Introduction
Due to the growth and improvement in living
standards, the changes in eating habits and lifestyles and the
increasingly aging population, the incidence of metabolic diseases,
such as diabetes and atherosclerosis (AS), is increasing each year.
According to statistics from the World Health Organization,
ischemic heart disease ranks first among the top ten fatal diseases
in 2011 (1). Ischemic heart disease
is pathologically and largely based on AS, and the majority of AS
cases are caused by a metabolic disturbance in glucolipids.
Previous studies have shown that nuclear receptors (NRs) are
closely associated with glucolipid metabolism in vivo
(2,3). Liver X receptor (LXR), as an important
member of the NR superfamily, participates in the regulatory
processes of a number of physical activities in vivo,
including the metabolism of cholesterol, glucose and fat,
inflammation and the maintenance of the metabolic balance. LXR has
attracted increasing attention due to its important function in
glucolipid metabolism (4). There are
two subtypes of LXR: LXRα and LXRβ. The expression of LXRα is
tissue-specific, with LXRα highly expressed in the liver,
intestine, kidney, adrenal gland, spleen, adipose tissue and
macrophages, while LXRβ is extensively expressed in tissue cells.
As previously demonstrated, application of a LXR agonist can reduce
the intracellular cholesterol content by decreasing the rate of
intestinal cholesterol absorption, increasing the level of bile
acid and decreasing the rate of reverse cholesterol transfer,
thereby ameliorating AS (5,6).
Due to their structural diversity, small natural
product molecules have become an essential source of small
molecular lead compounds. Magnolol is one of the key monomer
components of the traditional Chinese medicine, Magnolia
officinalis extract (7).
Magnolia officinalis has a long history as a traditional
Chinese medicine and is largely applied for dispelling a cold,
relieving headaches, inhibiting anxiety, treating diarrhea and
apoplexia, removing chest stuffiness, composing central nerves, and
as an anti-fungus and anti-ulcer treatment (8–10). In
addition, Jiangzhi Ninggan capsules, which exhibit lipid-lowering
effects, contain magnolol (11).
Magnolol has been demonstrated to interact with the retinoid X
receptor α (RXRα) and the peroxisome proliferator-activated
receptor γ, amongst other NRs associated with glucolipid metabolism
(12). Therefore, in the present
study, whether magnolol interacts with the LXR to regulate
LXR-targeted expression of downstream genes was investigated. The
aim of the study was to provide further understanding into the
lipid-lowering mechanism of magnolol, in order to offer a novel
idea for the research and development of anti-AS drugs.
Materials and methods
Cell culture
All the cells were cultured in an environment with
5% CO2 at 37°C. The HepG2 human hepatoma cell line
(American Type Culture Collection, Manassas, VA, USA) was cultured
in Minimal Essential Medium (MEM; Gibco Life Technologies, Grand
Island, NY, USA), supplemented with 10% fetal bovine serum (FBS;
Gibco Life Technologies), 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The THP-1 human
monocyte cell line (American Type Culture Collection) was cultured
in RPMI-1640 medium (Gibco Life Technologies) containing 10% FBS
with 10 mmol/l HEPES, 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich).
Transient cellular transfection and
luciferase reporter gene assay
Following HepG2 cell growth to a density of 30–50%,
the cells were seeded in a 24-well plate with non-serum MEM.
Lipofectamine 2000 transfection reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) was applied for the transfection
of the plasmids into the cells. The mammalian one-hybridization
experiment is a method extensively applied to identify
transcription factors at a molecular level. As an agonist of LXR,
T0901317 (J&K Scientific Ltd., Shanghai, China) is known to
effectively activate the transcriptional activity of the
LXRα-ligand binding domain (LBD) (13). Using the mammalian one-hybridization
method and T0901317 as a positive control, the effect of various
concentrations of magnolol (J&K Scientific Ltd.) on the
transcriptional activity of the LXRα-LBD was analyzed in order to
identify whether magnolol was able to directly combine with the
LXRα-LBD.
