Introduction
Aging is associated with the deterioration of a
number of physiological processes, leading to a decline in
functional capabilities, which ultimately impacts the health and
overall function of an organism. Among humans and other mammals,
these deteriorations occur primarily in the immune system, and
result in an increased susceptibility to various conditions,
including chronic inflammation, muscle loss, cancer and
age-associated degenerative disorders (1). Extensive research over the five
previous decades has focused on identifying the underlying
mechanisms of aging. In addition to understanding the mechanisms of
the aging process, one of the principal aims of research into aging
is the identification of intervention strategies or the development
of therapeutics that enhance longevity.
It is widely acknowledged that the limitation of
calorie intake, also known as calorie restriction (CR), may
increase the mean lifespan of an organism by up to 60%, while
reducing the incidence of degenerative disease (2). Although the positive effects of CR on
slowing the aging process and increasing the lifespan have been
demonstrated in a range of species, CR has not been widely adopted
as a preventative strategy due to the difficulty of following such
a strict dietary regime (3).
Therefore, there is a requirement for the identification of small
molecules that mimic the effect of CR without the application of a
strict diet, or that modulate the molecular pathways responsible
for the anti-aging effect produced by CR, in order to slow aging
and increase the lifespan of an organism (3). The molecular targets, sirtuin 1 (SIRT1)
and AMP-activated protein kinase (AMPK), are potential candidates.
SIRT1 is a mammalian ortholog of the yeast protein, silent
information regulator 2, and increased activity levels of SIRT1 by
activators, such as resveratrol, have been observed to extend the
lifespan in a number of species (4–6).
Similarly, AMPK has been recognized as a potential molecular target
for the regulation of longevity (7).
The progression of the aging process has been shown to correlate
with a reduction in the activity of SIRT1 and AMPK. Thus,
pharmacological interventions aimed at regulating SIRT1 and AMPK
may provide effective methods for improving health in aging
patients and extending their lifespan (4,5,7).
Medicinal plants have been used for millennia in
numerous cultures to prevent and treat of a variety of diseases.
However, the active constituents of these medicinal plants and
their precise mechanisms of action are not fully understood.
Humulus japonicus Siebold et Zucc, from the Cannabaceae
family, is an example of such plants. H. japonicus is a
perennial herb that grows commonly as a weed in Korea and China,
where it is also known as ʻJapanese hopʼ. In Western countries,
H. japonicus was previously imported for ornamental
purposes; however, the plant is considered to be an invasive plant
in numerous countries due to its notable survival capacity. In
traditional Chinese medicine, H. japonicus has been used to
treat pneumonia, diarrhea, hypertension, leprosy and tuberculosis.
In Korea, the leaves of H. japonicus have been used in the
treatment of pulmonary tuberculosis, tuberculosis cervical
lymphadenitis and hypertension (8,9). In
addition, previous studies have indicated that the extract of H.
japonicus (HJE) possesses antioxidative, antibacterial,
antimycobacterial, antimutagenic, anti-inflammatory and antitumor
properties (9–14). Over the previous five decades, a
number of the bioactive constituents from H. japonicus have
been identified and reported, including terpenes, lupulones,
phenolics and flavonoids (8,11,15,16).
To the best of our knowledge, the potential of HJE
to extend the lifespan and its effect on the aging process have not
yet been investigated. Thus, the aim of the present study was to
investigate the effect of HJE on lifespan, and to elucidate the
signaling pathways and active constituents involved in lifespan
extension. In addition, the antioxidant capacities of HJE and its
active constituents were evaluated, since reactive oxygen species
(ROS) are a major contributing factor to the aging process.
Materials and methods
Chemicals and reagents
Luteolin, luteolin 7-glucoside, quercetin,
quercitrin and resveratrol were obtained from Sigma-Aldrich (St.
Louis, MO, USA), dissolved in ethanol and stored at −20°C until
required. In addition,
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox),
3-morpholinosydnonimine hydrochloride (SIN-1) and
carboxy-H2DCFDA were obtained from Sigma-Aldrich.
