boone J, Koppo K and Bouckaert J: The VO2
response to submaximal ramp cycle exercise: Influence of ramp slope
and training status. Respir Physiol Neurobiol. 161:291–297. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Borg G: Psychophysical bases of perceived
exertion. Med Sci Sports Exerc. 14:377–381. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beaver WL, Wasserman K and Whipp BJ: A new
method for detecting anaerobic threshold by gas exchange. J Appl
Physiol (1985). 60:2020–2027. 1986.
|
|
22
|
Bard RL, Gillespie BW, Clarke NS, Egan TG
and Nicklas JM: Determining the best ventilatory efficiency measure
to predict mortality in patients with heart failure. J Heart Lung
Transplant. 25:589–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yancy CW, Jessup M, Bozkurt B, et al: 2013
ACCF/AHA guideline for the management of heart failure: a report of
the American College of Cardiology Foundation/American Heart
Association task force on practice guidelines. J Am Coll Cardiol.
62:e147–e239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chinese Society of Cardiology of Chinese
Medical Association and Editorial Board of Chinese Journal of
Cardiology. Chinese guideline for diagnosis and treatment of heart
failure 2014. Zhonghua Xin Xue Guan Bing Za Zhi. 42:98–122. 2014.In
Chinese.
|
|
25
|
Corrà U, Giordano A, Mezzani A, et al:
Prognostic significance of peak oxygen consumption ≤ 10 ml/kg/min
in heart failure: context vs. criteria. Int J Cardiol.
168:3419–3423. 2013. View Article : Google Scholar
|
|
26
|
O’Neill JO, Young JB, Pothier CE and Lauer
MS: Peak oxygen consumption as a predictor of death in patients
with heart failure receiving β-blockers. Circulation.
111:2313–2318. 2005. View Article : Google Scholar
|
|
27
|
Sarullo FM, Fazio G, Brusca I, et al:
Cardiopulmonary exercise testing in patients with chronic heart
failure: prognostic comparison from peak VO2 and
VE/VCO2 slope. Open Cardiovasc Med J. 4:127–134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arena R and Humphrey R: Comparison of
ventilatory expired gas parameters used to predict hospitalization
in patients with heart failure. Am Heart J. 143:427–432. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kleber FX, Vietzke G, Wernecke KD, et al:
Impairment of ventilatory efficiency in heart failure: prognostic
impact. Circulation. 101:2803–2809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ponikowski P, Francis DP, Piepoli MF, et
al: Enhanced ventilatory response to exercise in patients with
chronic heart failure and preserved exercise tolerance: marker of
abnormal cardiorespiratory reflex control and predictor of poor
prognosis. Circulation. 103:967–972. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guazzi M, Arena R and Myers J: Comparison
of the prognostic value of cardiopulmonary exercise testing between
male and female patients with heart failure. Int J Cardiol.
113:395–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Francis DP, Shamim W, Davies LC, et al:
Cardiopulmonary exercise testing for prognosis in chronic heart
failure: continuous and independent prognostic value from VE/VCO(2)
slope and peak VO(2). Eur Heart J. 21:154–161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arena R, Myers J, Abella J and Peberdy MA:
Influence of heart failure etiology on the prognostic value of peak
oxygen consumption and minute ventilation/carbon dioxide production
slope. Chest. 128:2812–2817. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arena RA, Guazzi M, Myers J and Abella J:
The prognostic value of ventilatory efficiency with beta-blocker
therapy in heart failure. Med Sci Sports Exerc. 39:213–219. 2007.
View Article : Google Scholar : PubMed/NCBI
|