In the mammalian one-hybrization experiment, 400 ng
pCMX-Gal4-DBD-LXRα-LBD, 400 ng UAS-TK-Luc, and 100 ng reference
pRL-SV40 plasmid were transfected (Promega Corporation, Madison,
WI, USA). In the transcriptional activation experiment, 400 ng
pcDNA3.1a-LXRα, 400 ng pcDNA3.1a-RXRα, 400 ng pGL2-basic-LXRE-Luc
plasmids and 100 ng reference plasmid pRL-SV40 were transfected
(Promega Corporation). pRL-SV40 was used as the negative control.
After cellular transfection for 6 h, the medium was changed to
complete medium, 10, 20 or 40 µM magnolol was added and incubated
for 18–24 h. The positive control received 50 nM T0901317 and the
negative control contained 10 µM DMSO. Following incubation, the
cells were washed with phosphate-buffered saline (PBS), and 100 µl
lysate was added to each well for cell lysis at 37°C for 20 min.
Subsequently, the activity of the firefly luciferase and reference
luciferase were detected, according to the manufacturer's
instructions (Promega Corporation, Madison, WI, USA).
Quantitative polymerase chain reaction
(PCR)
Prior to the PCR experiment, 160 nmol/l phorbol
ester (Sigma-Aldrich) was incubated with the THP-1 cells for 24 h
to induce cell differentiation into macrophages. The cells were
subsequently cultured in non-serum medium containing 50 µg/ml
oxidized low-density lipoprotein (Jingmei Biotechnology Co.,
Nanjing, China) for 48 h, and lipids were phagocyted, then formed
foam cells. After 24 h of treatment, the medium in the Petri dish
was removed and the cells were washed with PBS. The total RNA was
extracted using TRIzol reagent (Takara Biotechnology Co., Ltd.,
Dalian, China), according to the manufacturer's instructions. A
reverse transcription kit (Takara Biotechnology Co., Ltd.) was used
to convert the extracted RNA into cDNA, after which quantitative
PCR detection was conducted. The primer sequences were as follows:
Human ATP-binding cassette transporter A1 (ABCA1) forward,
5′-GATTGGCTTCAGGATGTCCATGTTGGAA-3′ and reverse,
5′-GTATTTTTGCAAGGCTACCAGTTACATTTGACAA-3′; human ATP binding
cassette transporter G1 (ABCG1) forward, GCCACTTTCGTGGGCCCAGTGA-3′
and reverse, 5′-TCTCATCACCAGCTGTGTTGCA-3′. β-Actin was used as the
reference gene in the genetic expression experiments, and the
β-actin primers were as follows: Forward, 5′-GCGGGAAATCGTGCGTGAC-3′
and reverse, 5′-CGTCATACTCCTGCTTGCTG-3′.
Sample cDNA underwent quantitative PCR amplification
using a SYBR fluorescent probe kit (Takara Biotechnology Co.,
Ltd.). The amplification procedure was as follows: Initial
denaturation at 95°C for 10 sec, followed by 40 cycles of 95°C for
5 sec, 60°C for 20 sec, 72°C for 10 sec and a final extension at
72°C for 10 min.
Statistical analysis
All experimental data are expressed as the mean ±
standard deviation. Statistical analysis was performed using
GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA,
USA). The statistical significance of differences was analyzed by
one way analysis of variance and Dunnett's post hoc test, where
P<0.05 was considered to indicate a statistically significant
difference.
