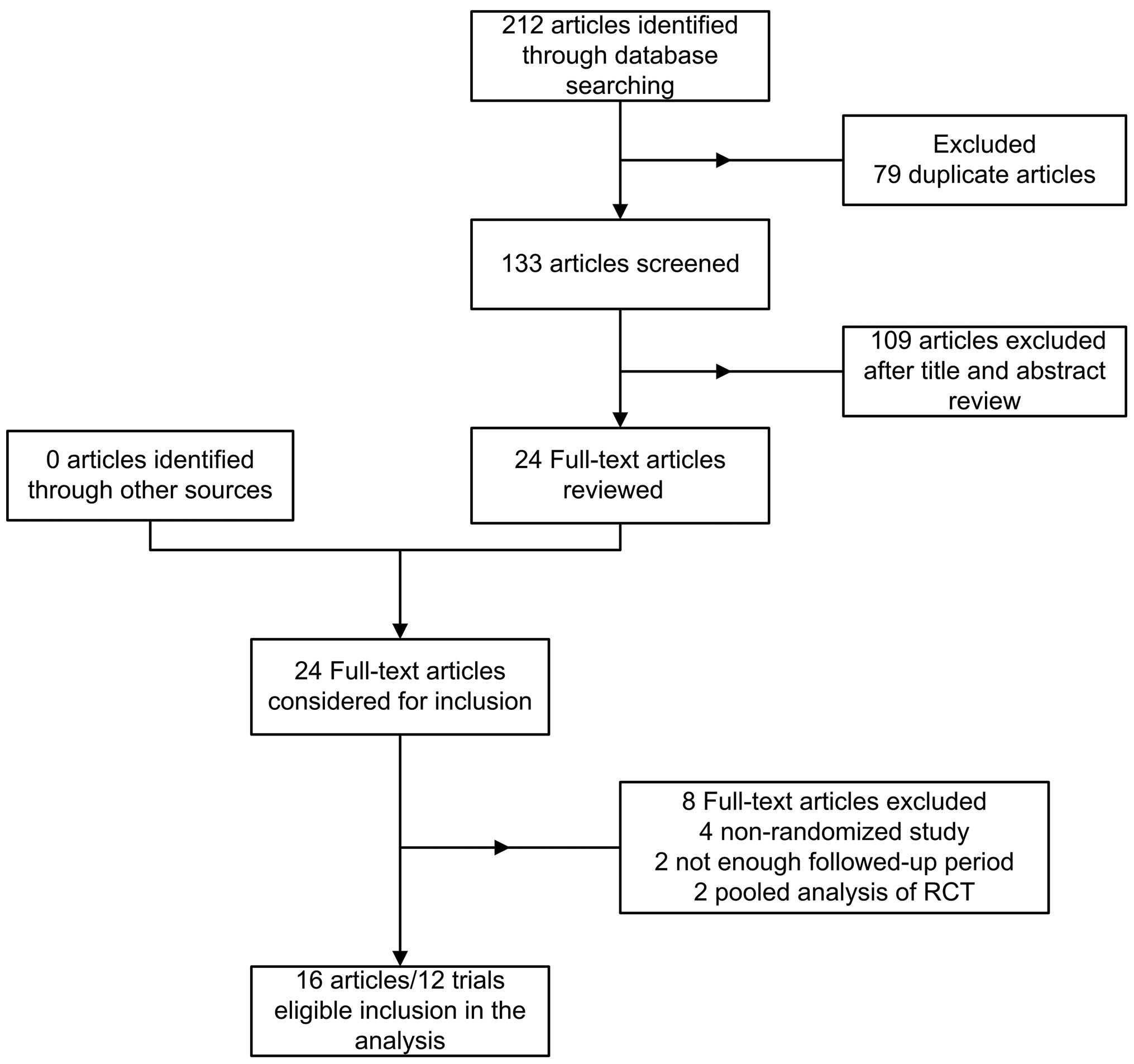
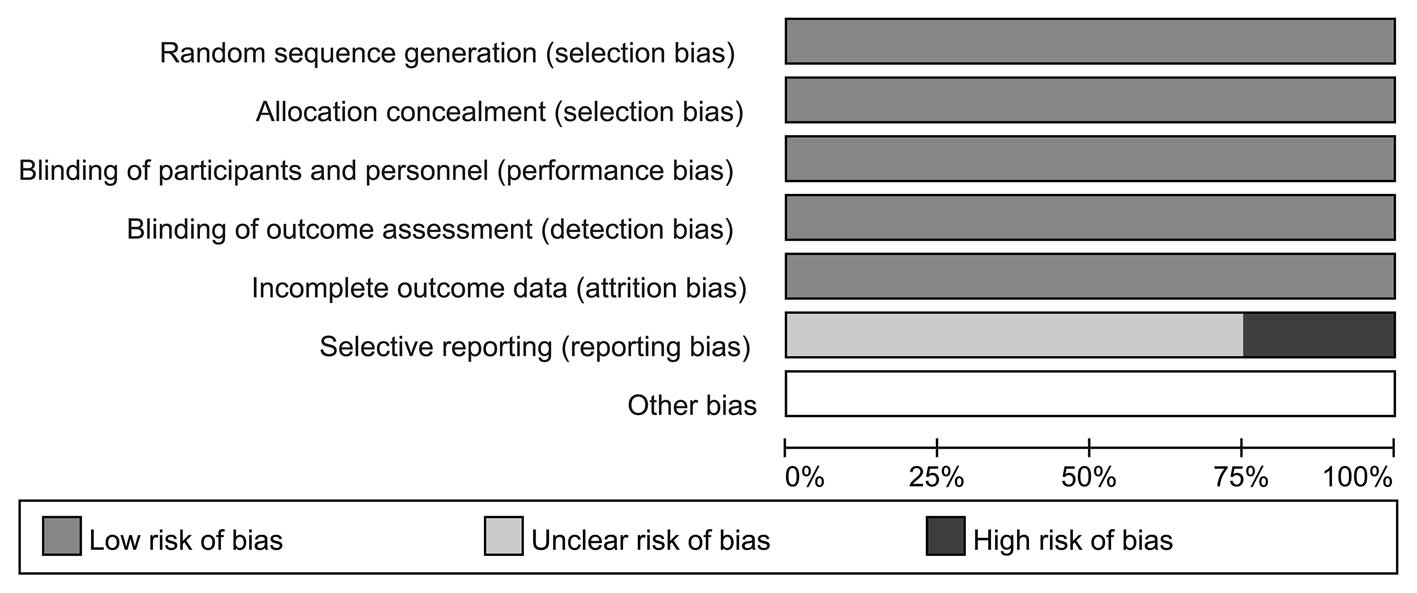
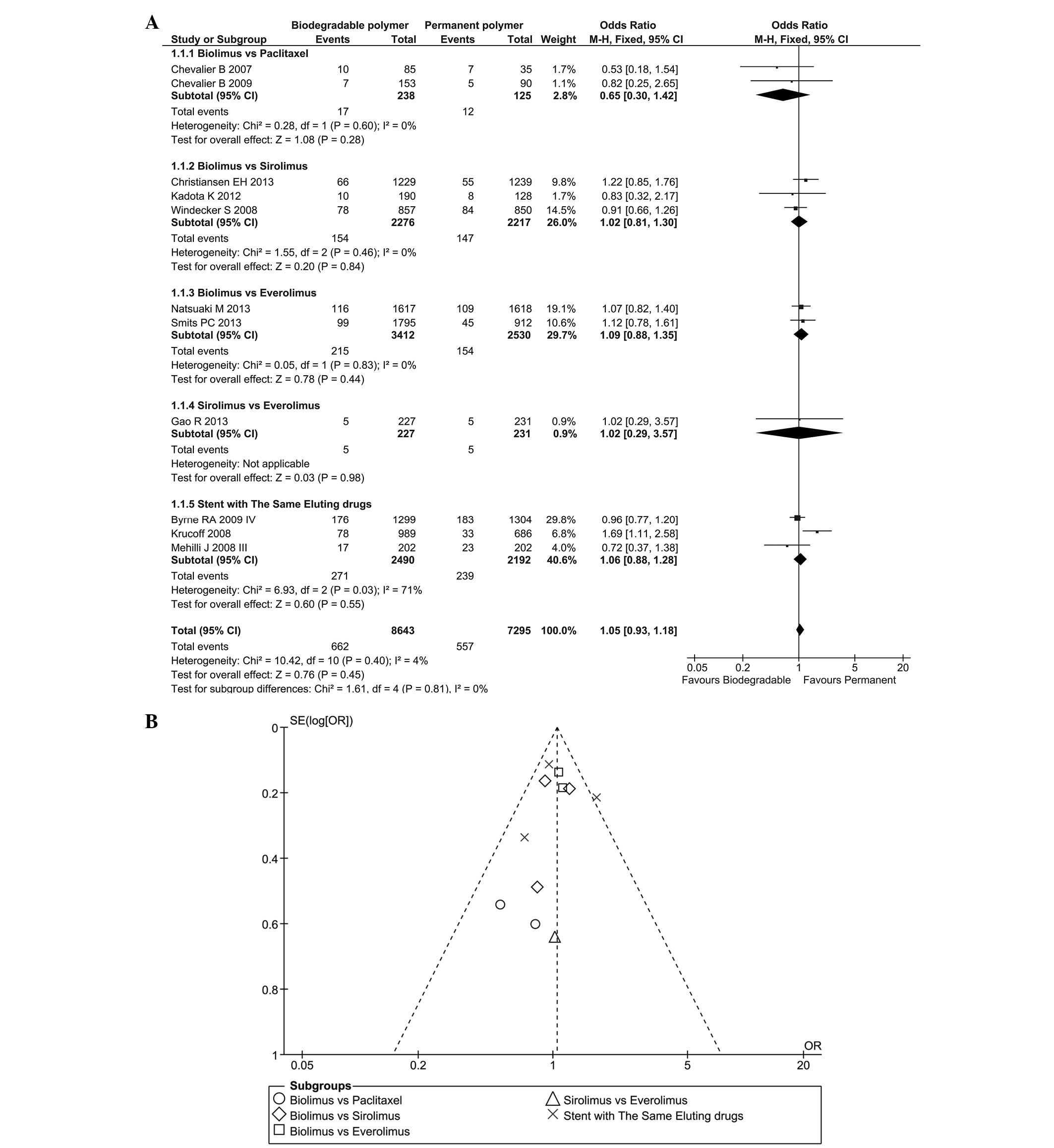
