Introduction
Magnetic resonance imaging (MRI) is a useful medical
imaging technique that utilizes the properties of nuclear magnetic
resonance (NMR) to image the nuclei of atoms inside the body in
detail and depth (1,2). With high resolution and good safety,
MRI is widely used as an efficient information source in medical
diagnosis of the whole body. Fluorescence imaging (FI) provides
better sensitivity than MRI, but cannot show tissues at different
levels. The combination of MRI and FI, as a dual imaging
application, should provide a balance between iconography and
histology. In addition, diagnosis and treatment are likely to be
more accurate and sensitive with an approach where tumors are
positioned with MRI and completely removed under the guidance of
FI.
The ability of contrast agents to alter the
relaxivity of the protons of water molecules that are coordinated
to tissue is the key of MRI (1). The
trivalent gadolinium ion (Gd3+) has seven unpaired
electrons in its outer electron shell, which makes it highly
paramagnetic with a short spin-lattice relaxation time
(longitudinal relaxation time, T1), and complexes of
Gd3+ have been widely used as MRI contrast agents
(3). Gd3+ has a similar
size to Ca2+ and so the former tends to interfere in the
metabolism of Ca2+ in the human body. Therefore, ligands
are necessary to reduce the toxicity. Functional groups on the
ligands can also be designed to bond with nanoparticles (NPs), in
order to extend the rotational correlation time and enhance
relaxivity. Ligands that are frequently used include diethylene
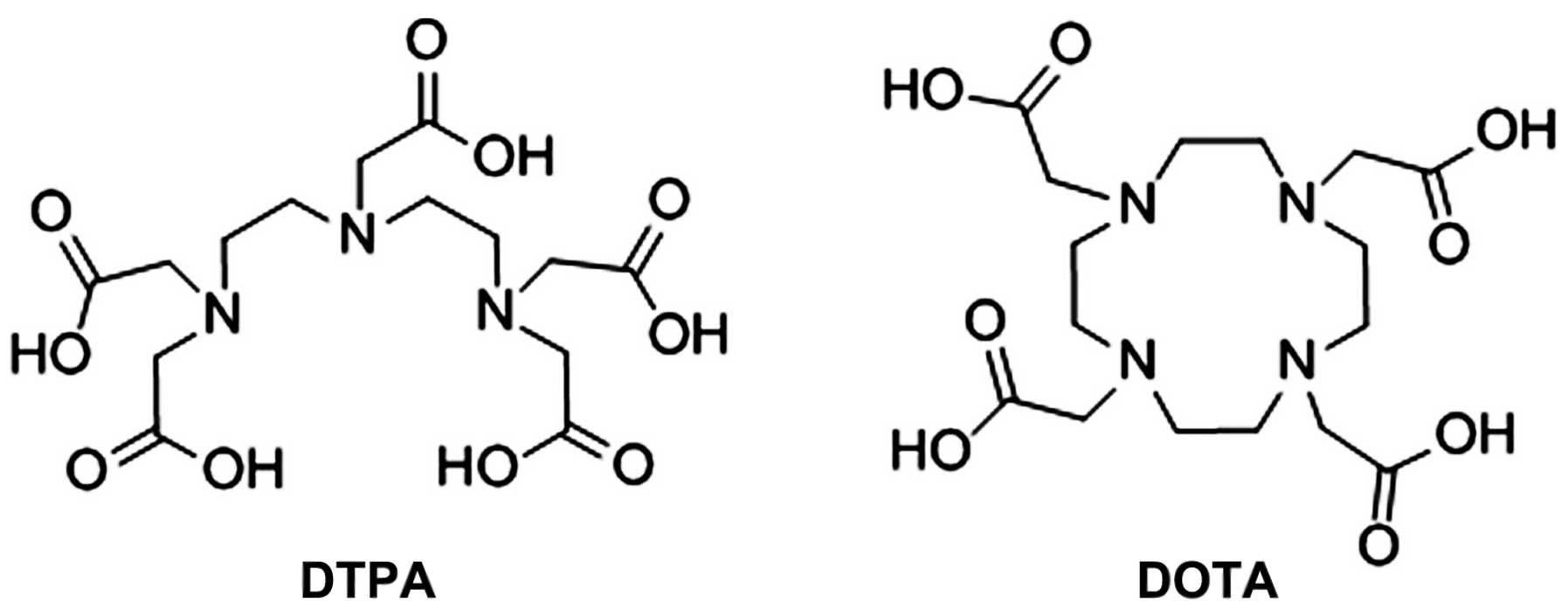
triamine pentacetic acid (DTPA),
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and
their derivatives (Fig. 1) (3,4).
Europium (Eu) is also lanthanide element, which is
adjacent to gadolinium in the periodic table, and the application
of its ions in MRI has also been the object of attention.
Eu3+ has six unpaired electrons in the outer electron
shell, which is indicative of good paramagnetism. It is
photoluminescent, can emit strong fluorescence (585–630 nm, red)
with a long life-time and is barely toxic. If the MRI and FI
functions of complexed Eu are both achieved, and the complexes
possess sensitivity and accuracy, a series of promising
applications are predicted. Xu et al presented a variety of
lanthanide oxide (Ln2O3) NPs and studied
their water proton relaxivities (5).
The authors coated Ln2O3 with D-glucuronic
acid to prepare ultrasmall NPs (with diameters of ∼2 nm), and
measured their longitudinal relaxivity (r1) and transverse
relaxivity (spin-spin relaxivity; r2). The results are listed in
Table I (6), and show that the D-glucuronic
acid-coated ultrasmall Eu2O3 NPs are not
outstanding in r1 or r2. This may be attributed to the spin
relaxation time of Eu3+ being too short to alter the
relaxivity of protons. To achieve MRI-FI dual imaging, the optimal
method is to blend Eu complexes with routine MRI contrast agents,
for example, Gd3+ complexes (5,7) and
Fe3O4 (8,9). Pinho
et al studied the dual imaging capabilities of
lanthanide-DTPA-grafted silica NPs, in which Eu3+ and
Gd3+ complexes were blended together
[SiO2@3-aminopropyl-triethoxysilane (APS)/DTPA:Gd:Eu]
(7). The results revealed that the
existence of Gd3+ did not disturb the photoluminescence
of Eu3+, while the addition of Eu3+ enhanced
the relaxivity of Gd3+.
 | Table I.Average particle diameter
(davg), r1, and r2 of D-glucuronic acid-coated
ultrasmall Ln2O3 nanoparticles and the M
values of Ln(III) in ultrasmall Ln2O3
nanoparticles (6). |
Table I.