Results
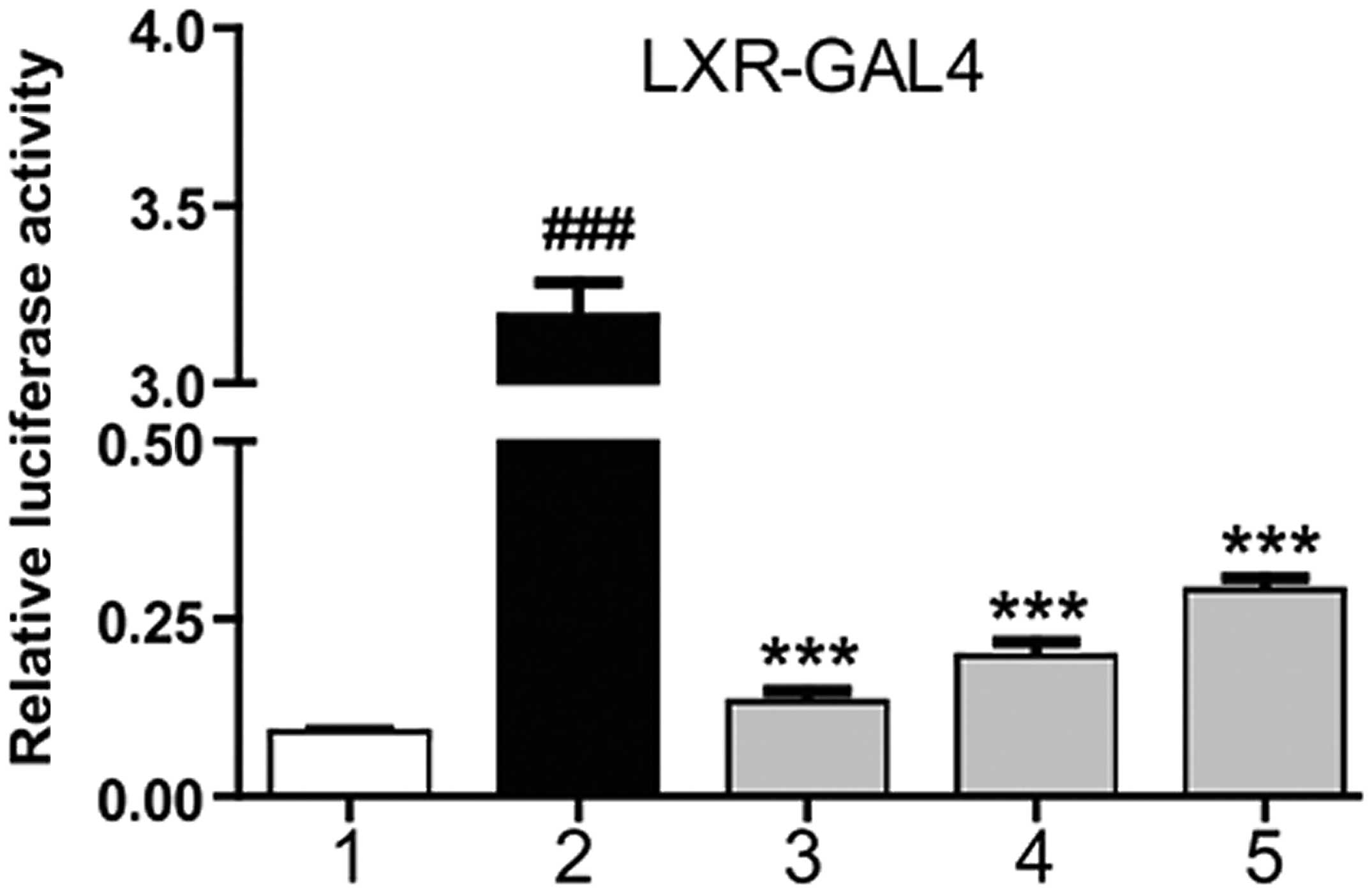
Magnolol is an agonist of LXRα
At 6 h after HepG2 cell transfection with the
corresponding plasmid, magnolol was applied at different
concentrations, as well as T0901317. After 18–24 h, the detection
of luciferase activity was performed. T0901317 was shown to
markedly stimulate the transcriptional activity of the LXRα-LBD,
while magnolol was shown to dose-dependently increase luciferase
activity. These results indicated that magnolol was able to
interact with the LXRα-LBD directly (Fig. 1). NRs are ligand-dependent
transcription factors, and magnolol was shown to combine with the
LXRα-LBD at a molecular scale, demonstrating that magnolol may
affect the expression of LXR downstream genes by combining with LXR
response elements (LXRE).