Dulbeccos modified Eagles medium (DMEM), fetal bovine serum (FBS)
and penicillin-streptomycin were purchased from GE Healthcare
(HyClone; Logan, UT, USA). Rabbit polyclonal antibodies against
phospho-AMPKα1/2 (Thr 172; cat. no. sc-33524), SIRT1 (cat. no.
sc-15404) transcription factor IIB (cat. no. sc-225) and mouse
monoclonal β-actin (cat. no. sc-47778), and goat anti-rabbit
IgG-horseradish peroxidase (HRP)-conjugated (cat. no. sc-2004) and
anti-mouse IgG-HRP-conjugated (cat. no. sc-2031) antibodies were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
2,7-Dichlorodihydrofluorescein diacetate (H2DCFDA) was
purchased from Invitrogen Life Technologies (Eugene, OR, USA).
Plant materials and extraction
Fresh leaves of Acanthopanax sessiliflorus,
Rubus crataegifolius (Bunge), Vitis thunbergii var.
sinuata and H. japonicus were collected in Busan
(Korea) and authenticated by Professor JS Choi at Pukyong National
University (Busan, Korea). These plant specimens were deposited in
Professor Hae Chung's laboratory (Pusan National University, Busan,
Korea). The fresh leaves of these plants were dried, chopped into
small pieces and refluxed with absolute ethanol (EtOH). The extract
of each plant was separated from the residues through Whatman No. 1
filter paper (GE Heathcare Life Sciences, Pittsburgh, PA, USA),
then concentrated to dryness to render the EtOH extract. The
extract was subsequently suspended in EtOH and stored at −20°C
until required.
Yeast strain and microbiological
methods
A BY4742 yeast strain (MATα his3Δ1 leu2Δ0 lys2Δ0
ura3Δ0; EUROSCARF, Frankfurt, Germany) was used for chronological
lifespan (CLS) measurements, as described previously (17). Yeast was grown to exponential phase
in rich yeast, peptone, dextrose medium or in synthetic defined
minimal medium (Sigma-Aldrich), both containing 2% glucose, which
were prepared as described by Sherman (18). CLS measurements were performed as
previously described (19).
Inhibition of total ROS
generation
The scavenging activity of the agents under
investigation was assessed using H2DCFDA, a fluorescent
oxidative stress indicator. For the measurement of ROS-scavenging
activity in a cell-free system, H2DCFDA was mixed with
esterase (pH 7.4) and incubated for 20 min at 37°C. The mixture was
then placed on ice in the dark until immediately prior to
measurement. H2DCFDA was hydrolyzed to non-fluorescent
2,7′-dichlorodihydrofluorescein (DCFH) by esterase (Sigma-Aldrich)
and subsequently oxidized to highly fluorescent
2,7-dichlorofluorescein by the ROS, ·O2−
(20). The fluorescence intensity of
the oxidized DCFH was quantified using a GENios fluorescence
microplate reader (Tecan Group Ltd., Männedorf, Switzerland) at
excitation and emission wavelengths of 485 and 530 nm,
respectively. Measurement was performed for 30 min, with or without
the addition of SIN-1 as an ·O2− donor. In
addition, a similar experiment was performed using Trolox as a
positive control to compare for antioxidant capacity.
Cell culture
Human fibroblast Hs27 (CRL-1634) cells were obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in DMEM containing 10% FBS, penicillin (100
U/ml) and streptomycin (100 µg/ml) at 37°C in a humidified
atmosphere of 5% CO2 in air. The fibroblast cells were
plated at 90–95% confluency for all the experiments.
Inhibitory activity on intracellular
ROS generation
Intracellular ROS generation was measured using
carboxy-H2DCFDA, a cell-permeable dye. This compound is
oxidized intracellularly by ROS to form fluorescent DCF. Briefly,
the Hs27 cells were incubated for 24 h in a 96-well plate. After
one day, the medium was replaced with fresh serum-free medium
containing HJE or flavinoids. The cells were pretreated with HJE or
flavonoids for 1 h and were then exposed to ultraviolet B (UVB),
according to designated experimental conditions. UVB irradiation
was carried out using a UV Crosslinker (CL-1000; UVP, LLC, Upland,
CA, USA) at the desired intensity (100 J/m2). Prior to
UVB exposure, the cells were washed with phosphate-buffered saline
(PBS) and resusupended in fresh PBS. Subsequently, the cells were
incubated with 10 µM carboxy-H2DCFDA for 10 min at 37°C,
and washed twice with PBS. Modulations in fluorescence intensity
were measured every 5 min for 30 min using a GENios fluorescence
plate reader, at excitation and emission wavelengths of 485 and 530
nm, respectively.