Average particle diameter
(davg), r1, and r2 of D-glucuronic acid-coated
ultrasmall Ln2O3 nanoparticles and the M
values of Ln(III) in ultrasmall Ln2O3
nanoparticles (6).
|
|
| Ma(µB) |
|
|
|---|
|
|
|
|
|
|
|---|
|
Ln2O3
nanoparticle | davg
(nm) | 5K | 300K | r1b (mM‒1
sec‒1) | r2b (mM‒1
sec‒1) |
|---|
|
Eu2O3 | 2.0 | 0.078 | 0.046 | 0.006 | 3.82 |
|
Gd2O3 | 2.4 | 6.42 | 0.24 | 4.25 | 27.11 |
|
Dy2O3 | 2.9 | 5.19 | 0.42 | 0.16 | 40.28 |
|
Ho2O3 | 2.4 | 4.66 | 0.39 | 0.13 | 31.24 |
|
Er2O3 | 2.9 | 4.52 | 0.34 | 0.06 | 14.74 |
However, in order to realize MRI-FI dual imaging
with Eu complexes in the absence of highly paramagnetic ions, it is
necessary to focus attention on improving the MRI properties of Eu
complexes.
Studies of Eu2+ complexes as MRI
contrast agents
Eu2+ has seven unpaired electrons in its
outer electron shell, as does Gd3+. However,
Eu2+ has a larger ion size than Gd3+ and a
lower charge, which gives Eu2+ a faster water-exchange
rate and guarantees a relatively high relaxivity (10). Eu2+ is also
photoluminescent; f→d transitions have been observed in
Eu2+, which have longer radiative emission lifetimes
than f→f transitions in Eu3+ (11). However, the relaxivity of
Eu2+-containing DTPA chelates has been found to be 20%
lower than that of Gd3+-DTPA complexes at 20 MHz, which
may be attributed to the fast ionic spin relaxation of
Eu2+ (12). Not all
Eu2+ complexes are restricted by ionic spin relaxation.
The relaxivity of Eu2+-DOTA complexes has been shown to
reach 4.74 mM−1sec−1 at 20 MHz and 298K, much
higher than that of Eu2+-DTPA complexes (13). A fast water-exchange rate of
Eu2+ is able to offset the disadvantage in ionic spin
relaxation time.
The biggest obstacle in the development of MRI
applications of Eu2+ complexes is the oxidative
stability of the ion. Eu2+ has a propensity for being
oxidized to Eu3+, which possesses low relaxivity and
relatively high toxicity. Coordination chemistry principles can be
used to oxidatively stabilize Eu2+ without weakening the
relaxivity and water-exchange rate.
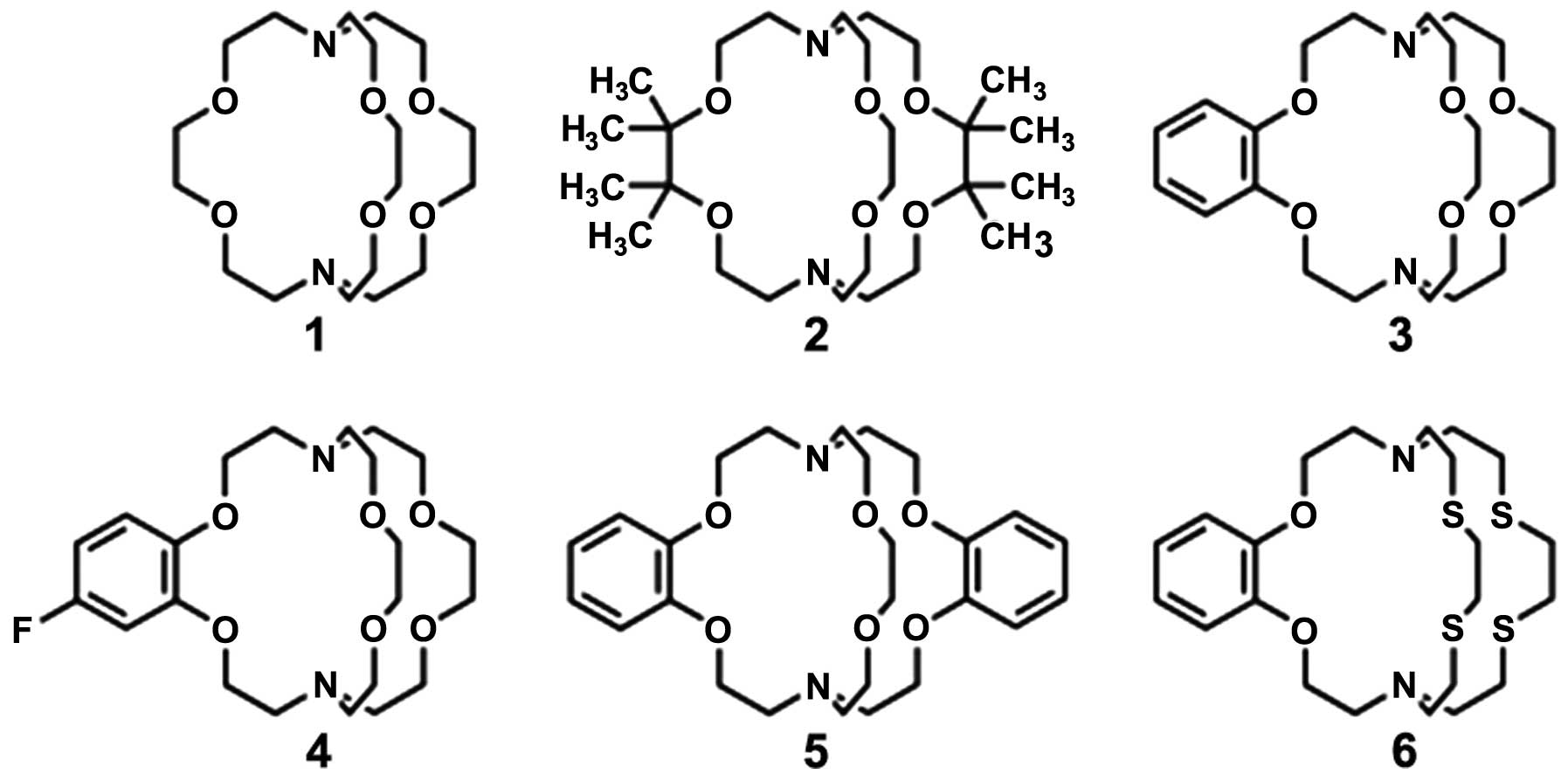
4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane
(crypt-222; ligand 1 in Fig. 2A) is
known to have an appropriate cage size for Eu2+, and the
crypt-222 complex
[Eu(crypt-222)(H2O)2]2+ is one of
the most redox-stable Eu2+ chelates (14). [Eu(crypt-222)]2+
coordinates immediately with two water molecules, and possesses an
appropriate water-exchange rate and ionic spin relaxation time.
