Introduction
Over the past few decades a large number of patients
have undergone percutaneous transluminal angioplasty (PTA), but
they have also suffered a high risk of restenosis following PTA
(30–50%) (1), which has been a
serious problem in interventional cardiology. The emergence of
bare-metal stents and subsequent drug-eluting stents (DES) eased
this problem, with the latter, in particular, greatly reducing the
restenosis risk to ~10% (2). With
an increasing number of patients receiving DES and more data
available from long-term follow-up studies, the safety of these
devices has been associated with a rise in the rate of late stent
thrombosis (LST) and very late stent thrombosis (VLST) (3,4).
Numerous animal and human studies have demonstrated that the
hypersensitive reaction to durable polymers on DES may play a major
role in the DES-induced inflammation and delayed vascular healing,
which subsequently causes LST and VLST following intervention
(5,6). Given these problems, biodegradable
polymer drug-eluting stents (BPDES) emerged, which are equipped
with biodegradable polymer drug carriers that degrade at the same
time as the drug is released until they completely disappear and
only the stent remains. This type of DES can, therefore, reduce or
eliminate the stimulatory effect of the polymer on the vessel so as
to, theoretically, reduce the incidence of LST and VLST (7); however, the degradation of
biodegradable polymers may succumb to negative factors, some of
which influence the velocity of degradation, either by accelerating
it or slowing it down (5). To
further study the influence of biodegradable polymers on stent
performance, a large number of controlled clinical studies have
been conducted to observe the clinical efficacy of BPDES. According
to a meta-analysis of 10 trials, BPDES significantly reduced late
lumen loss (LLL) and target vessel revascularization (TVR) but
without clear benefits on mortality, myocardial infarction (MI) and
LST rates when compared with permanent polymer drug eluting stents
(PPDES) at the one-year follow-up (8). Another meta-analysis of 22 clinical
trials did not show that BPDES were better than PPDES regarding the
incidence of definite or probable stent thrombosis (DpST) at one
year following implantation (9);
however, there has been no meta-analysis comparing the clinical
outcomes of BPDES and PPDES at >1 year follow-up. In addition,
the performances of various BPDES with different eluting drugs have
not been fully evaluated; therefore, the present meta-analysis was
conducted to try to rectify these omissions.
Materials and methods
Eligibility criteria and search
strategies
To be included in this meta-analysis, trials were
required to meet the following criteria: i) Randomized clinical
trials (RCTs) comparing a DES with a biodegradable polymer and a
DES with a permanent polymer in patients undergoing percutaneous
coronary intervention; ii) enrolment of >50 patients with
available follow-up data for at least one of the clinical
end-points or angiographic end-points at mid-term (≤9 months)
and/or long-term (≤16 months). PubMed, Web of Science, Medline and
The Cochrane Library were searched between January 2005 and January
2014 for RCTs on BPDES. The PubMed search strategy was formulated
as follows: (‘biodegradable polymer’ OR ‘bioabsorbable’) AND
(‘permanent’ OR ‘durable’) AND ‘clinical trials’. This search
strategy was translated to the corresponding vocabulary of Medline,
Web of Science and Cochrane Central Register of Controlled Trials.
No language restriction was applied and the search was kept updated
until January 2014.
Study selection and risk of bias
To select trials, the following steps were performed
following trial identification by the main search: i) Exclusion of
duplicates; ii) screening and selection of abstracts; iii)
assessment for eligibility through full-text articles; iv) final
inclusion. One author followed steps i) to ii) and another two
authors followed steps iii) to iv) independently. Disagreements
were resolved by discussion.
Three authors independently assessed the risk of
bias with the components recommended by the Cochrane Collaboration:
Random sequence generation; allocation concealment; blinding of
participants and personnel; blinding of outcome assessment;
incomplete outcome data; selective reporting and other sources of
bias (10). Trials with a high or
unclear risk for bias for any one of the first two or the fourth
components were identified as trials with a high risk of bias;
otherwise, they were identified as trials with a low risk of bias.
Trials were excluded if they lacked a clear statistical analysis or
did not adjust for potential confounders.
Data extraction
Two authors independently performed data extraction
on the trials. Any differences found were resolved by discussion.
The information was collected regarding the main clinical
characteristics (first author, year of publication, trial acronym,
event location, type of stent, number of participants and lesions,
age and gender, proportion of patients under the risk factors of
smoking, hypertension and diabetes, proportion with previous MI,
duration of treatment with thienopyridines and the maximum
follow-up period) and angiographic characteristics (location of
target lesion, reference vascular diameter, minimal lumen diameter
and target lesion length). For both biodegradable polymer stent
groups and permanent polymer stent groups, major adverse
cardiovascular events (MACE), including cardiac mortality, MI or
TVR were defined as the primary clinical outcomes. Other clinical
or angiographic outcomes of interest included DpST, LLL in the
stent and stenosis of lumen diameter (SLD) in the stent. In order
to better compare the differences between short- and long-term
follow-up results of BPDES and PPDES, the results are firstly
classified into the one-year follow-up group (with a period of 12
months) and the long-term follow-up group (with a period of >12
months) according to the length of the follow-up period.
Statistical analysis
Statistical analysis was performed using Review
Manager (version 5.2; Cochrane Collaboration, Copenhagen, Denmark).
Summary statistics of dichotomous variables were presented as odds
ratios (OR) and 95% confidence intervals (95% CI). Continuous
variables were calculated as weighted mean difference with 95% CI.
Following data pooling, statistical heterogeneity across trials was
identified and evaluated by Cochrane Q χ2 and
I2 statistics. Trivial heterogeneity was considered for
P-values >0.1 or I2<50%, and a fixed-effect model
would be used. A random-effect model replaced the fixed-effect
model if P<0.1 or I2>50%, which suggested
substantial and significant heterogeneity. The likelihood of
publication bias was assessed graphically by generating a funnel
plot for the primary end-points and angiographic outcomes. Subgroup
analysis was performed based on the eluting drugs. Following the
completion of data analysis, the GRADEPro system (Cochrane
Informatics and Knowledge Management, London, UK) was used for the
scoring of the main analysis results to assess the value of
each.