Cytosolic and nuclear extract
preparations
Cells were washed with ice-cold PBS and harvested. A
buffer containing 10 mM Tris (pH 8.0), 1.5 mM MgCl2, 1
mM DTT, 0.1% Nonidet P-40 and protease inhibitors was used to
extract the cytosolic fractions by centrifugation at 14,000 × g for
15 min at 4°C. Nuclear fractions were extracted from the resulting
pellets using a buffer containing 10 mM Tris (pH 8.0), 50 mM KCl,
100 mM NaCl and protease inhibitors. Aliquots of the cytosolic or
nuclear extracts were boiled in gel loading buffer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for 5 min.
Western blot analysis
In order to determine the expression levels of the
proteins under investigation, cell extracts were prepared and
western blot analysis was conducted. In brief, cell extracts
containing equal quantities of proteins (20 µg) were subjected to
8–10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and
transferred to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were probed with the primary
antibodies (1:1,000 dilutions) overnight at 4°C, followed by the
HRP-conjugated secondary antibodies (1:5,000 dilutions) for 1 h at
room temperature. Signals were detected using an enhanced
chemiluminescence reagent (AbFrontier Co., Ltd., Seoul, Korea).
Statistical analysis
Analysis of variance was used to analyze the
differences between each group, and Dunnetts multiple comparison
test was used to determine the differences between the mean values
of the groups. All statistical analyses were conducted using
GraphPad Prism version 5.02 (GraphPad Software, Inc., San Diego,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
HJE extends the yeast lifespan
Effects of HJE on the lifespan of yeast were
investigated. In traditional Korean medicine, plants with palmate
(hand-shaped) leaves are considered to possess health benefits.
Thus, extracts of A. sessiliflorus, a well-studied medicinal