However, [Eu(crypt-222)(H2O)2]2+
is not sufficiently stable for the development of MRI applications
in aqueous solution. However, it is potentially useful as a
pO2-responsive macromolecular MRI contrast agent
(14).
To obtain oxidatively stable aqueous Eu2+
complexes, Gamage et al carried out research and development
based on the coordination of crypt-222 (15). The goals were: i) to increase the
surrounding steric bulk to minimize interactions between
Eu2+ and its environment; ii) to reduce the Lewis
basicity of crypt-222 to favor Eu2+ over
Eu3+; iii) to change the cavity size of the cryptand to
match the size of the Eu2+ ion preferentially; and iv)
to modify the hard-soft, acid-base (HSAB) properties of crypt-222
to coordinate Eu2+ in preference to Eu3+. The
authors presented five ligands (ligands 2–6 in Fig. 2) with crypt-222 as the prototype.
The steric bulk of ligand 2 was increased by the
addition of methyl groups. Benzene rings were introduced to
decrease the ion-donating ability of the adjacent oxygen atoms of
ligands 3–5. The introduction of fluorine in ligand 4 and another
benzene ring in ligand 5 modulated the extent of ion withdrawal.
The existence of fused benzene moieties decreased the cavity size
of the cryptand and provided a cavity size closer to that of
Eu2+. In ligand 6, relatively soft sulfur-atom donors
were introduced to take the place of oxygen-atom donors, enabling
the influence of HSAB properties to be explored. In situ,
the authors mixed these ligands with
Eu(NO3)3·5H2O in aqueous solution.
The mixture was placed in a standard three-electrode cell while the
potential at the carbon electrode was held at −0.8 V to achieve
metal complexation. Cyclic voltammograms were obtained following
metallation for each complex in solution with ferrocene as an
internal standard. Anodic peak potentials for each complex were
obtained and are listed in Table
II.
 | Table II.Anodic peak potentials (Epa) of
various samples with respect to ferrocene/ferrocenium
(Fc/Fc+). |
Table II.
Anodic peak potentials (Epa) of
various samples with respect to ferrocene/ferrocenium
(Fc/Fc+).
| Sample | Epa vs.
Fc/Fc+ (V) | Sample | Epa vs.
Fc/Fc+ (V) |
|---|
| 1.
Eu(NO3)3 | −0.701±0.030 | 5. Eu−2 | −0.169±0.006 |
| 2. Eu−1 | −0.336±0.016 | 6. Eu−4 | −0.079±0.007 |
| 3. Eu−5 | −0.211±0.004 | 7. hemoglobin | −0.070±0.003 |
| 4. Eu−3 | −0.208±0.009 | 8. Eu−6 | −0.035±0.010 |
The results revealed that the new ligands all
increased the oxidative stability of Eu2+ to a certain
degree. There was almost no difference between the Eu complexes of
ligands 5 and 3, indicating that the addition of one benzene ring
was sufficient for stabilization. The Eu complex of ligand 6 had
the highest anodic peak potential, higher than that of
Fe2+-hemoglobin, suggesting that it was an efficient
ligand to prevent Eu2+ from oxidation. Garcia et
al also reported the stability of Eu complexes with ligands 1
and 3 in the presence of Ca2+, Mg2+ and
Zn2+ (16), and the study
indicated that the Eu2+ complexes remained stable in the
presence of Ca2+, Mg2+ and Zn2+ at
concentrations 1.87-20-fold higher than biological concentrations.
Eu2+ complexes have the potential to realize durable
biological oxidative stability, and provide satisfactory magnetic
and spectroscopic properties in vivo.
MRI of Eu3+ complexes based on
CEST
Chemical exchange saturation transfer (CEST) is a
novel mechanism for generating image contrast in MRI (1). Protons on ligands or water molecules
coordinated with a metal ion are saturated by a radiofrequency
pulse and then exchanged with bulk water molecules, presenting a
negative image. Imaging can be made more efficient by the
utilization of paramagnetic nuclei. The effects of these agents,
referred to as PARACEST agents, can be switched on and off
depending on the application of radiofrequency radiation.
Theoretically, with an optimal water-exchange rate, chemical shift
and relaxation properties, the detection limit of a single PARACEST
exchanging species is comparable to that of a single
Gd3+-based T1 imaging agent (17). The applications of CEST include the
visualization of living tissue structure, the imaging of metabolic
processes, the marking of cells and pH measurements (18).