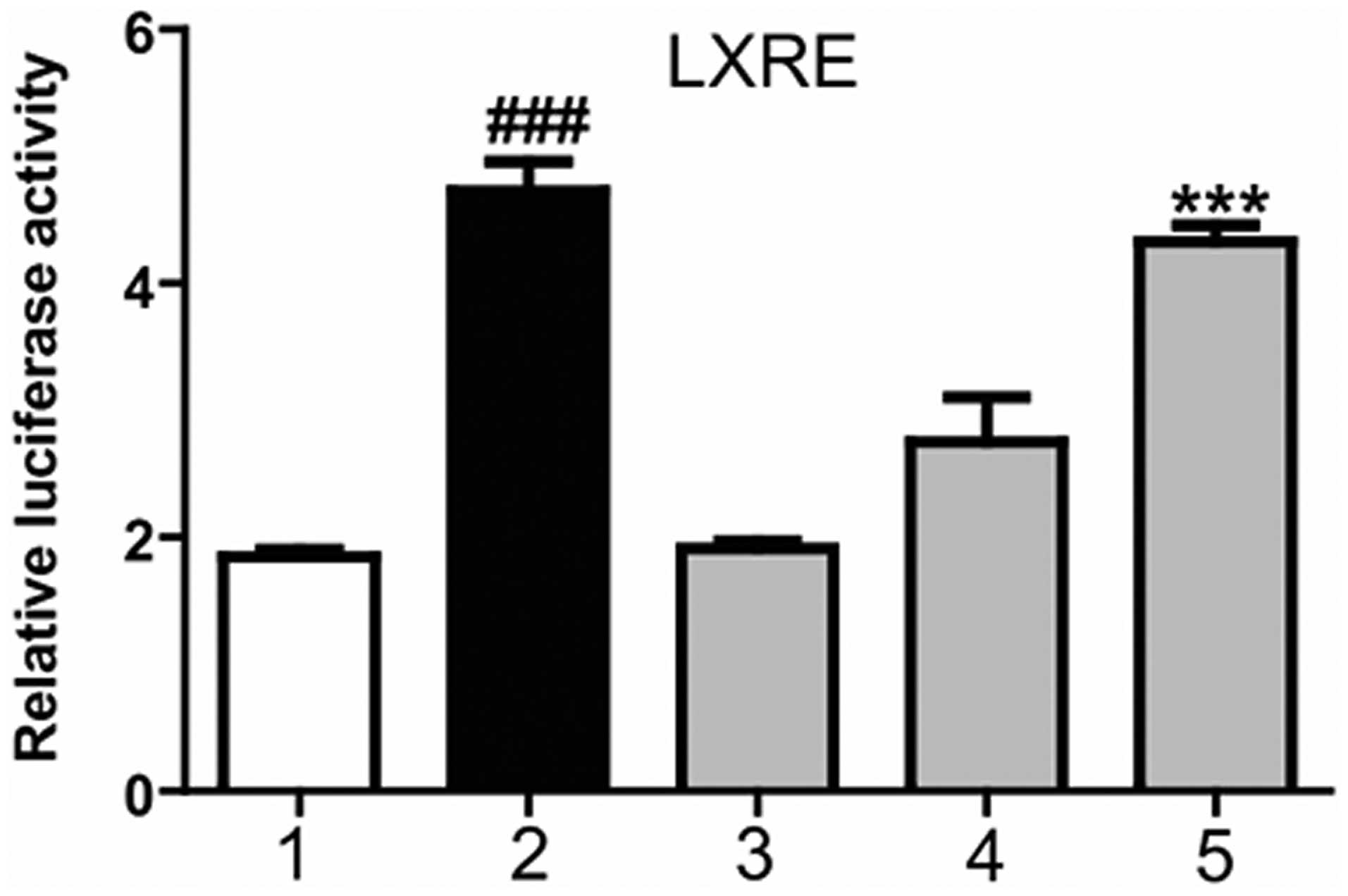
Magnolol increases the transcriptional
activation of LXRα
To function as transcription factor, LXR is required
to form a heterodimer with the RXR, with the combination in the
LXRE, in order to initiate downstream genetic expression. A
mammalian transcriptional activation experiment was conducted to
detect whether magnolol was able to promote the combination of the
LXR/RXR heterodimer and LXRE. At 6 h after HepG2 cell transfection
with the corresponding plasmids, the cells were treated with
magnolol at various concentrations or T0901317 (positive control)
for 24 h. As shown in Fig. 2,
T0901317 significantly increased the luciferase activity, promoted
the combination of the LXR/RXR heterodimer and LXRE, and increased
the transcriptional activity of the LXRE. Furthermore, magnolol was
shown to dose-dependently increase the transcriptional activity of
LXRE. These results indicated that magnolol was able to regulate
the combination of the LXR/RXR heterodimer and LXRE, thereby
affecting the expression of LXR downstream genes.