plant, V. thunbergii var. sinuata and R.
crataegifolius (Bunge) were also examined. To evaluate the
anti-aging effect of the plant extracts, yeast cells were
cultivated with the extracts for 25 days and the CLS was measured
by monitoring the number of colony-forming units (CFUs). The number
of viable yeast cells that were able to reproduce and form colonies
reduced in culture over time, regardless of the presence of plant
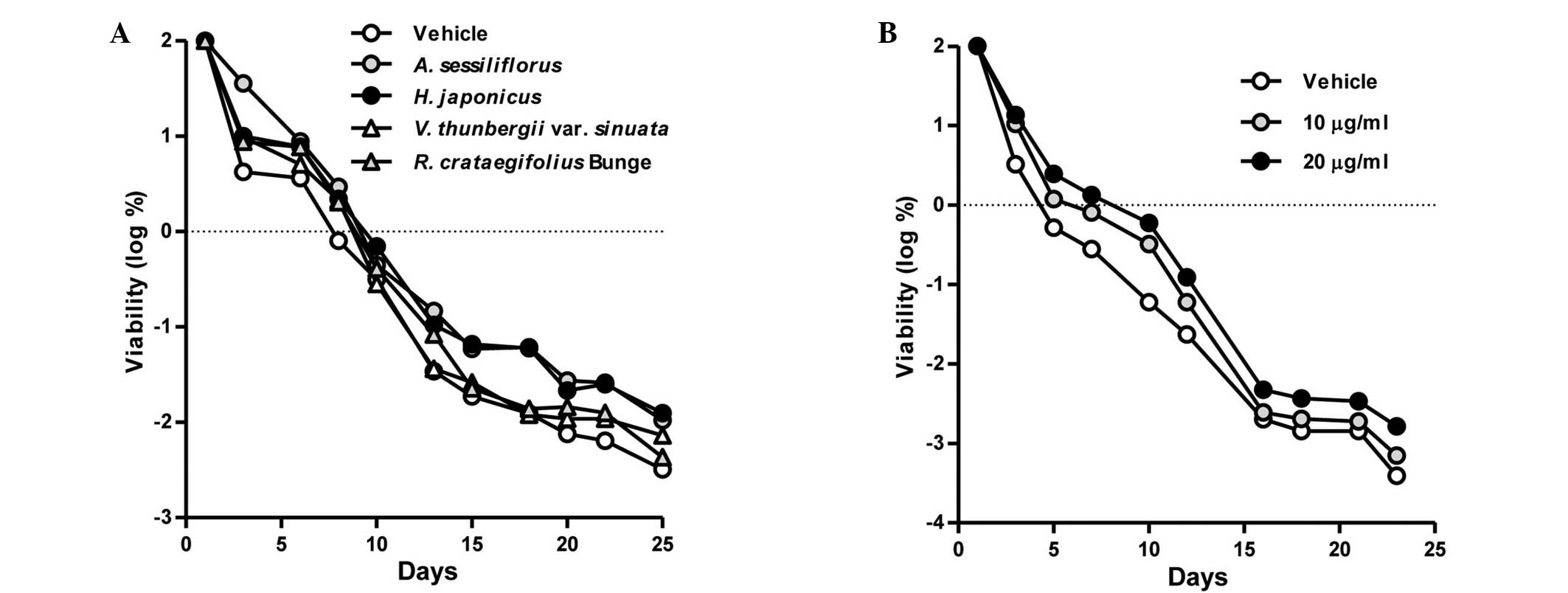
extracts. As presented in Fig. 1A,
the number of CFUs was higher in the HJE-treated cultures when
compared with untreated control cultures between days 10 and 25.
However, cultivation with the extracts from A.
sessiliflorus, V. thunbergii var. sinuata or
R. crataegifolius (Bunge) was shown to have no effect on the
yeast lifespan.
Therefore, the HJE exhibited comparatively notable
lifespan extension, and the effects of different concentrations of
HJE on the yeast lifespan were subsequently examined. The results
indicated that HJE increased the viability of yeast cells in a
concentration-dependent manner (Fig.
1B). Collectively, these results indicated that HJE exerts a
beneficial anti-aging effect.
HJE modulates the expression levels of
AMPK and SIRT1
A number of previous studies have demonstrated that
AMPK and SIRT1 serve important functions in the aging process
(21–23). As HJE was observed to exert a
beneficial effect on the lifespan of yeast, the effect of HJE on
AMPK and SIRT1 expression was subsequently examined. AMPK is the
principal energy sensor in eukaryotic cells and functions to
maintain cellular energy homeostasis and mitochondrial biogenesis
(24). Aging is associated with a
reduction in AMPK-induced mitochondrial biogenesis, and the
activation of AMPK has been observed to increase the lifespan of
fruit flies (25,26). The results of the present study
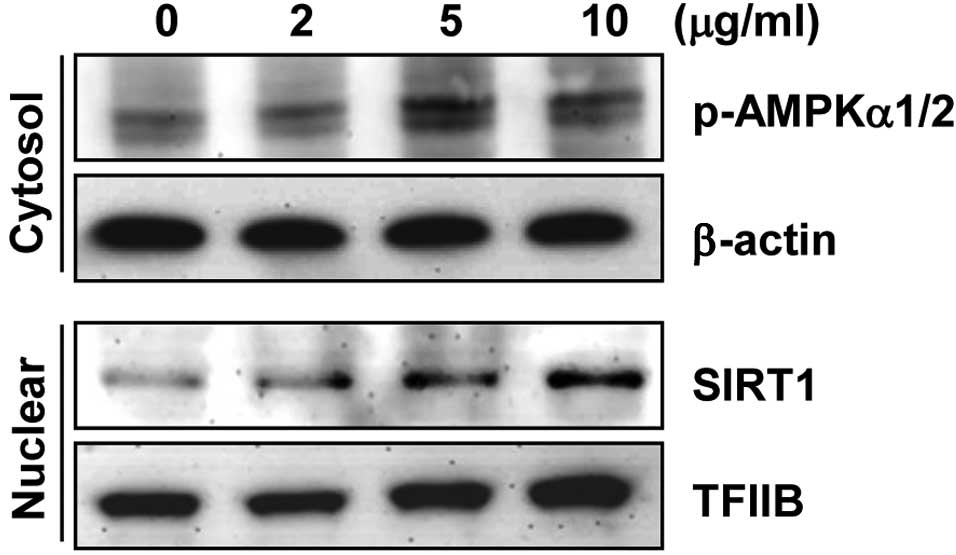
indicated that HJE readily activated AMPK (Fig. 2).