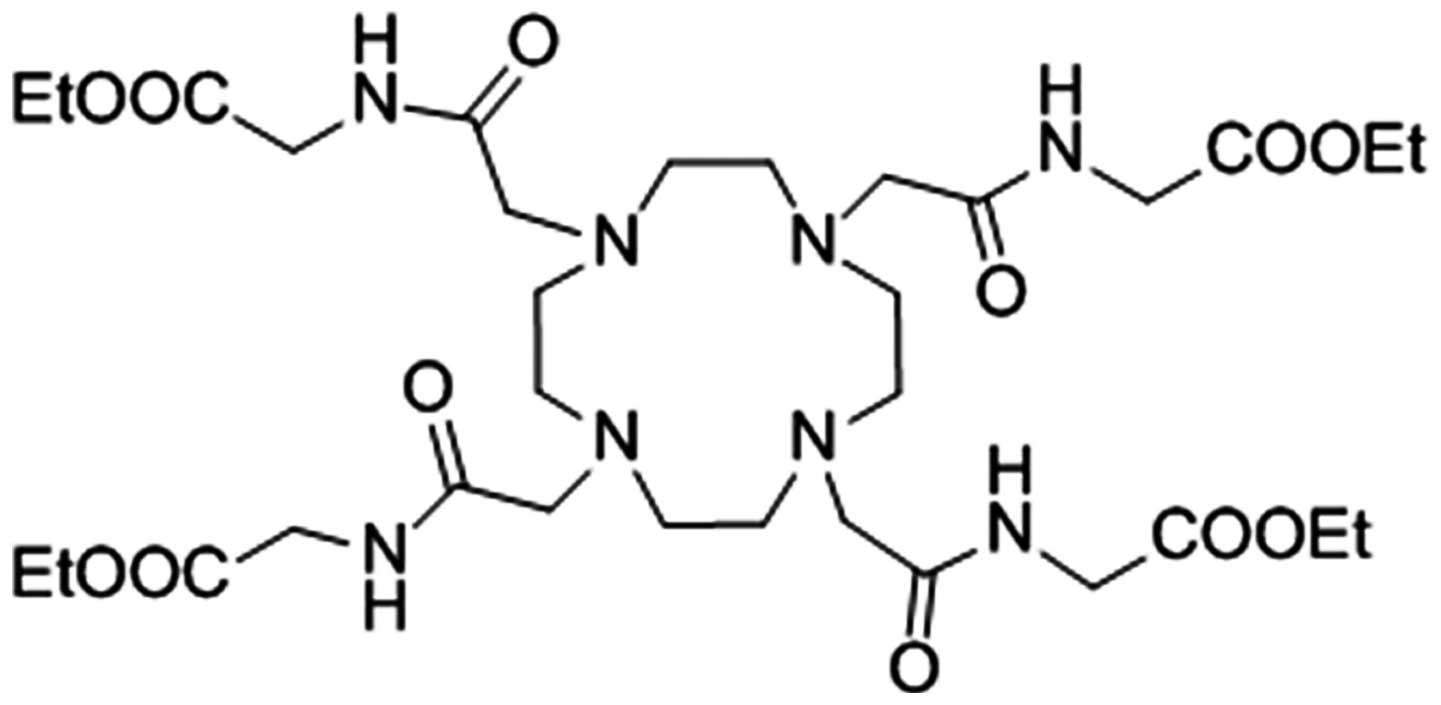
Zhang et al studied the CEST properties of a
complex of Eu3+ with a DOTA-tetraamide derivative
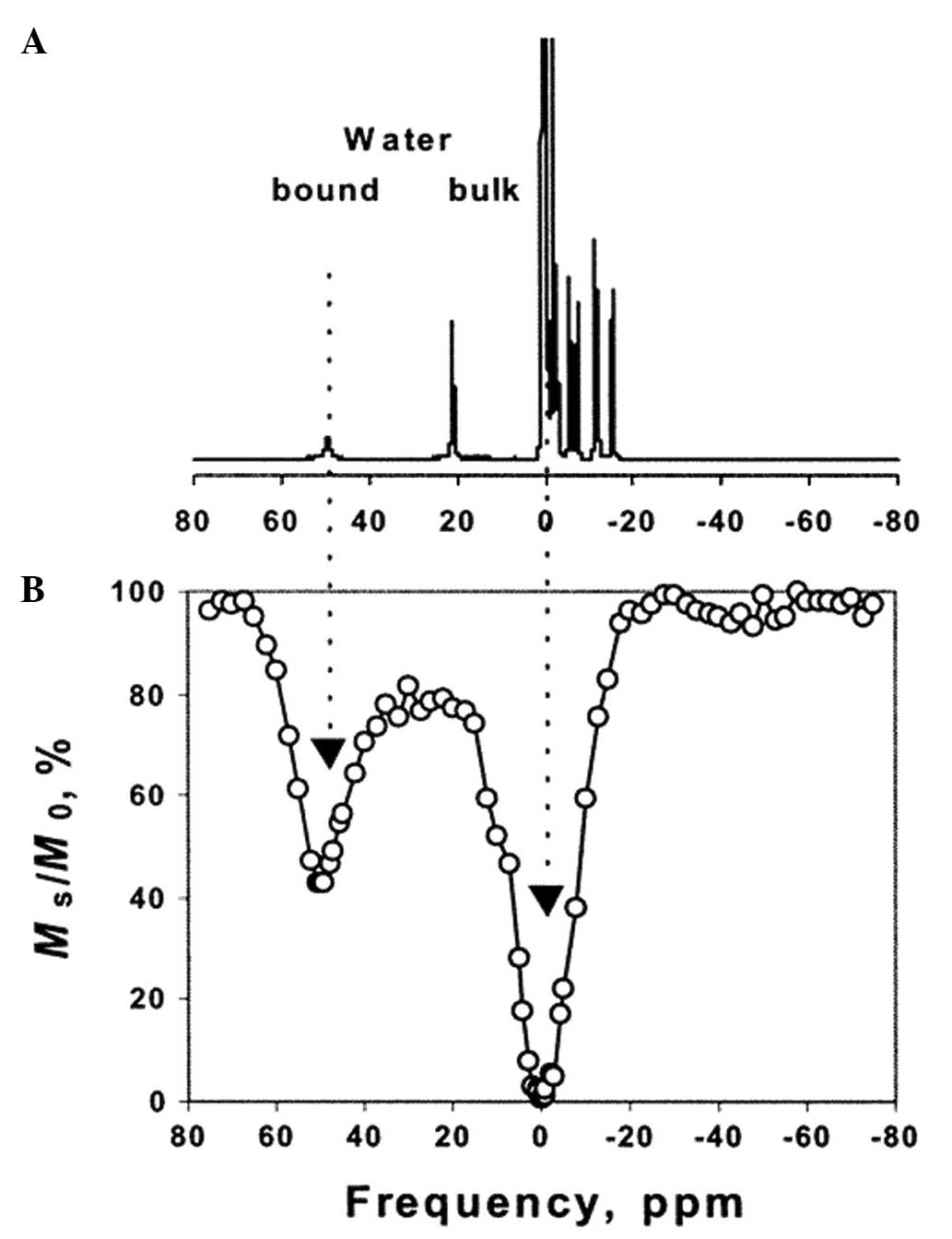
(ligand 7 in Fig. 3) (19). 1H NMR spectroscopy was
used to examine an aqueous solution of the Eu complex of ligand 7
(Fig. 4A). The peak corresponding to
complexed water shifted to ∼50 ppm. If protons were exchanged
between the complexed water and bulk water following the
application of radiofrequency energy, the signal intensities of
would change, revealing directly the ratio of saturated protons.
The ratio of saturated protons is defined as
Ms/M0, where Ms is the water
proton signal in the presence of saturation and M0 is
the signal under control conditions. CEST curves were drawn based
on the aforementioned method (Fig.