Results
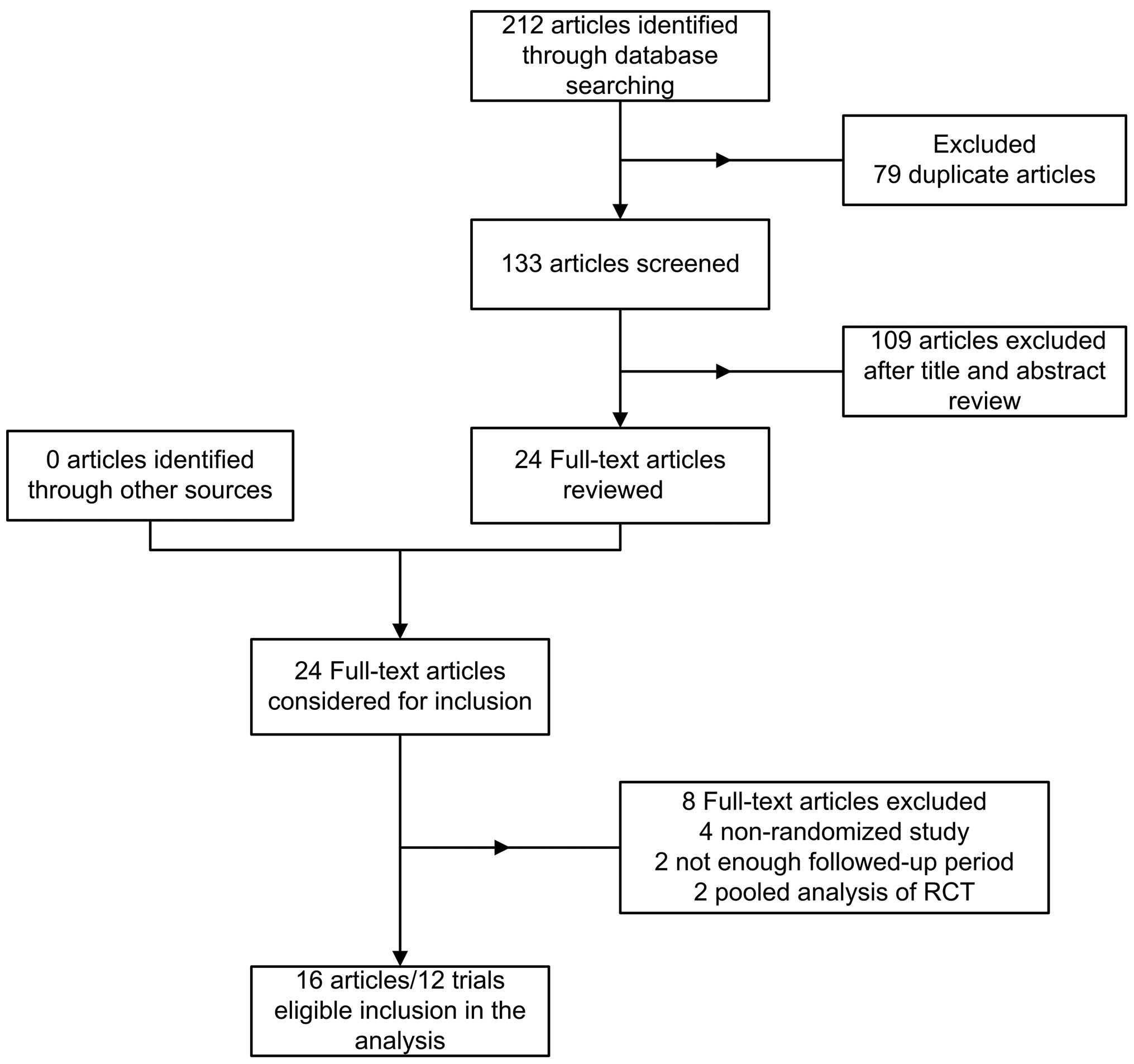
Trials and trial characteristics
Seventy-two papers were identified from PubMed, 69
from Web of Science, 17 from The Cochrane Library and 54 from
Medline. A total of 79 duplicates were excluded leaving 133 studies
identified by the main search. A further 109 papers were excluded
by reading the titles and abstracts so that 24 potentially relevant
papers were identified. Finally, a total of 16 articles concerning
12 RCTs (11–26) with a total of 15,938 patients with
coronary stenosis were included in the current meta-analysis. A
flow chart showing trial selection is shown in Fig. 1. Among these patients, BPDES were
used in 8,643 patients while PPDES were used in 7,295 patients. The
main demographic and clinical characteristics of the included
trials are summarized in Tables I
and II. No significant difference
was identified in the main characteristics of patients between the
biodegradable polymer (BP) and permanent polymer (PP) groups. The
mean age of the participants in individual trials ranged from 58 to
69 years with males representing the majority. The percentage of
diabetic patients was 29.1% among the BP group and 29.6% among the
PP group. The minimum duration of thienopyridine therapy following
stent implantation was variable between these trials; three months
in two trials (18,22), six months in five trials (11,14,15,19–21)
and 12 months in five trials (16,17,24–26).
The maximum follow-up period was from nine to 60 months. Data
extracted from trials with a >16 month follow-up period were
defined as long-term outcomes and were analyzed as an individual
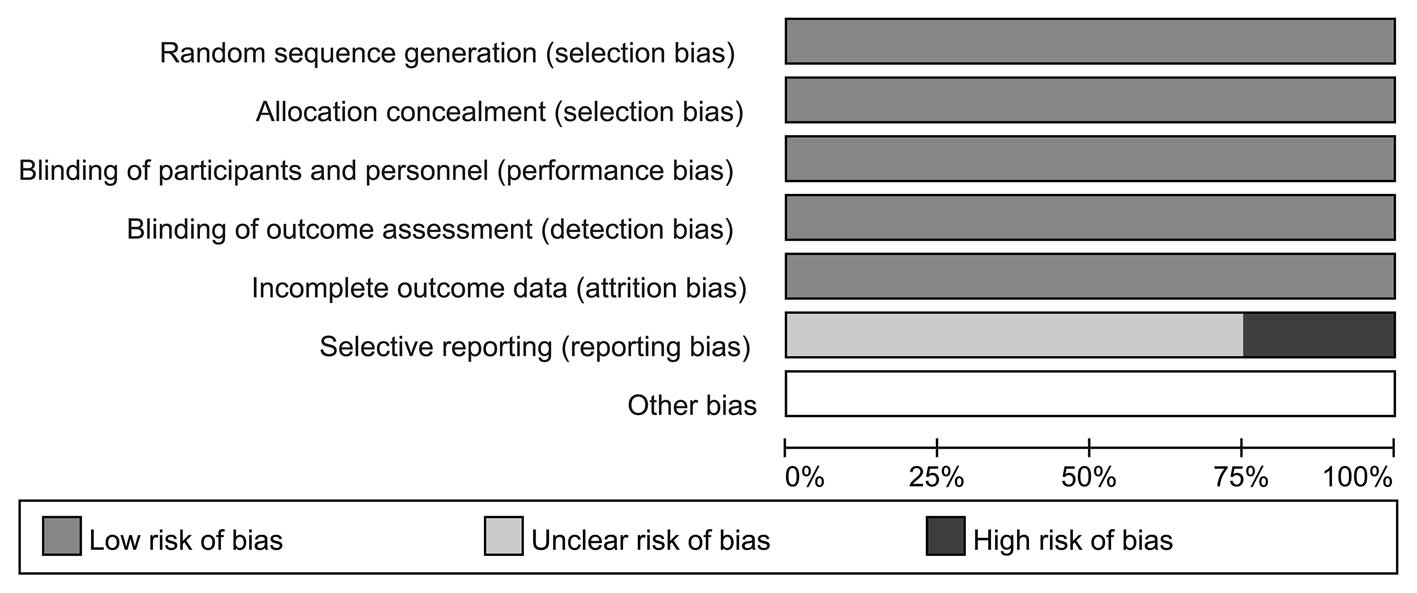
group. The risk of bias for all included studies is shown in
Fig. 2. The random sequence
generation and allocation concealment of RCTs were well described.
It was found that none of the trials blinded participants and
personnel. As blinding of participants and personnel had little
influence on outcome assessment, it was considered an insignificant
and low-risk source of bias. Three trials (19,24,26)
were judged to have a high risk of reporting bias due to the fact
that one of the outcomes of interest in the study was reported
incompletely; thus, it could not be included in this meta-analysis.
Other trials were considered to have an unclear risk of bias on
reporting bias as no clear information was found to judge them
either low risk or high risk.
 | Table IMain clinical characteristics of the
trials. |
Table I
Main clinical characteristics of the
trials.