SIRT1 is predominantly located in the nuclei and is
responsible for oxidative stress. In addition, a decrease in
nuclear SIRT1 levels has been previously reported in the hearts of
aged mice (27,28). Therefore, in the present study,
nuclear proteins were employed for the detection of SIRT1. The
results demonstrated that HJE treatment increased nuclear SIRT1
levels in a concentration-dependent manner (Fig. 2), indicating that HJE may extend the
lifespan by modulating the expression levels of AMPK and SIRT1.
However, further mechanistic experiments are required at the
molecular and organism levels.
HJE inhibits ROS generation
The effect of HJE on ROS generation was evaluated to
elucidate the mechanism underlying the HJE-mediated extension of
yeast lifespan. An equivalent concentration of Trolox, a
water-soluble vitamin E analog, was used as a positive control for
comparison with the inhibitory effect of HJE on SIN-1-induced ROS
in a cell-free system. As presented in Fig. 3A, HJE appeared to scavenge the ROS
generated by SIN-1 in a concentration-dependent manner. However,
the HJE-induced inhibition of ROS generation was lower compared
with that of Trolox.
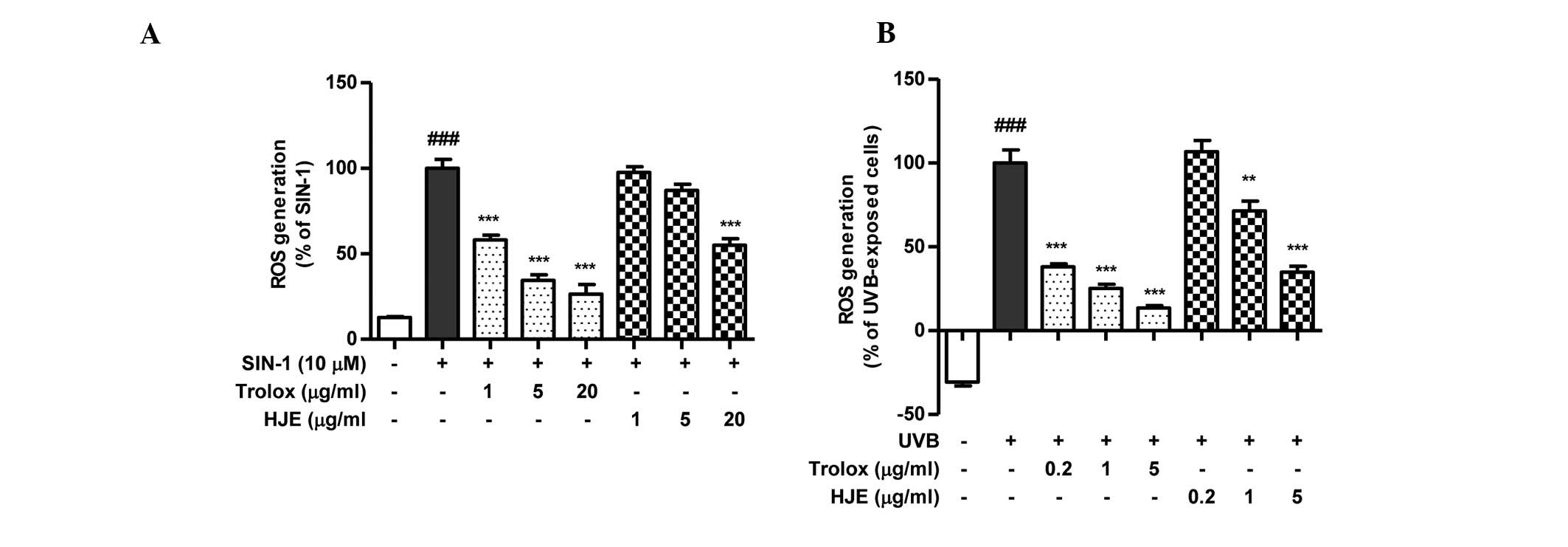 | Figure 3.Effects of HJE on ROS generation. The
inhibitory effect of HJE on ROS production was evaluated using a
2′,7′-dichlorodihydrofluorescein diacetate assay to detect the ROS.