4B). Lower MsM0 values indicated higher
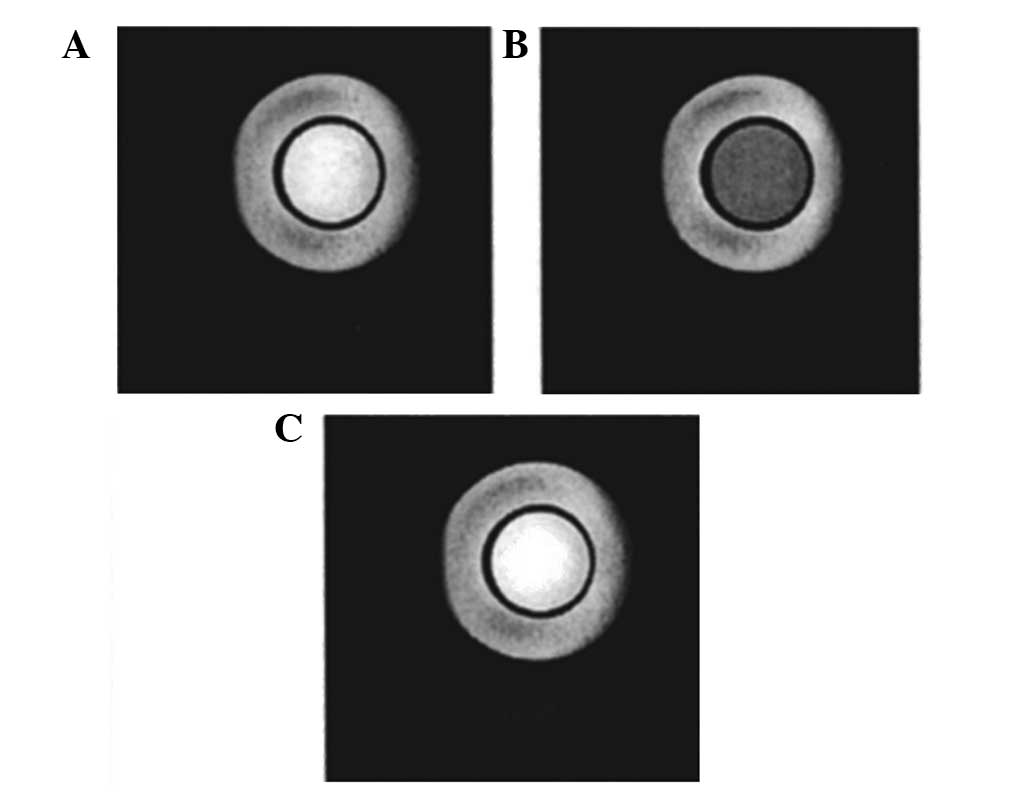
CEST effects. Magnetization transfer T1-weighted spin-echo images
for a phantom were obtained with no saturation (Fig. 5A), saturation at +9,800 Hz (Fig. 5B) and saturation at −9,800 Hz
(Fig. 5C). The image at +9,800 Hz
was dimmer than the others, demonstrating the weakening effect of
CEST on the magnetic resonance signals of protons, and the ability
to be switched on and off at will was achieved.
To lower the detection limit of PARACEST agents, Wu
et al reported the polymerization of PARACEST agents in
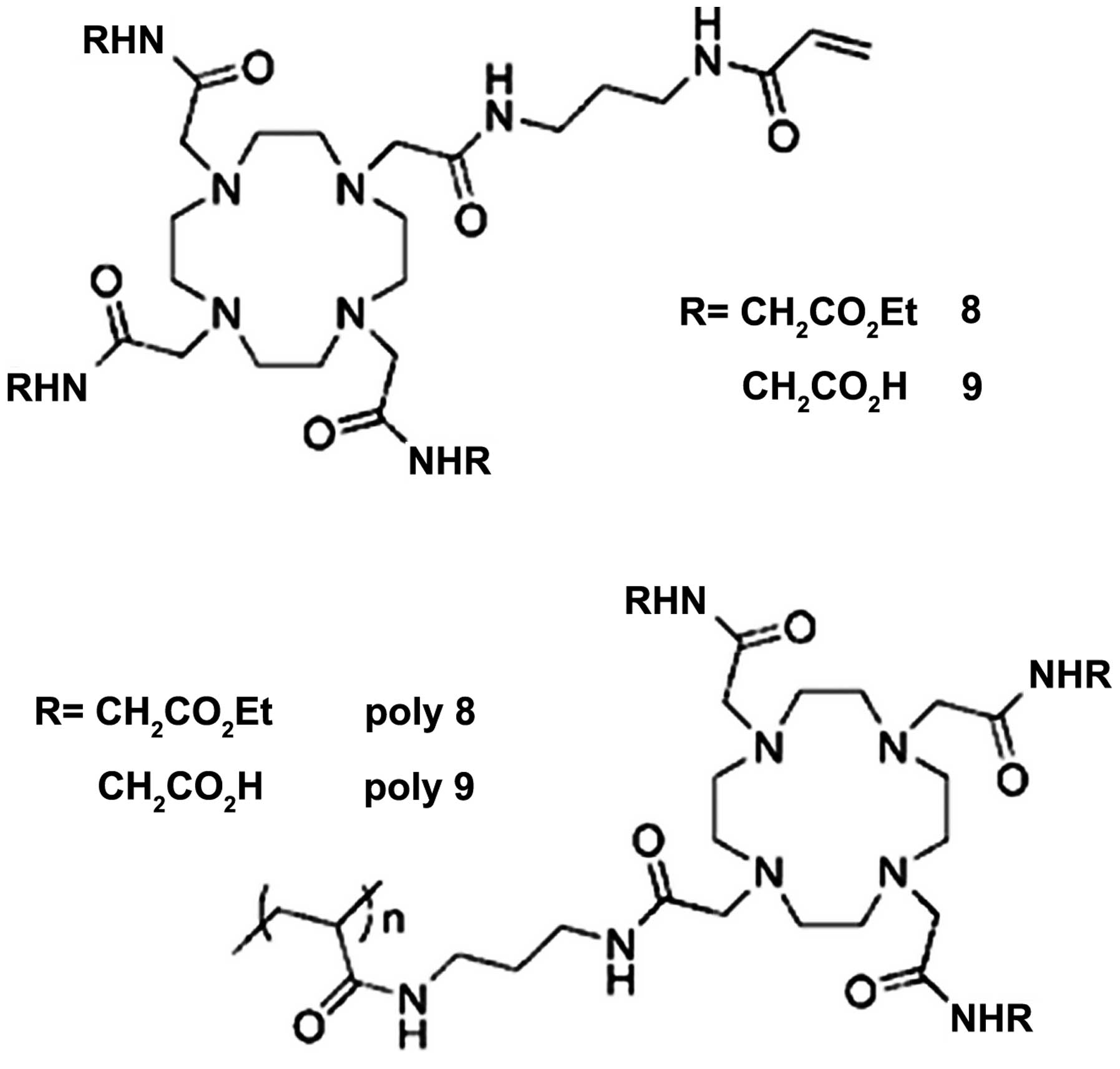
order to enhance MRI contrast sensitivity (17). The authors presented two kinds of
DOTA ligands with acrylamide substituents to coordinate with
Eu3+ (ligands 8 and 9 in Fig.