Magnolol increases the expression
levels of the LXRα downstream genes, ABCA1andABCG1
ABCA1 and ABCG1 are two essential proteins that
mediate cholesterol efflux in macrophages (14). In order to investigate whether
magnolol affects the expression of ABCA1 and ABCG1 via the LXR, and
subsequently ameliorates AS, the effect of magnolol on the
expression of ABCA1 and ABCG1 was investigated in THP-1
macrophage-derived foam cells using quantitative PCR. Magnolol was
applied at various concentrations to treat the cells. Magnolol was
demonstrated to markedly increase the mRNA expression levels of
ABCA1 and ABCG1, and the effect became more significant as the
magnolol concentration increased (Fig.
3). Therefore, the results indicated that magnolol
dose-dependently regulated the mRNA expression levels of ABCA1 and
ABCG1.
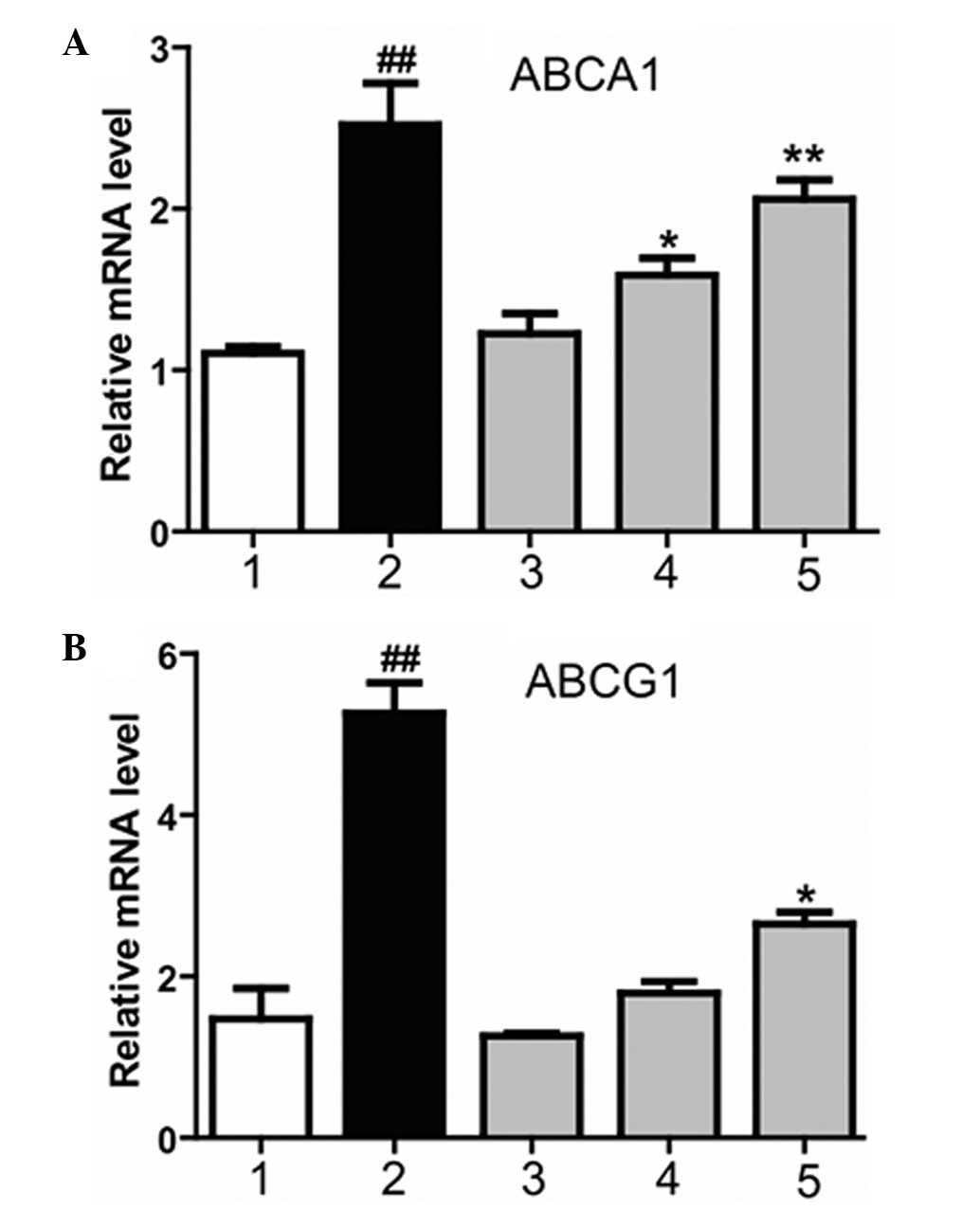 | Figure 3.Effect of magnolol on the mRNA
expression levels of (A) ABCA1 and (B) ABCG1, downstream genes of
the liver X receptor. 1, dimethyl sulfoxide negative control group;
2, T0901317 (2 µM) positive control group; 3, low-dose magnolol
group (10 µM), 4, medium-dose magnolol group (20 µM); 5, high-dose
magnolol group (40 µM). ##P<0.01, vs. negative
control group; **P<0.01 and *P<0.05, vs. negative control
group. ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP
binding cassette transporter G1. |
Discussion
In clinical practice, as a result of good
pharmacological effects, magnolol is widely applied in drugs with
Magnolia officinalis as the major component, such as
Huoxiang Zhengqi Shui, Banxia Houpo Tang, Dachengqi Tang, Tongfusan
and Jiangzhining liver capsules. Numerous studies have investigated
the pharmacological effects of magnolol, of which a number have
been demonstrated, including the inhibition of inflammation to
protect endothelial cells from damage, relieving acute inflammatory
pain, inhibiting cellular mutation by suppressing mutant enzyme
activity, and increasing the combination degree of tranquilizers
and the γ-aminobutyric acid (GABA) receptor by increasing the
expression of the GABA receptor; thus, magnolol ultimately achieves
central sedative and anxiolytic effects (14–16). In
addition, magnolol has been shown to inhibit skin photoaging by