Trolox, a well-known scavenger of ROS, was used as positive
control. (A) Scavenging activity of HJE on ROS generation induced
by 10 µM SIN-1 was measured in vitro.
###P<0.001, vs. untreated control; **P<0.01 and
***P<0.001, vs. 10 µM SIN-1-treated control. (B) Hs27 cells were
pretreated with HJE extract for 1 h and further treated with 10
J/m2 UVB. ###P<0.001, vs. untreated
control; ***P<0.001 vs. UVB-treated control. Results were
analyzed by one-factor analysis of variance and are expressed as
the mean ± standard error. ROS, reactive oxygen species; HJE,
Humulus japonicus extract; SIN-1, 3-morpholinosydnonimine
hydrochloride; Trolox,
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid; UVB,
ultraviolet B. |
As the antioxidative capacity of the testing
molecules may have differed between cell-free and intracellular
systems, the antioxidative effect of HJE was examined in Hs27 human
fibroblast cells. UVB radiation has been reported to induce ROS
generation, resulting in cellular senescence, and the role of SIRT1
in UVB-induced skin aging is well-established (29,30).
Thus, UVB was used to induce ROS generation in the Hs27 skin
fibroblast cells. Pretreatment with HJE (5 µg/ml) resulted in a
significant reduction (34.8%) in the generation of ROS when
compared with the UVB-exposed control cells (Fig. 3B). These results indicated that HJE
effectively scavenged ROS in the cell-free system and
UVB-stimulated fibroblast cells.
HJE-derived flavonoids suppress ROS
generation
H. japonicus is known to contain a number of
flavonoids and phenolics that are responsible for various
biological activities (8,11,12). In
order to investigate whether the antioxidative capacity of HJE is
mediated by the aforementioned active constituents, the effects of
luteolin, luteolin 7-glycoside, quercetin and quercitrin
(structures shown in Fig. 4A) on
oxidative stress were determined. The ROS scavenging activities of