6), and synthesized polymeric ligands by the respective radical
polymerization of ligands 8 and 9 (poly 8 and poly 9 in Fig. 6). The CEST spectra of Eu complexes of
ligands 9 and poly 9 were almost identical at equal Eu3+
concentrations (Fig. 7), suggesting
that the polymerization had no impact of the exchange of protons. A
comparison among the maximum CEST for each concentration of the Eu
complexes of ligand 9 and poly 9 with different initiator ratios
(Fig. 8) revealed that
polymerization efficiently lowered the detection limit.
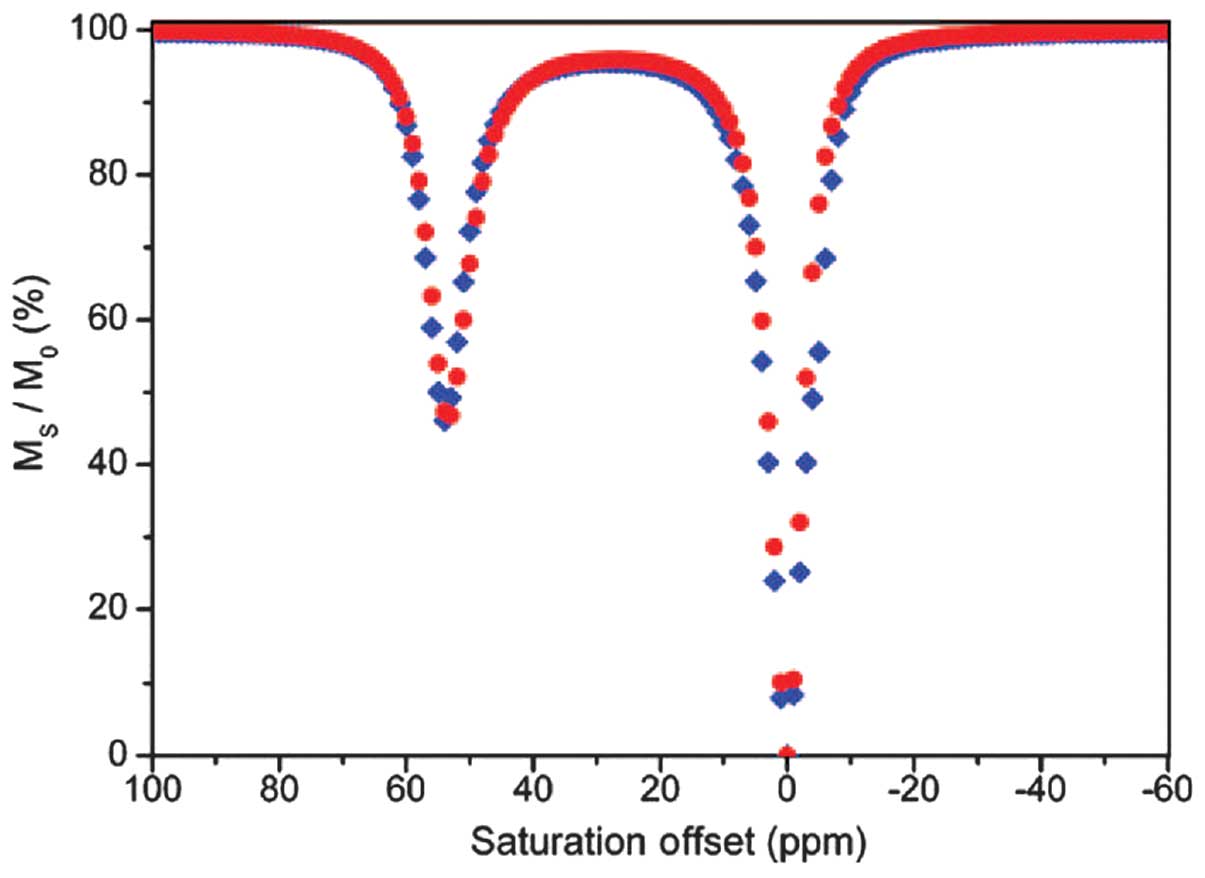 | Figure 7.Chemical exchange saturation transfer
spectra of Eu-9 (red) and Eu-poly 9 (2%; blue); concentration of
Eu3+, 30 mM. Eu-9 is a complex of europium
(Eu3+) with an acrylamide-substituted derivative of
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (ligand
9), and Eu-poly 9 is a complex of Eu3+ with a polymer of
ligand 9. Ms/M0, ratio of saturated protons;
Ms, water proton signal in the presence of saturation;
M0, signal under control conditions; ppm, parts per
million. |
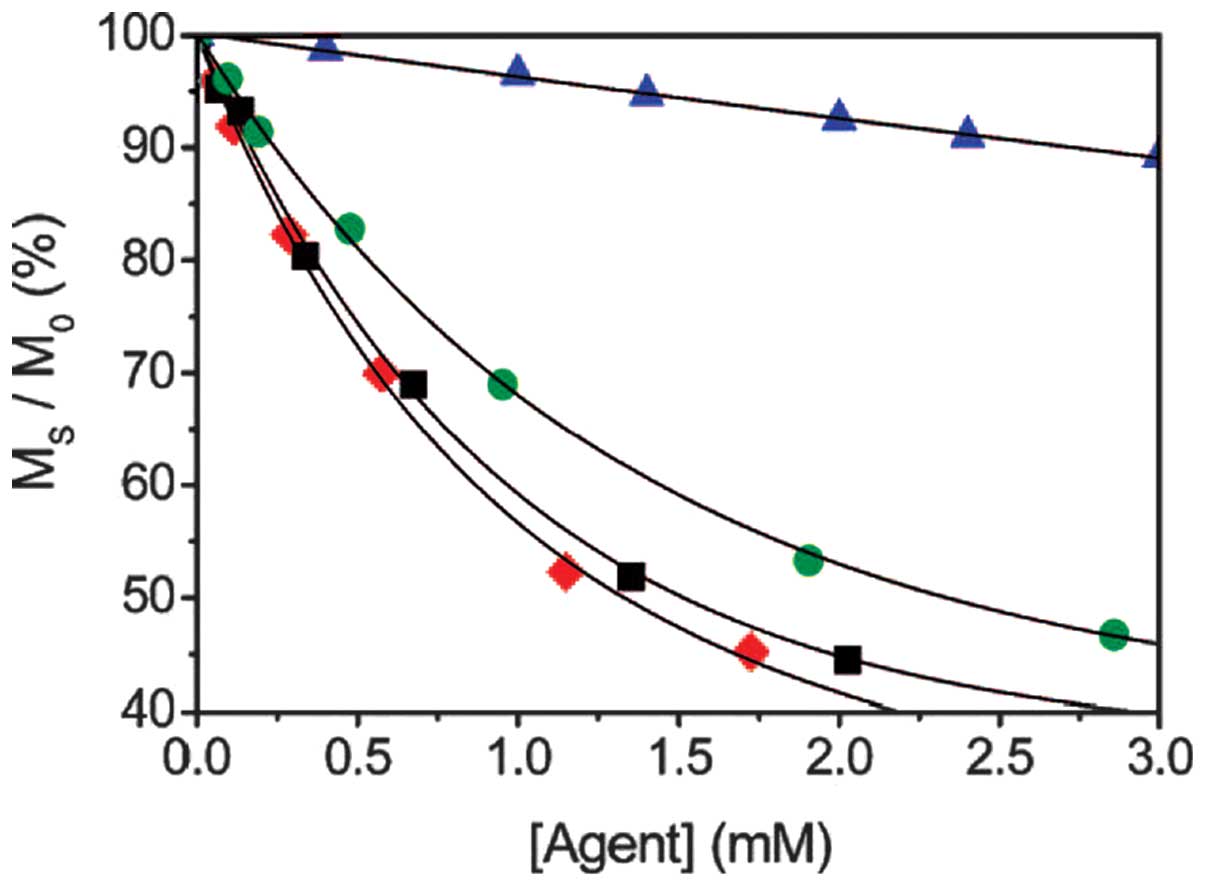 | Figure 8.Maximum chemical exchange saturation
transfer per concentration of agent [agent] for Eu-9 (▲) and
Eu-poly 9 (•, 2%; ▪, 5%; ♦,10%). Eu-9 is a complex of europium
(Eu3+) with an acrylamide-substituted derivative of
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (ligand
9), and Eu-poly 9 is a complex of Eu3+ with a polymer of
ligand 9. Ms/M0, ratio of saturated protons;
Ms, water proton signal in the presence of saturation;
M0, signal under control conditions. |
In another article from these authors,
2-methylacrylic acid (MAA),
2-(acryloylamino)-2-methyl-1-propanesulfonic acid (AMPS) and
N-isopropylacrylamide (NIPAM) were respectively used to
copolymerize with ligand 9, to form the polymers DMAA, DAMPS and
DNIPAM (D: ligand 9), respectively (Fig.
9), in order to investigate the effect of water-exchange rate
on the CEST properties of Eu3+ complexes (20).
Unlike Gd3+-based T1 imaging agents that
rely on rapid water exchange between metal ion-bound water and bulk
solvent, the signals of PARACEST agents become quenched as the
bound protons on the agent are not readily saturated at a rapid