| | | Stent type | No. of patients
(lesions) | Mean agea, years | Male, % |
|---|
| | |
|
|
|
|
|---|
| First author, year
(ref) | Trial acronym | Event location | BPS | PPS | BPS | PPS | BPS | PPS | BPS | PPS |
|---|
Byrne, 2009 (10)
Byrne, 2011 (11)
Kufner, 2011 (19) | ISAR-TEST IV | Munich,
Germany | Sirolimus | Sirolimus or
everolimus | 1299 (1689) | 1304 (1683) | 66.7±10.7 | 66.8±11.1 | 78.2 | 81.7 |
Byrne, 2009
(12)
Mehilli, 2008 (20) | ISAR-TEST III | Munich,
Germany | Sirolimus | Sirolimus | 202 (239) | 202 (242) | 66.5±11.6 | 65.0±10.7 | 75.3 | 76.8 |
| Chevalier, 2007
(13) | Nobori I | Europe, Asia,
Australia | Biolimus | Paclitaxel | 85 (95) | 35 (42) | 65.0±11.0 | 63.0±11.0 | 69.0 | 66.0 |
| Chevalier, 2009
(14) | Nobori I phase
2 | Europe, Asia,
Australia | Biolimus | Paclitaxel | 153 (174) | 90 (98) | 62.7±10.3 | 63.2±11.2 | 74.5 | 68.9 |
| Christiansen, 2013
(15) | SORT OUT V | Western
Denmark | Biolimus | Sirolimus | 1229 (1532) | 1239 (1555) | 65.0±10.6 | 65.2±10.3 | 74.6 | 75.1 |
| Gao, 2013 (16) | TARGET I | China | Sirolimus | Everolimus | 227 | 231 | 58.7±9.4 | 59.6±9.4 | 69.2 | 68.4 |
| Kadota, 2012
(17) | - | Japan | Biolimus | Sirolimus | 198 (218) | 137 (150) | 67.1±10.3 | 67.7±9.3 | 71.6 | 72.0 |
| Krucoff, 2008
(18) | COSTAR II | USA, Belgium,
Germany, New Zealand | Paclitaxel | Paclitaxel | 989 (1212) | 686 (846) | 63.5±10.8 | 63.7±10.6 | 73.1 | 71.1 |
| Natsuaki, 2013
(21) | NEXT | Japan | Biolimus | Everolimus | 1617 (2059) | 1618 (2010) | 69.1±9.8 | 69.3±9.8 | 77.0 | 77.0 |
| Smits, 2013
(23) | COMPARE II | Europe | Biolimus | Everolimus | 1795 (2638) | 912 (1387) | 63.0±11.1 | 62.7±11.0 | 74.4 | 74.3 |
Windecker, 2008
(24)
Serruys, 2013 (22) | LEADERS | Europe | Biolimus | Sirolimus | 857 (1256) | 850 (1213) | 64.6±10.8 | 64.5±10.7 | 75.0 | 74.6 |
| Zhang, 2013
(25) | - | China | Sirolimus | Sirolimus | 341 | 321 | 67.5±9.8 | 65.9±11.1 | 69.2 | 68.5 |
 | Table IIMain angiographic baseline
characteristics of the included trials. |
Table II
Main angiographic baseline
characteristics of the included trials.
| LAD, n (%) | LCX, n (%) | RCA, n (%) | RDa, mm | MLDa, mm | Lesion
lengtha, mm |
|---|
|
|
|
|
|
|
|
|---|
| First author, year
(ref) | BPS | PPS | BPS | PPS | BPS | PPS | BPS | PPS | BPS | PPS | BPS | PPS |
|---|
Byrne, 2009
(10)
Byrne, 2011 (11)
Kufner, 2011 (19) | 753 (44.7) | 748 (44.3) | 454 (27.0) | 453 (26.8) | 476 (28.3) | 488 (28.9) | 2.79±0.47 | 2.80±0.52 | 0.98±0.50 | 0.98±0.51 | 14.8±8.6 | 15.0±8.8 |
Byrne, 2009
(12)
Mehilli, 2008 (20) | 110 (46.0) | 104 (43.0) | 53 (22.2) | 69 (28.5) | 76 (31.8) | 69 (28.5) | 2.74±0.52 | 2.75±0.51 | 1.06±0.42 | 1.13±0.49 | 13.9±7.2 | 14.6±7.0 |
| Chevalier, 2007
(13) | 52 (54.7) | 28 (54.8) | 21 (22.1) | 9 (19.0) | 22 (23.2) | 13 (26.2) | 2.70±0.44 | 2.71±0.52 | 1.06±0.24 | 1.12±0.38 | 11.35±4.51 | 11.03±4.75 |
| Chevalier, 2009