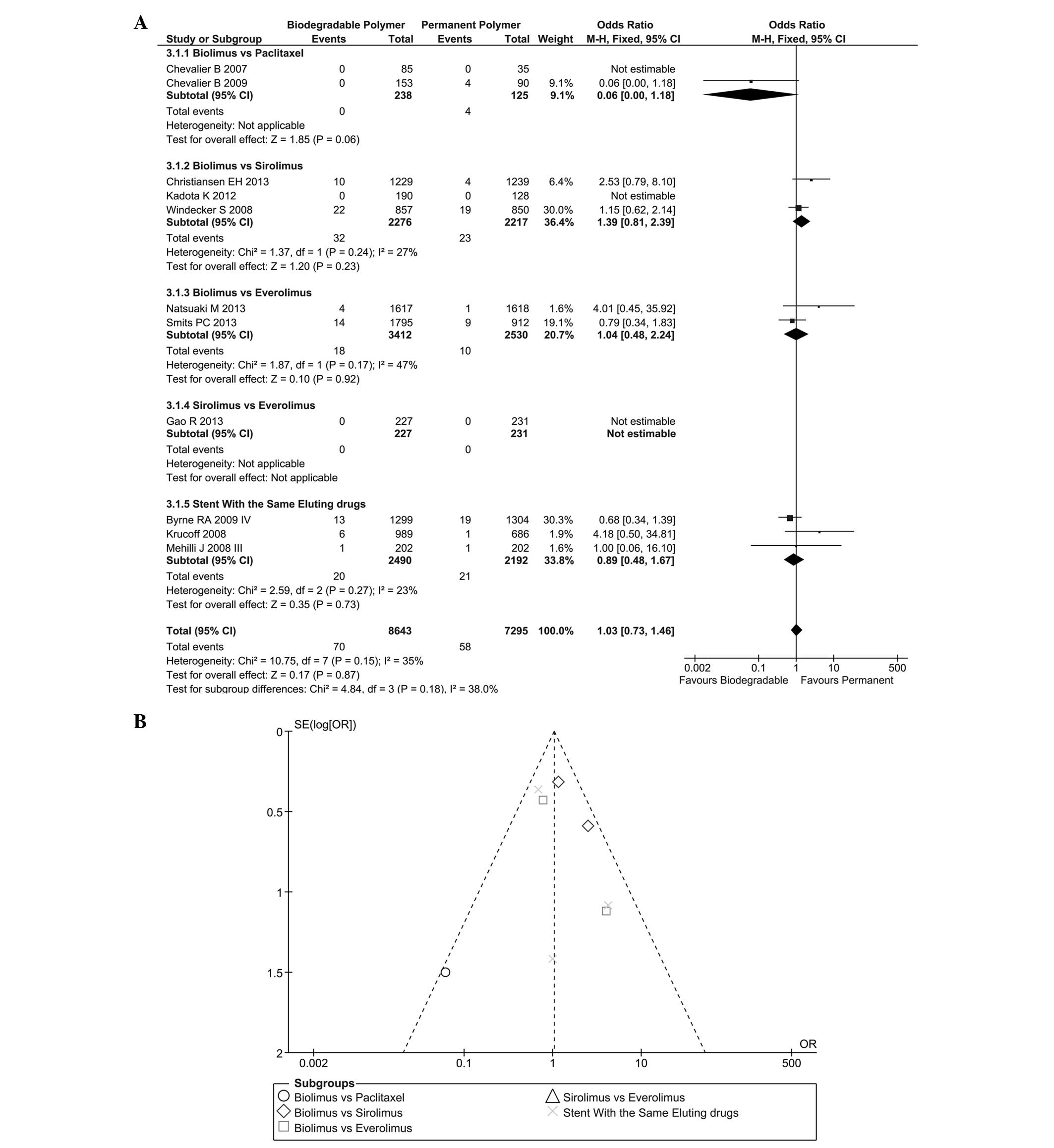
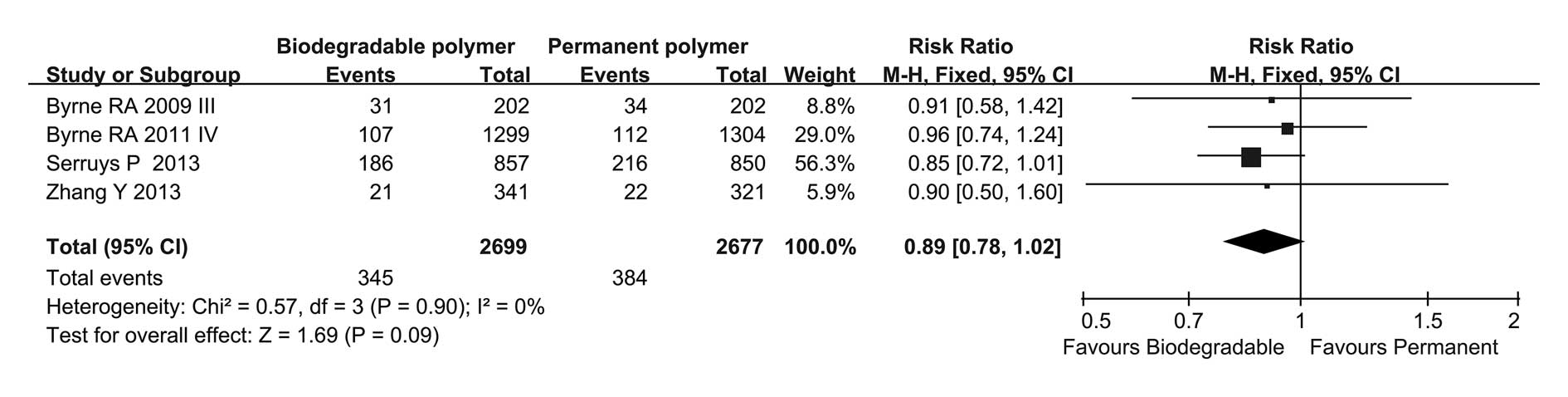
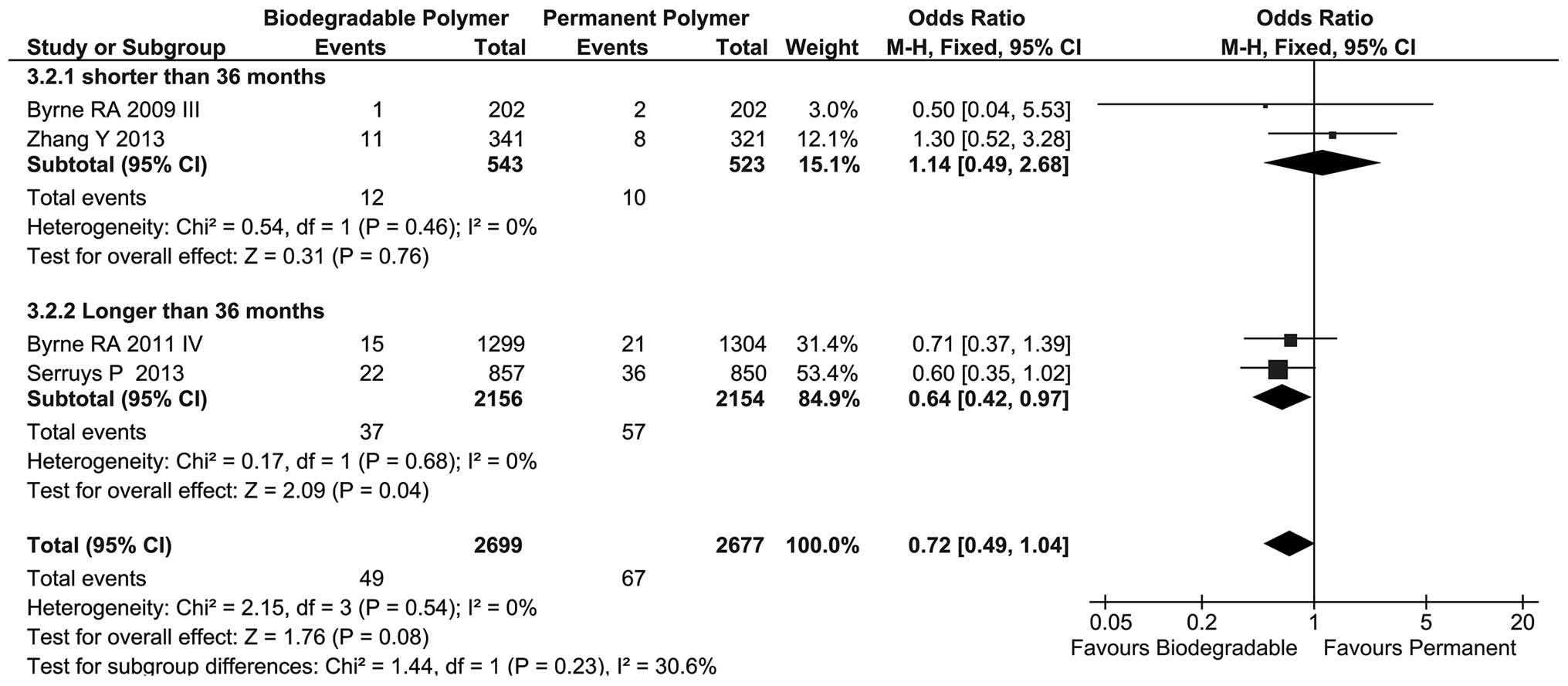