water-exchange rate; they require moderate-to-slow water exchange
rates for optimal performance. From the CEST results of an Eu
complex of DMAA at different pH values (Fig. 10), the signals were attenuated as
the pH was reduced from 8 to 4. The carboxyl group was stable
enough to bond with water at lower pH and water exchange between
water and the complexes was promoted. Copolymerization also
rendered the complexes responsive to temperature. The
water-exchange rate increased as the temperature rose. However,
unlike DMAA, which is hydrophilic along the full length of the
polymer, the interaction between water and the side groups of
DNIPAM was attenuated when the temperature rose, and the side
groups tended to aggregate against water, which avoided an
unfavorably rapid exchange rate. The aforementioned factors explain
why changes in the signals generated by the Eu-DNIPAM complex were
more moderate than those generated by Eu-DMAA as the temperature
changed. The results of these studies indicate that
Eu3+-based CEST agents are promising for use in pH- and
temperature-responsive MRI applications.
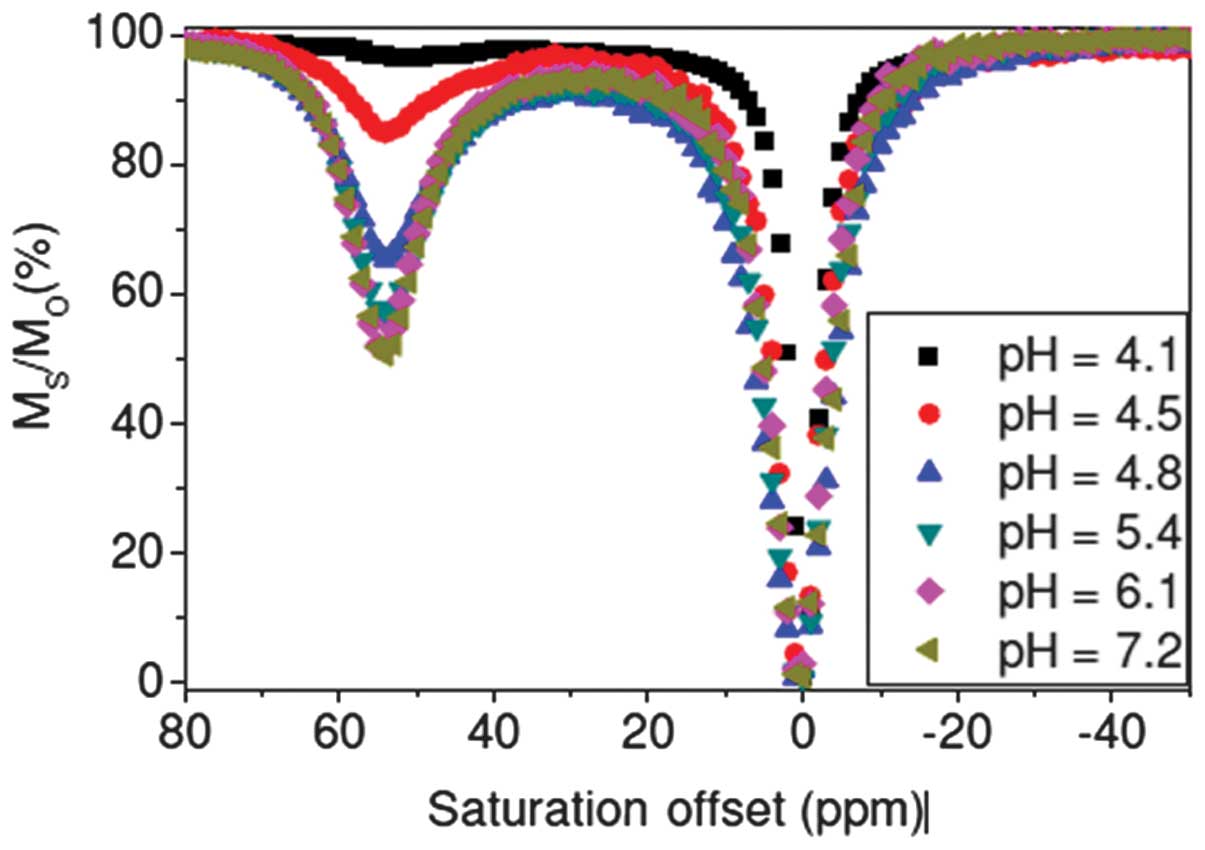 | Figure 10.Chemical exchange saturation transfer
curves of Eu-DMAA at different pH values. Eu-DMAA is a complex of
europium (Eu3+) with a copolymer of an
acrylamide-substituted derivative of
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (ligand 9)
and 2-methylacrylic acid (MAA). Ms/M0, ratio
of saturated protons; Ms, water proton signal in the
presence of saturation; M0, signal under control
conditions; ppm, parts per million. |
Conclusion and prospects
Eu3+ and Eu2+ possesses six
and seven unpaired electrons in the 4f orbital, respectively, which
is indicative of paramagnetism. However, the short ionic spin
relaxation time of Eu3+ and the propensity of
Eu2+ to be oxidized result in the MRI properties of Eu
complexes being inferior to those of Gd complexes. The design of
ligands and enhancement by CEST are the main approaches at present
to compensate for the deficiencies of Eu ions, enhance their
properties as MRI contrast agents and eventually realize MRI-FI
dual imaging with further efforts.