(14) | 62 (35.6) | 46 (46.9) | 41 (23.6) | 19 (19.4) | 71 (40.8) | 33 (33.7) | NA | NA | NA | NA | NA | NA |
| Christiansen, 2013
(15) | 623 (40.7) | 636 (40.9) | 355 (23.2) | 350 (22.5) | 508 (33.2) | 535 (34.4) | 3.2±0.34 | 3.3±0.33 | NA | NA | 18.0±3.75 | 18.00±4.50 |
| Gao, 2013 (16) | 147 (64.8) | 139 (60.2) | 39 (17.2) | 42 (18.2) | 41 (18.1) | 50 (21.6) | 2.87±0.47 | 2.90±0.50 | 0.96±0.40 | 0.95±0.42 | 15.7±7.1 | 15.7±6.7 |
| Kadota, 2012
(17) | 83 (39.9) | 62 (44.7) | 56 (27.1) | 33 (24.0) | 68 (33.0) | 43 (31.3) | 2.68±0.57 | 2.68±0.54 | NA | NA | 12.64±5.52 | 12.82±6.81 |
| Krucoff, 2008
(18) | 528 (39.9) | 529 (40.3) | 369 (27.9) | 402 (30.6) | 426 (32.2) | 383 (29.2) | 2.77±0.47 | 2.75±0.48 | 0.86±0.40 | 0.89±0.41 | 15.4±6.5 | 15.1±6.5 |
| Natsuaki, 2013
(21) | 795 (49.0) | 774 (48.0) | 405 (25.0) | 435 (27.0) | 552 (34.0) | 517 (32.0) | 2.62±0.60 | 2.61±0.57 | 0.77±0.44 | 0.75±0.42 | 19.5±12.8 | 19.3±13.1 |
| Smits, 2013
(23) | 1078 (40.9) | 550 (39.7) | 602 (22.8) | 356 (25.7) | 882 (33.4) | 448 (32.3) | 2.9±0.5 | 2.9±0.5 | NA | NA | 16.8±9.8 | 17.7±10.6 |
Windecker, 2008
(24)
Serruys, 2013 (22) | 467 (37.2) | 482 (39.7) | 352 (28.0) | 286 (23.6) | 386 (30.7) | 399 (32.9) | 2.60±0.61 | 2.60±0.57 | 0.91±0.50 | 0.95±0.52 | 12.7±8.1 | 12.4±8.5 |
| Zhang, 2013
(25) | 216 (48.4) | 217 (53.0) | 90 (20.2) | 65 (20.3) | 140 (31.4) | 129 (31.5) | NA | NA | NA | NA | 29.2±16.6 | 24.8±14.5 |
Clinical outcomes at
one-year-follow-up
MACE
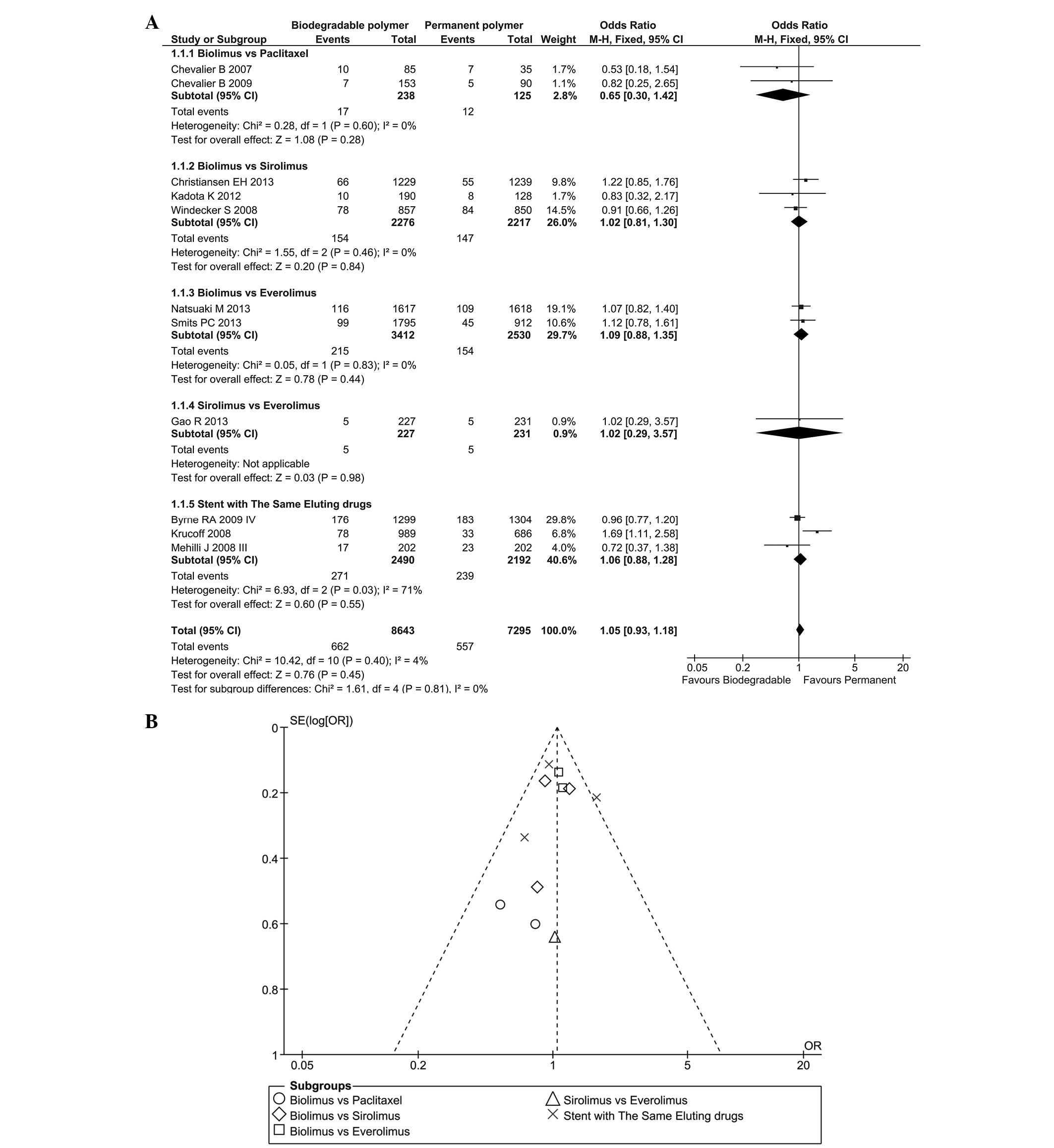
MACE data were acquired from 11 RCTs between nine
and 16 months following the stent installation. As there was no
significant heterogeneity (P=0.40, I2=4%), a fixed
effect model was used (total OR=1.05, 95% CI=0.93–1.18, P=0.45;
Fig. 3A) and the results revealed
that the incidence rate of MACE was similar in the BP and PP groups
at the one-year follow-up. This outcome was observed in each
subgroup, and the difference between subgroups was low
(I2=0%). A funnel plot was used to assess the likelihood
of publication bias, as presented in Fig. 3B.
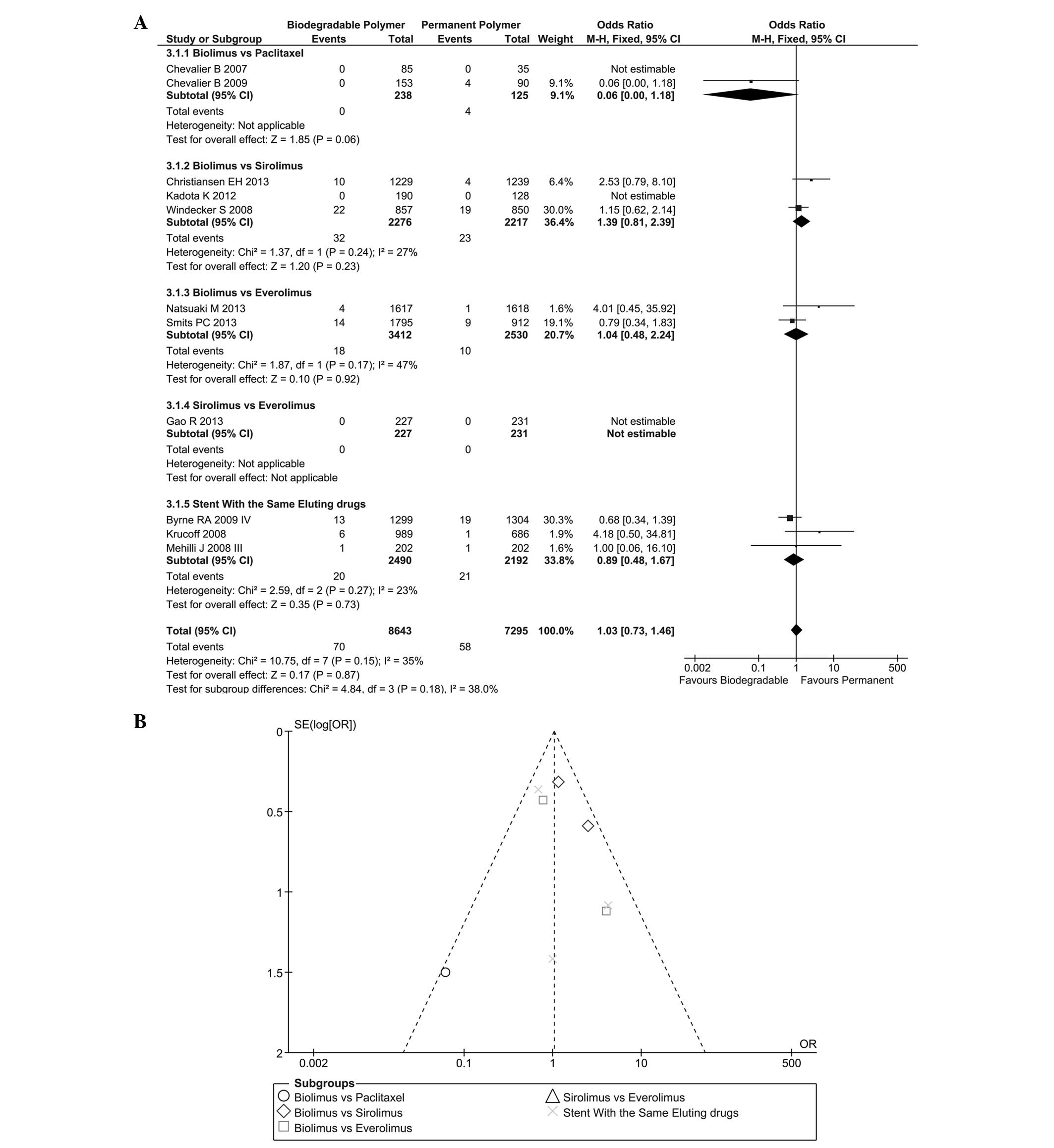
DpST
DpST at the one-year time-point is reported in
Fig. 4A. No significant
heterogeneity was found (I2=35%; P=0.15) among these
trials so a fixed effect model was selected. No significant
difference was found between these two groups for DpST (OR=1.03,
95%CI=0.73–1.46, P=0.87). The subgroup analysis of different
eluting drugs showed comparable results and inter-group
heterogeneity was low (I2=38%). The likelihood of
publication bias is shown in Fig.
4B.
Clinical outcomes at
long-term-follow-up
MACE
MACE data were acquired from four RCTs regarding
long-term follow-up (Fig. 5). No
heterogeneity was found (P=0.90, I2=0%) among these
trials so a fixed effect model was selected (total OR=0.89, 95%
CI=0.78–1.02, P=0.09). Results showed no significant difference
between these two groups.