constraining the nuclear transcription factor, nuclear factor-κB
(17). However, there are
comparatively less studies investigating the lipid-lowering
mechanism of magnolol. One of the research areas in AS is the
regulatory systems of LXRα; thus, the present study hypothesized
that magnolol interacts with LXRα to aid lipid-lowering.
In the present study, using a HepG2 cell line,
magnolol was shown to directly combine with the NR, LXRα-LBD, and
increase LXR transcriptional activation. In addition, using a THP-1
cell line, magnolol was demonstrated to regulate the expression of
ABCA1 and ABCG1, which are downstream genes of LXR. Previous
studies have indicated that the expression of ABCA1, ABCG1 and
ABCG5/8, LXR downstream genes, can affect the formation of
high-density lipoprotein (HDL) (18–21),
while the latest treatment method for AS is to increase the level
of HDL cholesterol in the serum (22,23).
Therefore, the results of the present study indicate that magnolol
may promote cholesterol efflux by increasing the expression levels
of ABCA1 and ABCG1, inhibiting the formation of foam cells and
regulating the HDL level, to subsequently ameliorate AS. However,
whether magnolol can ameliorate AS in a high fat-induced model or
AS gene knock-out model requires further investigation.
References
|
1
|
Lozano R, Naghavi M, Foreman K, et al:
Global and regional mortality from 235 causes of death for 20 age
groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vacca M, Degirolamo C, Mariani-Costantini
R, Palasciano G and Moschetta A: Lipid-sensing nuclear receptors in
the pathophysiology and treatment of the metabolic syndrome. Wiley
Interdiscip Rev Syst Biol Med. 3:562–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calkin AC and Tontonoz P: Transcriptional
integration of metabolism by the nuclear sterol-activated receptors
LXR and FXR. Nat Rev Mol Cell Biol. 13:213–224. 2012.PubMed/NCBI
|
|
4
|
Chawla A, Boisvert WA, Lee CH, et al: A
PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in
cholesterol efflux and atherogenesis. Mol Cell. 7:161–171. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Venkateswaran A, Laffitte BA, Joseph SB,
et al: Control of cellular cholesterol efflux by the nuclear
oxysterol receptor LXR alpha. Proc Natl Acad Sci USA.