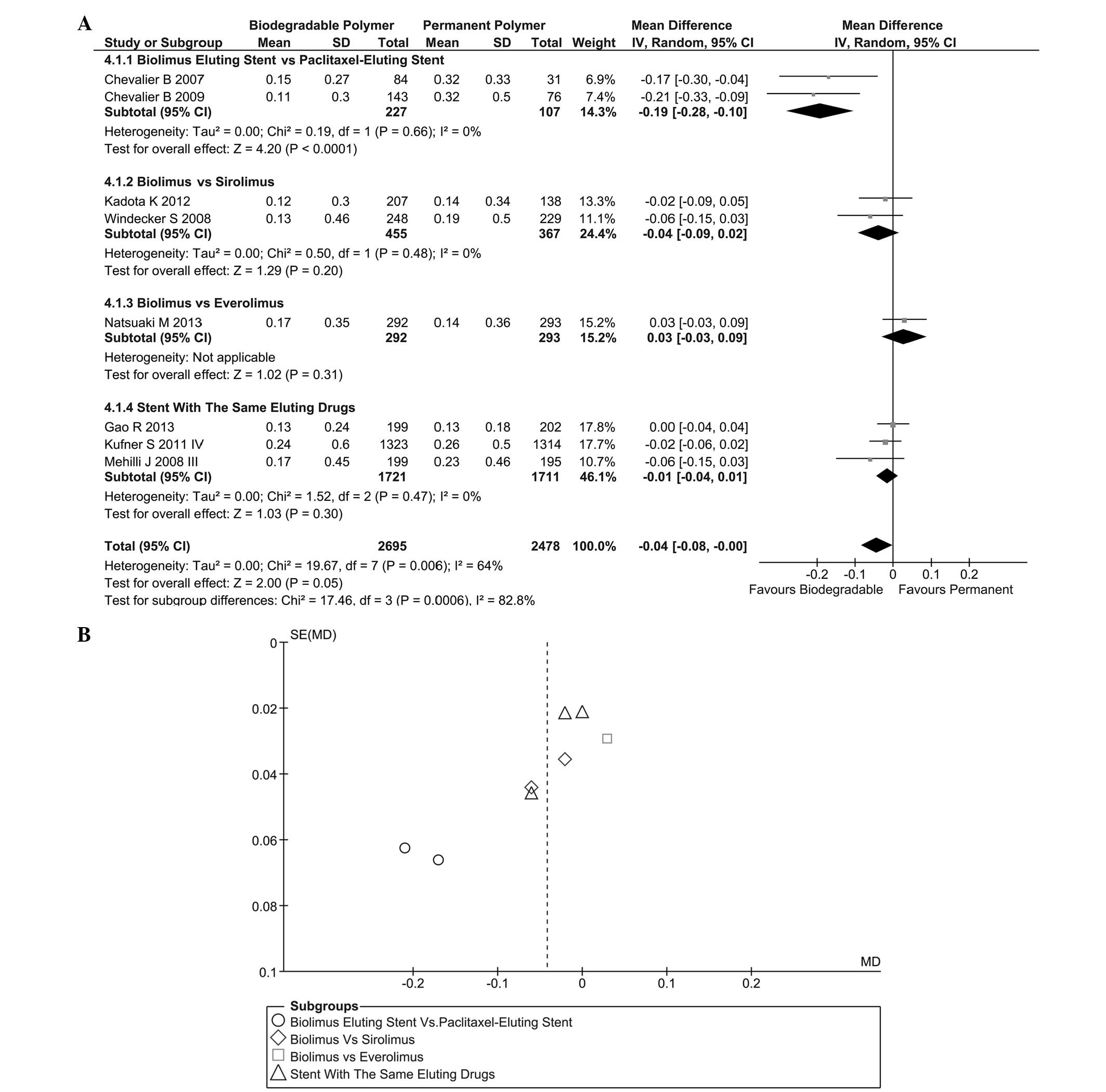
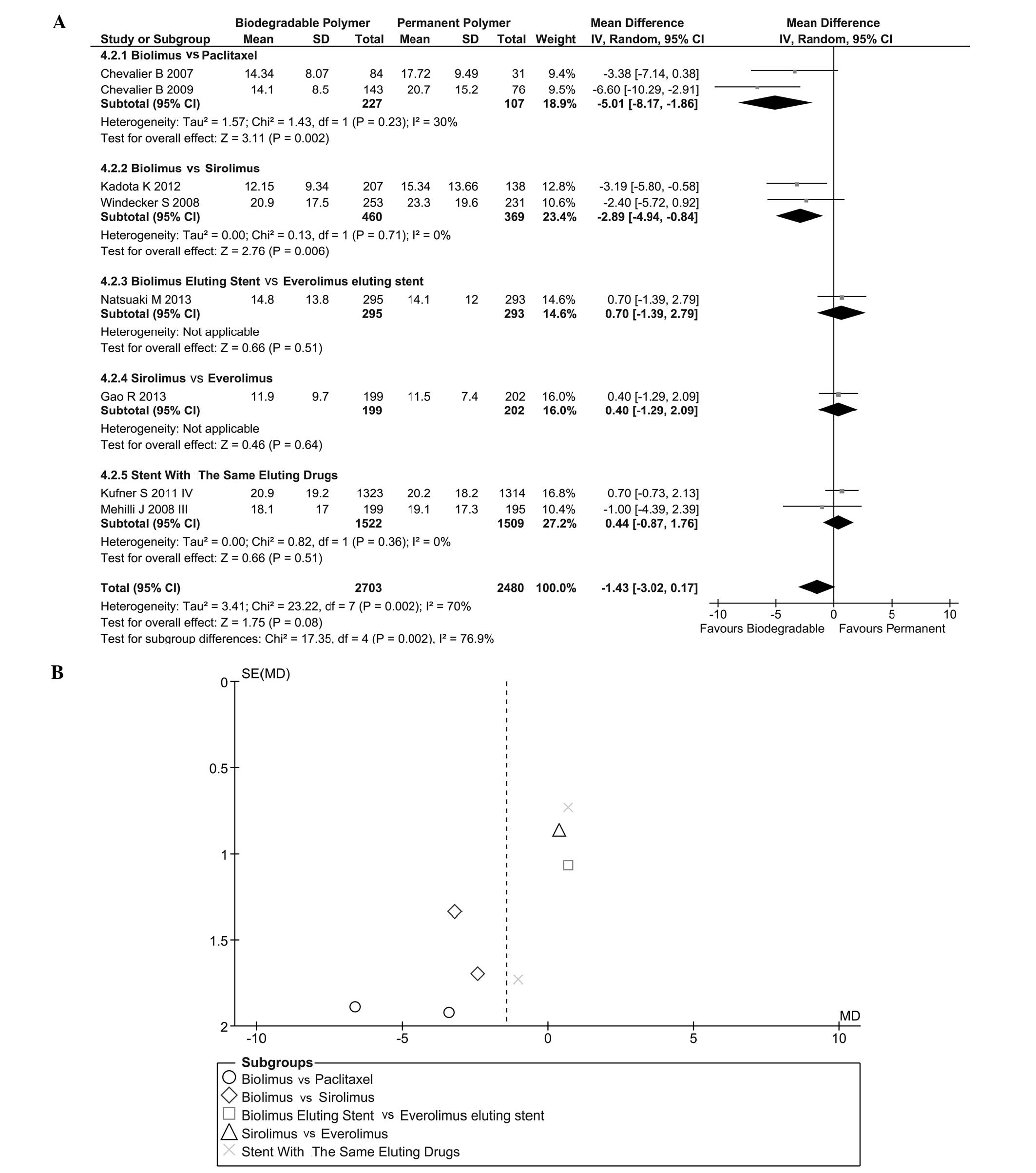
|
1
|
Fischman DL, Leon MB, Baim DS, et al;
Stent Restenosis Study Investigators. A randomized comparison of
coronary-stent placement and balloon angioplasty in the treatment
of coronary artery disease. N Engl J Med. 331:496–501. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palmerini T, Biondi-Zoccai G, Della Riva
D, et al: Stent thrombosis with drug-eluting and bare-metal stents:
evidence from a comprehensive network meta-analysis. Lancet.