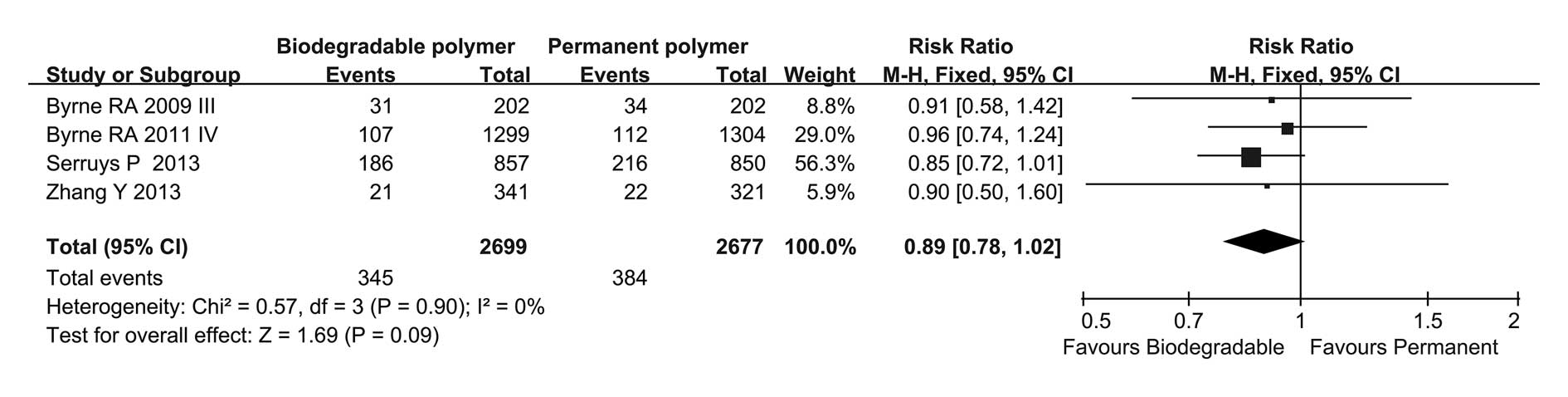
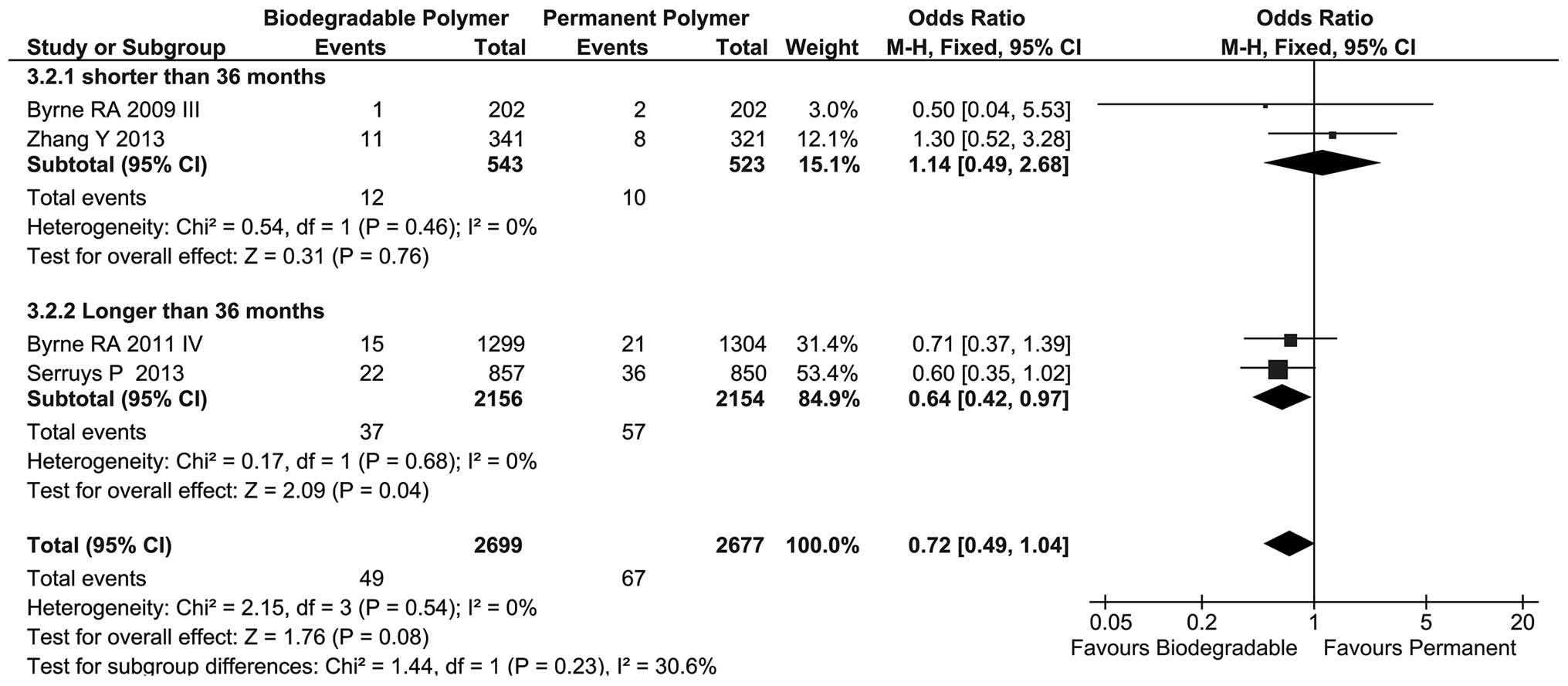
DpST
Regarding long-term follow-up, the incidence of DpST
in these two groups was similar but the BP group showed a tendency
to reduced DpST compared with that in the PP group (OR=0.72, 95%
CI=0.49–1.04, P=0.08; Fig. 6) and
no heterogeneity was found among these four trials
(I2=0%). Subgroup analysis showed a statistically
significant difference between the BP and PP groups at >36
months follow-up (OR=0.64, 95% CI=0.42–0.97, P=0.04).
Angiographic outcomes
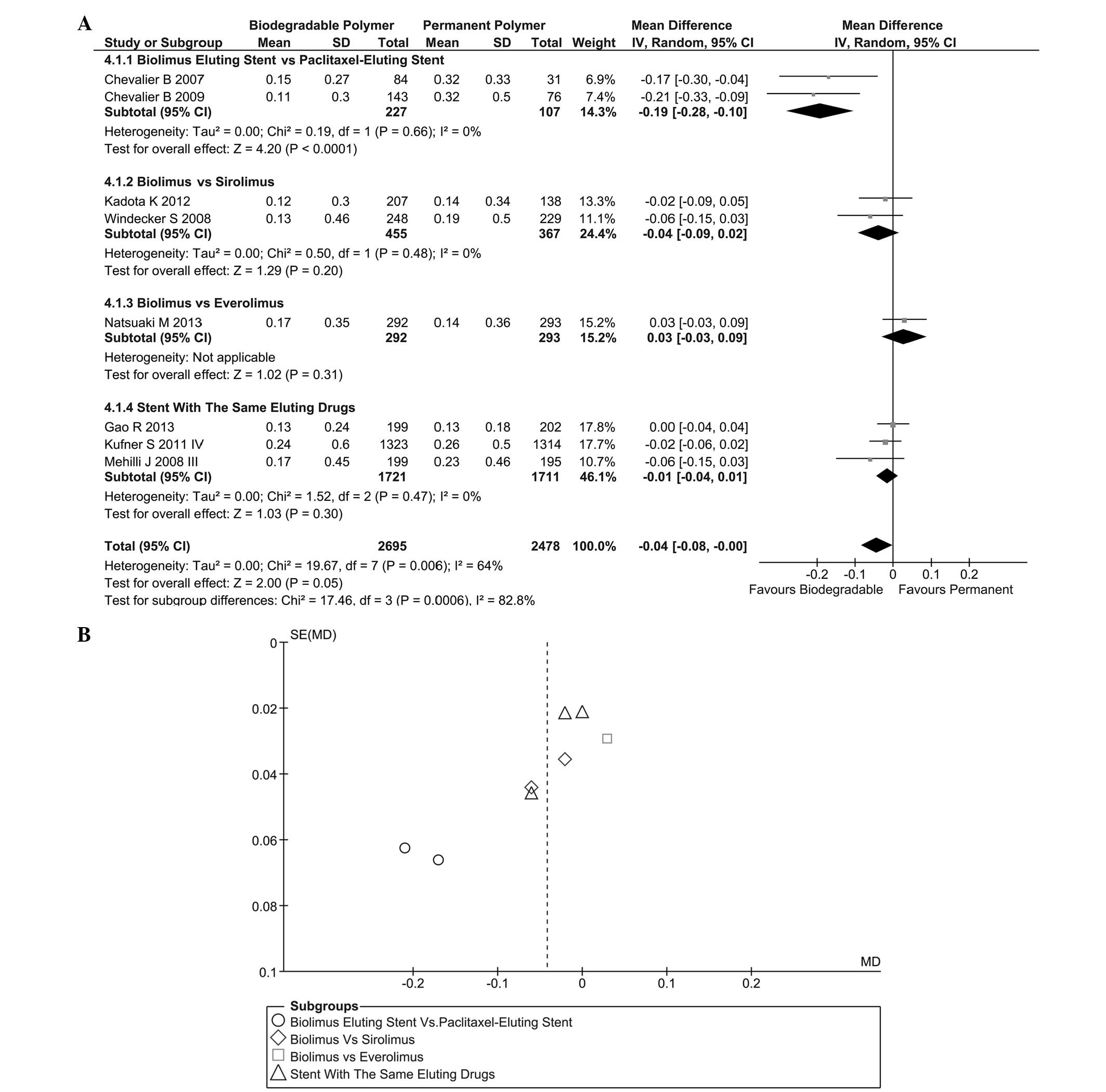
LLL in stent
Regarding the 6–12-month follow-up, results of LLL
in stent were acquired in eight trials, as shown in Fig. 7A. Heterogeneity was found
(I2=64%, P=0.006) among these trials and a randomized
effect model was selected. The results [instrumental variable
(IV)=−0.04, 95% CI=−0.08–0.00, P=0.05] indicated that the
difference between the BP and PP groups was considered to be
statistically significant. Subgroup analysis showed that the
biolimus-eluting stent (BES) was superior to the paclitaxel-eluting
stent (PES) (IV=−0.19, 95% CI=−0.28 to −0.10, P<0.001). A funnel
plot of this result is shown in Fig.