Acknowledgements
This study was supported by National Basic Research
Program of China (973 Program, no. 2014CB744505), National Natural
Science Foundation of China (no. 81430040) and Scientific Research
Foundation of Health Bureau of Zhejiang Province in China (no.
2014PYA010).
References
|
1
|
Dorweiler JD, Nemykin VN, Ley AN, Pike RD
and Berry SM: Structural and NMR characterization of Sm(III),
Eu(III) and Yb(III) complexes of an amide based polydentate ligand
exhibiting paramagnetic chemical exchange saturation transfer
abilities. Inorg Chem. 48:9365–9376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang LY, Chen QY and Wei B: MRI contrast
agent for the diagnosis of tumor. Hua Xue Jin Zhan. 22:186–193.
2010.[(In Chinese)].
|
|
3
|
Liu Y and Zhang N: Gadolinium loaded
nanoparticles in theranostic magnetic resonance imaging.
Biomaterials. 33:5363–5375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee GH, Chang YM and Kim TJ: Blood-pool
and targeting MRI contrast agents: From Gd-chelates to
Gd-nanoparticles. Eur J Inorg Chem. 2012:1924–1933. 2012.
View Article : Google Scholar
|
|
5
|
Xu W, Kattel K, Park JY, Chang Y, Kim TJ
and Lee GH: Paramagnetic nanoparticle T1 and T2 MRI contrast
agents. Phys Chem Chem Phys. 14:12687–12700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kattel K, Park JY, Xu W, et al: A facile
synthesis, in vitro and in vivo MR studies of D-glucuronic
acid-coated ultrasmall Ln2O3 (Ln = Eu, Gd,
Dy, Ho and Er) nanoparticles as a new potential MRI contrast agent.
ACS Appl Mater Interfaces. 3:3325–3334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pinho SL, Faneca H, Geraldes CF, Delville
MH, Carlos LD and Rocha J: Lanthanide-DTPA grafted silica
nanoparticles as bimodal-imaging contrast agents. Biomaterials.
33:925–935. 2012.PubMed/NCBI
|
|
8
|
Wang W, Zou M and Chen K: Novel
Fe3O4@YPO4: Re (Re = Tb, Eu)
multifunctional magnetic-fluorescent hybrid spheres for biomedical
applications. Chem Commun (Camb). 46:5100–5102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xi P, Cheng K, Sun X, Zeng Z and Sun S:
Magnetic Fe3O4 nanoparticles coupled with a
fluorescent Eu complex for dual imaging applications. Chem Commun
(Camb). 48:2952–2954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia J and Allen MJ: Developments in the
coordination chemistry of europium (II). Eur J Inorg Chem.
2012:4550–4563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JM, Jeong YK, Sohn Y and Kang JG:
Synthesis and photophysical properties of an Eu(II)-complex/PS
blend: Role of Ag nanoparticles in surface-enhanced luminescence.
Langmuir. 28:9842–9848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seibig S, Tóth É and Merbach AE:
Unexpected differences in the dynamics and in the nuclear and
electronic relaxation properties of the isoelectronic
[EuII(DTPA)(H2O)]3- and
[GdIII(DTPA) (H2O)]2− complexes
(DTPA = diethylenetriaminepentaacetate). J Am Chem Soc.
122:5822–5830. 2000. View Article : Google Scholar
|
|
13
|
Burai L, Tóth É, Moreau G, Sour A,
Scopelliti R and Merbach AE: Novel macrocyclic EuII
complexes: Fast water exchange related to an extreme M-Owater
distance. Chemistry. 9:1394–1404. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burai L, Scopelliti R and Tóth É:
EuII-cryptate with optimal water exchange and electronic
relaxation: A synthon for potential pO2 responsive
macromolecular MRI contrast agents. Chem Commun (Camb).
20:2366–2367. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gamage NDH, Mei YJ, Garcia J and Allen MJ:
Oxidatively stable, aqueous europium(II) complexes through steric
and electronic manipulation of cryptand coordination chemistry.
Angew Chem Int Ed Engl. 49:8923–8925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garcia J, Kuda-Wedagedara AN and Allen MJ:
Physical properties of Eu2+-containing cryptates as
contrast agents for ultra-high field magnetic resonance imaging.
Eur J Inorg Chem. 2012:2135–2140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Y, Zhou Y, Ouari O, et al: Polymeric
PARACEST agents for enhancing MRI contrast sensitivity. J Am Chem
Soc. 130:13854–13855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu CM and Jin JY: Research progress of
CEST agent in MR imaging. Guoji Yixue Fangshexue Zazhi. 32:475–477.
2009.[(In Chinese)].
|
|
19
|
Zhang S, Winter P, Wu K and Sherry AD: A
novel europium(III)-based MRI contrast agent. J Am Chem Soc.
123:1517–1518. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Zhao P, Kiefer GE and Sherry AD:
Multifunctional polymeric scaffolds for enhancement of PARACEST
contrast sensitivity and performance: Effects of random copolymer
variations. Macromolecules. 43:6616–6624. 2010. View Article : Google Scholar : PubMed/NCBI
|