379:1393–1402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liistro F and Colombo A: Late acute
thrombosis after paclitaxel eluting stent implantation. Heart.
86:262–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brodie B, Pokharel Y, Fleishman N,
Bensimhon A, Kissling G, et al: Very late stent thrombosis after
primary percutaneous coronary intervention with bare-metal and
drug-eluting stents for ST-segment elevation myocardial infarction:
A 15-year single-center experience. JACC Cardiovasc Interv.
4:30–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vorpahl M, Finn AV, Nakano M and Virmani
R: The bioabsorption process: tissue and cellular mechanisms and
outcomes. EuroIntervention. 5(Suppl F): F28–F35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson GJ, Nakazawa G, Schwartz RS,
Huibregtse B, Poff B, et al: Comparison of inflammatory response
after implantation of sirolimus- and paclitaxel-eluting stents in
porcine coronary arteries. Circulation. 120:141–149. 1–2. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Byrne RA, Joner M and Kastrati A: Polymer
coatings and delayed arterial healing following drug-eluting stent
implantation. Minerva Cardioangiol. 57:567–584. 2009.PubMed/NCBI
|
|
8
|
Lupi A, Rognoni A, Secco GG, Lazzero M,
Nardi F, et al: Biodegradable versus durable polymer drug eluting
stents in coronary artery disease: Insights from a meta-analysis of
5834 patients. Eur J Prev Cardiol. 21:411–424. 2014. View Article : Google Scholar
|
|
9
|
Ahmed TA, Bergheanu SC, Stijnen T, Plevier
JW, Quax PH and Jukema JW: Clinical performance of drug-eluting
stents with biodegradable polymeric coating: A meta-analysis and
systematic review. EuroIntervention. 7:505–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions. The Cochrane
Collaboration; 2009
|
|
11
|
Byrne RA, Kastrati A, Kufner S, Massberg
S, Birkmeier KA, et al; Intracoronary Stenting and Angiographic
Results. Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4)
Trial Investigators: Randomized, non-inferiority trial of three
limus agent-eluting stents with different polymer coatings: the
Intracoronary Stenting and Angiographic Results: Test Efficacy of 3
Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J.
30:2441–2449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Byrne RA, Kastrati A, Massberg S,
Wieczorek A, Laugwitz KL, et al; ISAR-TEST-4 Investigators.
Biodegradable polymer versus permanent polymer drug-eluting stents
and everolimus- versus sirolimus-eluting stents in patients with
coronary artery disease: 3-year outcomes from a randomized clinical
trial. J Am Coll Cardiol. 58:1325–1331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Byrne RA, Kufner S, Tiroch K, Massberg S,
Laugwitz KL, et al; ISAR-TEST-3 Investigators. Randomised trial of
three rapamycin-eluting stents with different coating strategies
for the reduction of coronary restenosis: 2-year follow-up results.
Heart. 95:1489–1494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chevalier B, Serruys PW, Silber S, Garcia
E, Suryapranata H, et al: Randomised comparison of Nobori, biolimus
A9-eluting coronary stent with a Taxus(R), paclitaxel-eluting
coronary stent in patients with stenosis in native coronary
arteries: The Nobori 1 trial. EuroIntervention. 2:426–434.
2007.PubMed/NCBI
|
|
15
|
Chevalier B, Silber S, Park SJ, Garcia E,
Schuler G, et al: Randomized comparison of the Nobori Biolimus
A9-eluting coronary stent with the Taxus Liberté paclitaxel-eluting
coronary stent in patients with stenosis in native coronary
arteries: the NOBORI 1 trial-Phase 2. Circ Cardiovasc Interv.
2:188–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christiansen EH, Jensen LO, Thayssen P,
Tilsted HH, Krusell LR, et al; Scandinavian Organization for
Randomized Trials with Clinical Outcome (SORT OUT) V Investigators.