7B.
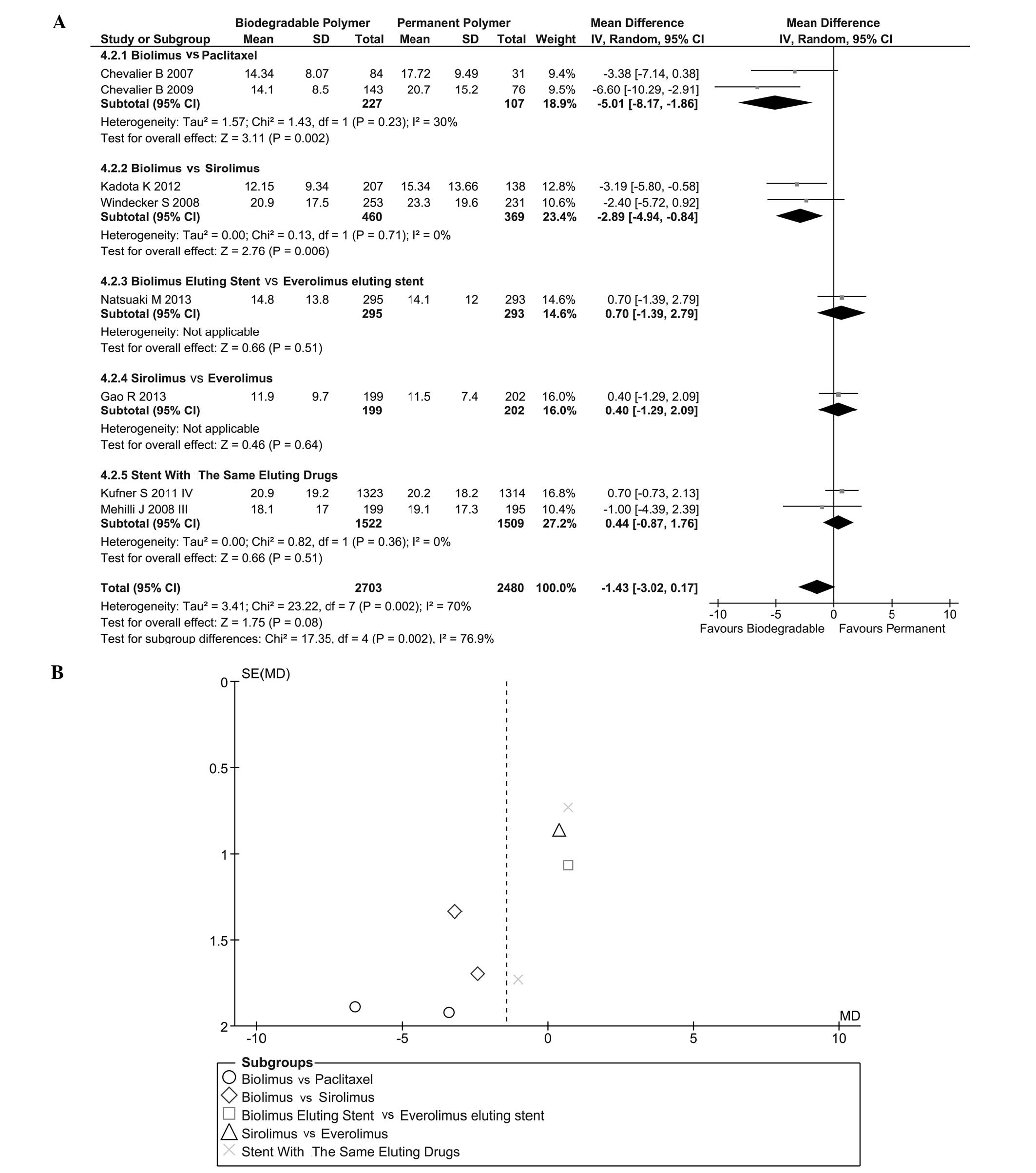
Stenosis of lumen diameter (SLD)
SLD (in stent) is shown in Fig. 8A. Heterogeneity was found
(I2=70%, P=0.002) and a randomized effect model was used
(IV=−1.43, 95% CI=−30.2–0.17, P=0.08). Subgroup analysis indicated
that BES could effectively decrease the severity of SLD in stent
compared with PES (IV=−5.01, 95% CI=−8.17 to −1.86, P<0.01) and
sirolimus-eluting stent (SES) (IV=−2.89, 95% CI=−4.94 to −0.84,
P<0.01). This result indicated that a BES was more effective in
reducing SLD in stent than other drug-eluting stents. Heterogeneity
between these groups was significant (I2=94.6%). The
likelihood of publication bias was assessed by funnel plot
(Fig. 8B).