97:12097–12102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naik SU, Wang X, Da Silva JS, et al:
Pharmacological activation of liver X receptors promotes reverse
cholesterol transport in vivo. Circulation. 113:90–97. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun AL, Feng L and Liu RM: Preparative
isolation and purification of honokiol and magnolol from Magnolia
officinalis Rehd. et Wils by high-speed countercurrent
chromatography. Fen Xi Hua Xue Bian Ji Bu. 33:1016–1018. 2005.[(In
Chinese)].
|
|
8
|
Wang ZQ, Mi W, Liu XB, et al: The in vitro
growth-inhibitory effect of Magnolia officinalis Rehd. et Wils.
(MOR) on bacteria. Shizhen Guo Yi Guo Yao. 18:27632007.[(In
Chinese)].
|
|
9
|
Zhang WW, Li Y, Wang XQ, et al: Effects of
magnolol and honokiol derived from traditional Chinese herbal
remedies on gastrointestinal movement. World J Gastroenterol.
11:4414–4418. 2005.PubMed/NCBI
|
|
10
|
Ren SC, Fan YC and Li CC: Inhibitory
effects of 30 kinds of Chinese herbal medicine on fungi in food.
Adv Mater Res. 343-344:737–742. 2012.
|
|
11
|
Yang QH, Yang HW, Xie F and Zhang YP:
Experimental study of Jiangzhi Ninggan capsules on rat model with
fatty liver disease. Liaoning Zhong Yi Zazhi Bianji Bu.
35:1420–1422. 2008.[(In Chinese)].
|
|
12
|
Kotani H, Tanabe H, Mizukami H, Amagaya S
and Inoue M: A naturally occurring rexinoid, honokiol, can serve as
a regulator of various retinoid x receptor heterodimers. Biol Pharm
Bull. 35:1–9. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Houck KA, Borchert KM, Hepler CD, Thomas
JS, Bramlett KS, Michael LF and Burris TP: T0901317 is a dual
LXR/FXR agonist. Mol Genet Metab. 83:184–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CR, Tan R, Qu WM, et al: Magnolol, a
major bioactive constituent of the bark of Magnolia officinalis,
exerts antiepileptic effects via the GABA/benzodiazepine receptor
complex in mice. Br J Pharmacol. 164:1534–1546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar S, Guru SK, Pathania AS, Kumar A,
Bhushan S and Malik F: Autophagy triggered by magnolol derivative
negatively regulates angiogenesis. Cell Death Dis. 4:e8892013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y and Tang F: Advance in latest
studies on pharmacological effects of magnolol. Zhongguo Zhong Yao
Za Zhi. 37:3526–3530. 2012.[(In Chinese)]. PubMed/NCBI
|
|
17
|
Lee YJ, Lee YM, Lee CK, Jung JK, Han SB
and Hong JT: Therapeutic applications of compounds in the Magnolia
family. Pharmacol Ther. 130:157–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vaughan AM and Oram JF: ABCA1 and ABCG1 or
ABCG4 act sequentially to remove cellular cholesterol and generate
cholesterol-rich HDL. J Lipid Res. 47:2433–2443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grefhorst A, Oosterveer MH, Brufau G,
Boesjes M, Kuipers F and Groen AK: Pharmacological LXR activation
reduces presence of SR-B1 in liver membranes contributing to
LXR-mediated induction of HDL-cholesterol. Atherosclerosis.
222:382–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X and Paigen B: Genetics of variation
in HDL cholesterol in humans and mice. Circ Res. 96:27–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yasuda T, Grillot D, Billheimer JT, Briand
F, Delerive P, Huet S and Rader DJ: Tissue-specific liver X
receptor activation promotes macrophage reverse cholesterol
transport in vivo. Arterioscler Thromb Vasc Biol. 30:781–786. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Linsel-Nitschke P and Tall AR: HDL as a
target in the treatment of atherosclerotic cardiovascular disease.
Nat Rev Drug Discov. 4:193–205. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Degoma EM and Rader DJ: Novel HDL-directed
pharmacotherapeutic strategies. Nat Rev Cardiol. 8:266–277. 2011.
View Article : Google Scholar : PubMed/NCBI
|