Biolimus-eluting biodegradable polymer-coated stent versus durable
polymer-coated sirolimus-eluting stent in unselected patients
receiving percutaneous coronary intervention (SORT OUT V): A
randomised non-inferiority trial. Lancet. 381:661–669. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao RL, Xu B, Lansky AJ, Yang YJ, Ma CS,
et al; TARGET I Investigators. A randomised comparison of a novel
abluminal groove-filled biodegradable polymer sirolimus-eluting
stent with a durable polymer everolimus-eluting stent: Clinical and
angiographic follow-up of the TARGET I trial. EuroIntervention.
9:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kadota K, Muramatsu T, Iwabuchi M, Saito
S, Hayashi Y, et al: Randomized comparison of the Nobori biolimus
A9-eluting stent with the sirolimus-eluting stent in patients with
stenosis in native coronary arteries. Catheter Cardiovasc Interv.
80:789–796. 2012. View Article : Google Scholar
|
|
19
|
Krucoff MW, Kereiakes DJ, Petersen JL, et
al; COSTAR II Investigators Group. A novel bioresorbable polymer
paclitaxel-eluting stent for the treatment of single and
multivessel coronary disease: Primary results of the COSTAR (Cobalt
Chromium Stent With Antiproliferative for Restenosis) II study. J
Am Coll Cardiol. 51:1543–1552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kufner S, Massberg S, Dommasch M, Byrne
RA, Tiroch K, et al; Intracoronary Stenting and Angiographic
Results. Test Efficacy of 3 Limus-Eluting Stents Trial
Investigators: Angiographic outcomes with biodegradable polymer and
permanent polymer drug-eluting stents. Catheter Cardiovasc Interv.
78:161–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mehilli J, Byrne RA, Wieczorek A, Iijima
R, Schulz S, et al; Intracoronary Stenting and Angiographic
Restenosis Investigators-Test Efficacy of Rapamycin-Eluting Stents
with Different Polymer Coating Strategies (ISAR-TEST-3). Randomized
trial of three rapamycin-eluting stents with different coating
strategies for the reduction of coronary restenosis. Eur Heart J.
29:1975–1982. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Natsuaki M, Kozuma K, Morimoto T, Kadota
K, Muramatsu T, et al; NEXT Investigators. Biodegradable polymer
biolimus-eluting stent versus durable polymer everolimus-eluting
stent: a randomized, controlled, noninferiority trial. J Am Coll
Cardiol. 62:181–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serruys PW, Farooq V, Kalesan B, de Vries
T, Buszman P, et al: Improved safety and reduction in stent
thrombosis associated with biodegradable polymer-based
biolimus-eluting stents versus durable polymer-based
sirolimus-eluting stents in patients with coronary artery disease:
Final 5-year report of the LEADERS (Limus Eluted From A Durable
Versus ERodable Stent Coating) randomized, noninferiority trial.
JACC Cardiovasc Interv. 6:777–789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smits PC, Hofma S, Togni M, Vázquez N,
Valdés M, et al: Abluminal biodegradable polymer biolimus-eluting
stent versus durable polymer everolimus-eluting stent (COMPARE II):
A randomised, controlled, non-inferiority trial. Lancet.
381:651–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Windecker S, Serruys PW, Wandel S, Buszman
P, Trznadel S, et al: Biolimus-eluting stent with biodegradable
polymer versus sirolimus-eluting stent with durable polymer for
coronary revascularisation (LEADERS): A randomised non-inferiority
trial. Lancet. 372:1163–1173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Shen J, Li Z, Zhu A, Yuan Y, et
al: Two-year clinical outcomes of different drug-eluting stents
with different polymer coating strategies in coronary artery heart
disease: A multi-centre, randomised, controlled clinical trial. Int
J Cardiol. 168:2646–2652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moses JW, Leon MB, Popma JJ, Fitzgerald
PJ, Holmes DR, et al; SIRIUS Investigators. Sirolimus-eluting
stents versus standard stents in patients with stenosis in a native
coronary artery. N Engl J Med. 349:1315–1323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stone GW, Ellis SG, Cannon L, Mann JT,
Greenberg JD, et al; TAXUS V Investigators. Comparison of a
polymer-based paclitaxel-eluting stent with a bare metal stent in
patients with complex coronary artery disease: a randomized
controlled trial. JAMA. 294:1215–1223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakazawa G, Finn AV, Vorpahl M, Ladich ER,
Kolodgie FD and Virmani R: Coronary responses and differential
mechanisms of late stent thrombosis attributed to first-generation
sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol.
57:390–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Waha A, Stefanini GG, King LA, Byrne
RA, Serruys PW, et al: Long-term outcomes of biodegradable polymer
versus durable polymer drug-eluting stents in patients with
diabetes a pooled analysis of individual patient data from 3
randomized trials. Int J Cardiol. 168:5162–5166. 2013. View Article : Google Scholar : PubMed/NCBI
|