Evaluation of results
Following analysis of the data from RCTs by Review
Manager, the GRADEPro system was used to evaluate the results. In
conclusion, the quality of each result concerning long-term follow
up was high. Details of the evaluation are shown in Table III.
 | Table IIIResults evaluated by the GRADE
system. |
Table III
Results evaluated by the GRADE
system.
| A, Cardiac events,
target lesion revascularization and thromobosis |
|---|
|
|---|
| Outcomes | Comparative
risksa (95% CI) | Relative effect
(95% CI) | No. of participants
(no. of studies) | Quality of evidence
(GRADE) |
|---|
|
|---|
| Assumed risk PPS
per 1000 | Corresponding risk
BPS per 1000 |
|---|
| Major adverse
cardiac events at one-year follow-upa | | | OR 1.05
(0.93–1.18) | 15938 (11) | ++++ (high) |
| Study
population | 76 | 80 (71–89) | | | |
| Moderate | 63 | 66 (58–74) | | | |
| Target lesion
revascularization at one-year follow-upb | | | OR 0.98
(0.84–1.15) | 14263 (10) | +++−
(moderate) |
| Study
population | 47 | 46 (39–53) | | | |
| Moderate | 40 | 39 (34–46) | | | |
| Definite or
portable stent thrombosis at one-year follow-upb | | | OR 1.03
(0.73–1.46) | 15818 (10) | +++−
(moderate) |
| Study
population | 8 | 8 (6–12) | | | |
| Moderate | 3 | 3 (2–4) | | | |
| Major adverse
cardiac events at long term follow-upc | | | OR 0.89
(0.78–1.02) | 5376 (4) | ++++ (high) |
| Study
population | 143 | 128 (112–146) | | | |
| Moderate | 127 | 113 (99–130) | | | |
| Target lesion
revascularization at long term follow-upd | | | OR 0.92
(0.78–1.07) | 5376 (4) | ++++ (high) |
| Study
population | 110 | 101 | (86–118) | | |
| Moderate | 107 | 98 | (83–114) | | |
| Definite stent or
portable thrombosis at long term follow-upc | | | OR 0.72
(0.51–1.04) | 5396 (4) | ++++ (high) |
| Study
population | 27 | 20 (14–28) | | | |
| Moderate | 17 | 12 (9–18) | | | |
|
| B, Late lumen loss
and stenosis |
|
| Outcomes | Absolute difference
in outcome | No. of
participants | Quality of evidence
(GRADE) |
|
| Late lumen loss in
stent (mm)e | Mean late lumen
loss in stent in the intervention groups was 0.04 (0.08–0.00)
lower | 5173 (8) | ++−− (low) |
| Stenosis of lumen
diameter in stent (%)e | Mean stenosis of
lumen diameter in stent in the intervention groups was 1.43 lower
(3.02 lower to 0.17 higher) | 5183 (8) | ++−− (low) |
Discussion
DES are a major breakthrough in the field of PTA,
since they have markedly reduced the rate of acute stent thrombosis
and the requirement for repeated revascularization procedures
compared with bare-metal stents (27,28).
As the use of DES for artery stenosis has increased, an increasing
amount of attention has been paid to the potential inflammatory
response, which occurs due to the polymers used for the delivery of
the anti-restenotic agents (7).
BPDES were designed to solve this problem. There have been clinical
trials evaluating the safety and efficacy of BPDES but the present
study, to the best of our knowledge, is the first meta-analysis to
compare the clinical performances of BPDES and PPDES in patients
with coronary stenosis at short- and long-term follow-up
periods.
In this analysis, the RCTs that were considered
included numerous countries and regions; therefore, they covered
the different races of the world. The analyzed BPDES group
contained three types of stents with different eluting drugs:
Sirolimus (11,17,21,26),
biolimus (14–16,18,22,24,25)
and paclitaxel (19). The PPDES
group was also equipped with three different drugs: Everolimus
(17,22,24),
paclitaxel (14,15,19)
and sirolimus (11,16,18,21,25,26).
The analysis of clinical events at the one-year
follow-up showed that the incidence of clinical events due to BPDES
was not significantly different from that due to PPDES. More
accurately, the BPDES were noninferior to PPDES in safety profile
one year following DES implantation. In the present study, the
incidence of various clinical events in the analyzed RCTs was
maintained at a low level, which provided evidence for the safety
of modern stents.
Nine RCTs reported angiographic outcomes. It was
found that the degree of LLL in patients receiving BPDES was
significantly lower than that in patients receiving PPDES. The
Nobori I (14) and Nobori I phase
2 trials (16) reported an
advantage in using BPDES, while other trials did not identify any
significant difference between BPDES and PPDES. In addition to LLL,
the analysis of the SLD did not show any significant difference in
the degree of SLD overall but demonstrated that the BES was
superior to the PES (P=0.002) and the SES (P=0.006). Coupled with
the conclusion from previous clinical events (29), BPDES did not significantly alter
the reliability following stent implantation with the application
of biodegradable polymer compared with PPDES. In the analysis of
angiographic outcomes, high heterogeneity was found in these
trials. This phenomenon prompted caution regarding the angiographic
conclusion.
BPDES were proposed to improve the long-term safety
of DES as they was designed to reduce the incidence of LST and
VLST. Recently, a long-term outcome of a pooled analysis of BPDES
versus PPDES in patients with diabetes from three RCTs showed that
BPDES were associated with comparable overall clinical outcomes at
a four-year follow-up, and rates of DpST were significantly lower
with BPDES (30). In the present
meta-analysis, the incidence of MACE and DpST was not found to be
significantly different between BPDES and PPDES on long-term
follow-up. By comparing the analysis data between long- and
one-year follow-up results, the coronary stenosis patients with
BPDES implantation had a tendency towards a lower incidence of
clinical events, particularly DpST (P=0.05). In the RCTs with a
>12 month follow-up period, two trials (13,26)
had a follow-up period of ≤24 months. In the subgroup analysis on
the incidence of DpST, as expected, >36 months following stent
implantation, BPDES showed a beneficial effect on the reduction of
DpST episodes compared with the use of PPDES. This provided
evidence for the correct basic mechanism used in BPDES design: A
reduction in the time the polymer is in contact with tissue reduced
the incidence of DpST. In addition, this result suggested that
re-endothelialization was important for reducing the risk of DpST.
As mentioned previously, however, in the included RCTs, only four
RCTs had a follow-up period >24 months. In these four RCTs, only
two RCTs had a follow-up period >36 months; therefore the
conclusion based on trials with a long-term follow up requires
further research for confirmation.
The main deficiencies in the present study are as
follows: Firstly, the inclusion criteria did not specifically
subdivide the lesion types of the patients in detail; therefore,
the study lacked specific targets. As a result, the type of
coronary stenosis the BPDES were more suitable for could not be
determined; thus, it was not possible to provide a specific
recommendation for clinical practice. Secondly, only four RCTs had
a follow-up period >12 months and only two out of four RCTs had
a follow-up period >36 months. The limited data did not make the
analysis very reliable; the conclusion may be regarded as a
reference with further research required to confirm the
results.
In conclusion, the results of the present
meta-analysis suggest that BPDES were noninferior to PPDES in
short-term results but superior to PPDES in long-term results.
According to the clinical outcomes and angiographic outcomes at
short-term follow-up, BPDES and PPDES exhibited no significant
differences overall but the BES was superior to the PES. Subgroup
analysis, however, demonstrated that the BES with a biodegradable
polymer was superior to the PES with a permanent polymer. In this
first meta-analysis comparing the data from long-term follow-up,
the BPDES were found to exhibit an increased safety profile over
time, particularly for incidences of LST and VLST in which the
BPDES were superior to PPDES. This was consistent with the original
purpose of BPDES design. Additional prolonged follow-up data,
however, is required for an adequate comparison of the safety and
efficacy between BPDES and PPDES to be conducted, in order to
provide adequate and strong evidence for the selection of stents in
clinical practice.
References
|
1
|
Fischman DL, Leon MB, Baim DS, et al;
Stent Restenosis Study Investigators. A randomized comparison of
coronary-stent placement and balloon angioplasty in the treatment
of coronary artery disease. N Engl J Med. 331:496–501. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palmerini T, Biondi-Zoccai G, Della Riva
D, et al: Stent thrombosis with drug-eluting and bare-metal stents:
evidence from a comprehensive network meta-analysis. Lancet.
379:1393–1402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liistro F and Colombo A: Late acute
thrombosis after paclitaxel eluting stent implantation. Heart.
86:262–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brodie B, Pokharel Y, Fleishman N,
Bensimhon A, Kissling G, et al: Very late stent thrombosis after
primary percutaneous coronary intervention with bare-metal and
drug-eluting stents for ST-segment elevation myocardial infarction:
A 15-year single-center experience. JACC Cardiovasc Interv.
4:30–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vorpahl M, Finn AV, Nakano M and Virmani
R: The bioabsorption process: tissue and cellular mechanisms and
outcomes. EuroIntervention. 5(Suppl F): F28–F35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson GJ, Nakazawa G, Schwartz RS,
Huibregtse B, Poff B, et al: Comparison of inflammatory response
after implantation of sirolimus- and paclitaxel-eluting stents in
porcine coronary arteries. Circulation. 120:141–149. 1–2. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Byrne RA, Joner M and Kastrati A: Polymer
coatings and delayed arterial healing following drug-eluting stent
implantation. Minerva Cardioangiol. 57:567–584. 2009.PubMed/NCBI
|
|
8
|
Lupi A, Rognoni A, Secco GG, Lazzero M,
Nardi F, et al: Biodegradable versus durable polymer drug eluting
stents in coronary artery disease: Insights from a meta-analysis of
5834 patients. Eur J Prev Cardiol. 21:411–424. 2014. View Article : Google Scholar
|
|
9
|
Ahmed TA, Bergheanu SC, Stijnen T, Plevier
JW, Quax PH and Jukema JW: Clinical performance of drug-eluting
stents with biodegradable polymeric coating: A meta-analysis and
systematic review. EuroIntervention. 7:505–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions. The Cochrane
Collaboration; 2009
|
|
11
|
Byrne RA, Kastrati A, Kufner S, Massberg
S, Birkmeier KA, et al; Intracoronary Stenting and Angiographic
Results. Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4)
Trial Investigators: Randomized, non-inferiority trial of three
limus agent-eluting stents with different polymer coatings: the
Intracoronary Stenting and Angiographic Results: Test Efficacy of 3
Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J.
30:2441–2449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Byrne RA, Kastrati A, Massberg S,
Wieczorek A, Laugwitz KL, et al; ISAR-TEST-4 Investigators.
Biodegradable polymer versus permanent polymer drug-eluting stents
and everolimus- versus sirolimus-eluting stents in patients with
coronary artery disease: 3-year outcomes from a randomized clinical
trial. J Am Coll Cardiol. 58:1325–1331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Byrne RA, Kufner S, Tiroch K, Massberg S,
Laugwitz KL, et al; ISAR-TEST-3 Investigators. Randomised trial of
three rapamycin-eluting stents with different coating strategies
for the reduction of coronary restenosis: 2-year follow-up results.
Heart. 95:1489–1494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chevalier B, Serruys PW, Silber S, Garcia
E, Suryapranata H, et al: Randomised comparison of Nobori, biolimus
A9-eluting coronary stent with a Taxus(R), paclitaxel-eluting
coronary stent in patients with stenosis in native coronary
arteries: The Nobori 1 trial. EuroIntervention. 2:426–434.
2007.PubMed/NCBI
|
|
15
|
Chevalier B, Silber S, Park SJ, Garcia E,
Schuler G, et al: Randomized comparison of the Nobori Biolimus
A9-eluting coronary stent with the Taxus Liberté paclitaxel-eluting
coronary stent in patients with stenosis in native coronary
arteries: the NOBORI 1 trial-Phase 2. Circ Cardiovasc Interv.
2:188–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christiansen EH, Jensen LO, Thayssen P,
Tilsted HH, Krusell LR, et al; Scandinavian Organization for
Randomized Trials with Clinical Outcome (SORT OUT) V Investigators.
Biolimus-eluting biodegradable polymer-coated stent versus durable
polymer-coated sirolimus-eluting stent in unselected patients
receiving percutaneous coronary intervention (SORT OUT V): A
randomised non-inferiority trial. Lancet. 381:661–669. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao RL, Xu B, Lansky AJ, Yang YJ, Ma CS,
et al; TARGET I Investigators. A randomised comparison of a novel
abluminal groove-filled biodegradable polymer sirolimus-eluting
stent with a durable polymer everolimus-eluting stent: Clinical and
angiographic follow-up of the TARGET I trial. EuroIntervention.
9:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kadota K, Muramatsu T, Iwabuchi M, Saito
S, Hayashi Y, et al: Randomized comparison of the Nobori biolimus
A9-eluting stent with the sirolimus-eluting stent in patients with
stenosis in native coronary arteries. Catheter Cardiovasc Interv.
80:789–796. 2012. View Article : Google Scholar
|
|
19
|
Krucoff MW, Kereiakes DJ, Petersen JL, et
al; COSTAR II Investigators Group. A novel bioresorbable polymer
paclitaxel-eluting stent for the treatment of single and
multivessel coronary disease: Primary results of the COSTAR (Cobalt
Chromium Stent With Antiproliferative for Restenosis) II study. J
Am Coll Cardiol. 51:1543–1552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kufner S, Massberg S, Dommasch M, Byrne
RA, Tiroch K, et al; Intracoronary Stenting and Angiographic
Results. Test Efficacy of 3 Limus-Eluting Stents Trial
Investigators: Angiographic outcomes with biodegradable polymer and
permanent polymer drug-eluting stents. Catheter Cardiovasc Interv.
78:161–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mehilli J, Byrne RA, Wieczorek A, Iijima
R, Schulz S, et al; Intracoronary Stenting and Angiographic
Restenosis Investigators-Test Efficacy of Rapamycin-Eluting Stents
with Different Polymer Coating Strategies (ISAR-TEST-3). Randomized
trial of three rapamycin-eluting stents with different coating
strategies for the reduction of coronary restenosis. Eur Heart J.
29:1975–1982. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Natsuaki M, Kozuma K, Morimoto T, Kadota
K, Muramatsu T, et al; NEXT Investigators. Biodegradable polymer
biolimus-eluting stent versus durable polymer everolimus-eluting
stent: a randomized, controlled, noninferiority trial. J Am Coll
Cardiol. 62:181–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serruys PW, Farooq V, Kalesan B, de Vries
T, Buszman P, et al: Improved safety and reduction in stent
thrombosis associated with biodegradable polymer-based
biolimus-eluting stents versus durable polymer-based
sirolimus-eluting stents in patients with coronary artery disease:
Final 5-year report of the LEADERS (Limus Eluted From A Durable
Versus ERodable Stent Coating) randomized, noninferiority trial.
JACC Cardiovasc Interv. 6:777–789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smits PC, Hofma S, Togni M, Vázquez N,
Valdés M, et al: Abluminal biodegradable polymer biolimus-eluting
stent versus durable polymer everolimus-eluting stent (COMPARE II):
A randomised, controlled, non-inferiority trial. Lancet.
381:651–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Windecker S, Serruys PW, Wandel S, Buszman
P, Trznadel S, et al: Biolimus-eluting stent with biodegradable
polymer versus sirolimus-eluting stent with durable polymer for
coronary revascularisation (LEADERS): A randomised non-inferiority
trial. Lancet. 372:1163–1173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Shen J, Li Z, Zhu A, Yuan Y, et
al: Two-year clinical outcomes of different drug-eluting stents
with different polymer coating strategies in coronary artery heart
disease: A multi-centre, randomised, controlled clinical trial. Int
J Cardiol. 168:2646–2652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moses JW, Leon MB, Popma JJ, Fitzgerald
PJ, Holmes DR, et al; SIRIUS Investigators. Sirolimus-eluting
stents versus standard stents in patients with stenosis in a native
coronary artery. N Engl J Med. 349:1315–1323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stone GW, Ellis SG, Cannon L, Mann JT,
Greenberg JD, et al; TAXUS V Investigators. Comparison of a
polymer-based paclitaxel-eluting stent with a bare metal stent in
patients with complex coronary artery disease: a randomized
controlled trial. JAMA. 294:1215–1223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakazawa G, Finn AV, Vorpahl M, Ladich ER,
Kolodgie FD and Virmani R: Coronary responses and differential
mechanisms of late stent thrombosis attributed to first-generation
sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol.
57:390–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Waha A, Stefanini GG, King LA, Byrne
RA, Serruys PW, et al: Long-term outcomes of biodegradable polymer
versus durable polymer drug-eluting stents in patients with
diabetes a pooled analysis of individual patient data from 3
randomized trials. Int J Cardiol. 168:5162–5166. 2013. View Article : Google Scholar : PubMed/NCBI
|