the HJE-derived flavonoids, at concentrations of 0.2, 1 and 5 µM,
on the cell-free system are presented in Fig. 4B. Among the four tested compounds
from HJE, luteolin 7-glucoside exhibited the highest ROS
scavenging capacity.
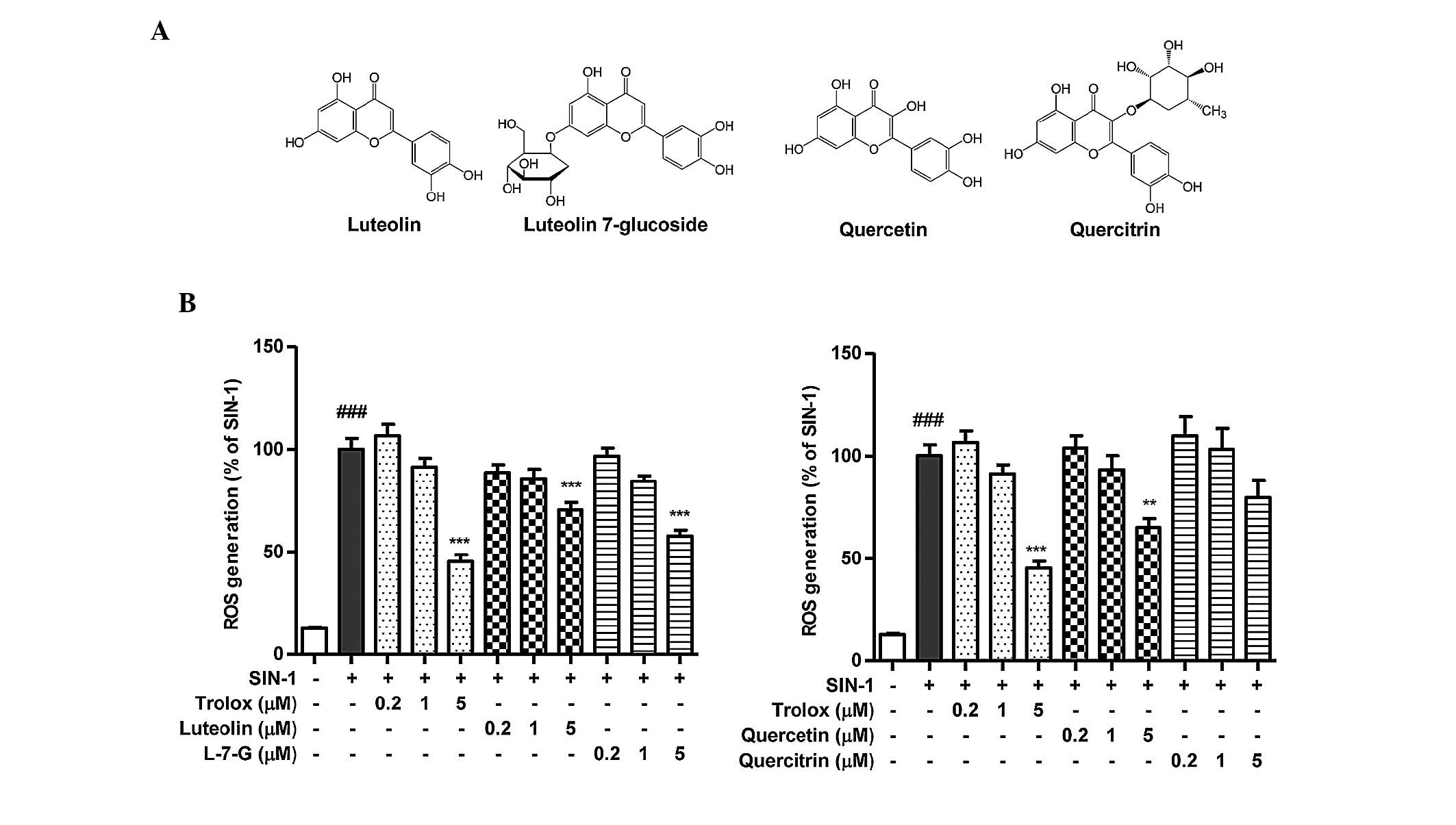 | Figure 4.In vitro ROS scavenging
activity of the active constituents of Humulus japonicus.
The inhibitory effects of the active constituents from H.
japonicus on ROS production were evaluated using a
2′,7′-dichlorodihydrofluorescein diacetate assay. (A) Chemical
structures of the active constituents of H. japonicus. (B)
Scavenging activity of the H. japonicus-derived flavonoids
on ROS generation induced by 10 µM SIN-1 was measured in
vitro. ###P<0.001, vs. untreated control;
**P<0.01 and ***P<0.001, vs. 10 µM SIN-1-treated control.
Results were analyzed by one-factor analysis of variance and are
expressed as the mean ± standard error. ROS, reactive oxygen
species; L-7-G, luteolin 7-glucoside; Trolox,
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid; SIN-1,
3-morpholinosydnonimine hydrochloride. |
Next, the intracellular antioxidative effect of
HJE-derived flavonoids was investigated. UVB radiation was used to
induce ROS generation in order to assess the capacities of the
HJE-derived flavonoids to inhibit intracellular ROS. The scavenging
activities of the active constituents on UVB-induced ROS in
pretreated Hs27 cells are shown in Fig.
5. In particular, luteolin was observed to exert a marked ROS
scavenging effect on intracellular ROS, while luteolin 7-glucoside
was most effective at scavenging ROS in the cell-free system. Thus,
the results indicated clear differences in the antioxidative
capacity among the HJE-derived flavonoids. The rank order was as
follows: Luteolin > luteolin 7-glucoside = quercetin >
quercitrin in the intracellular system. These results clearly
demonstrated that the potent antioxidative properties of HJE may be
mediated by flavonoids.
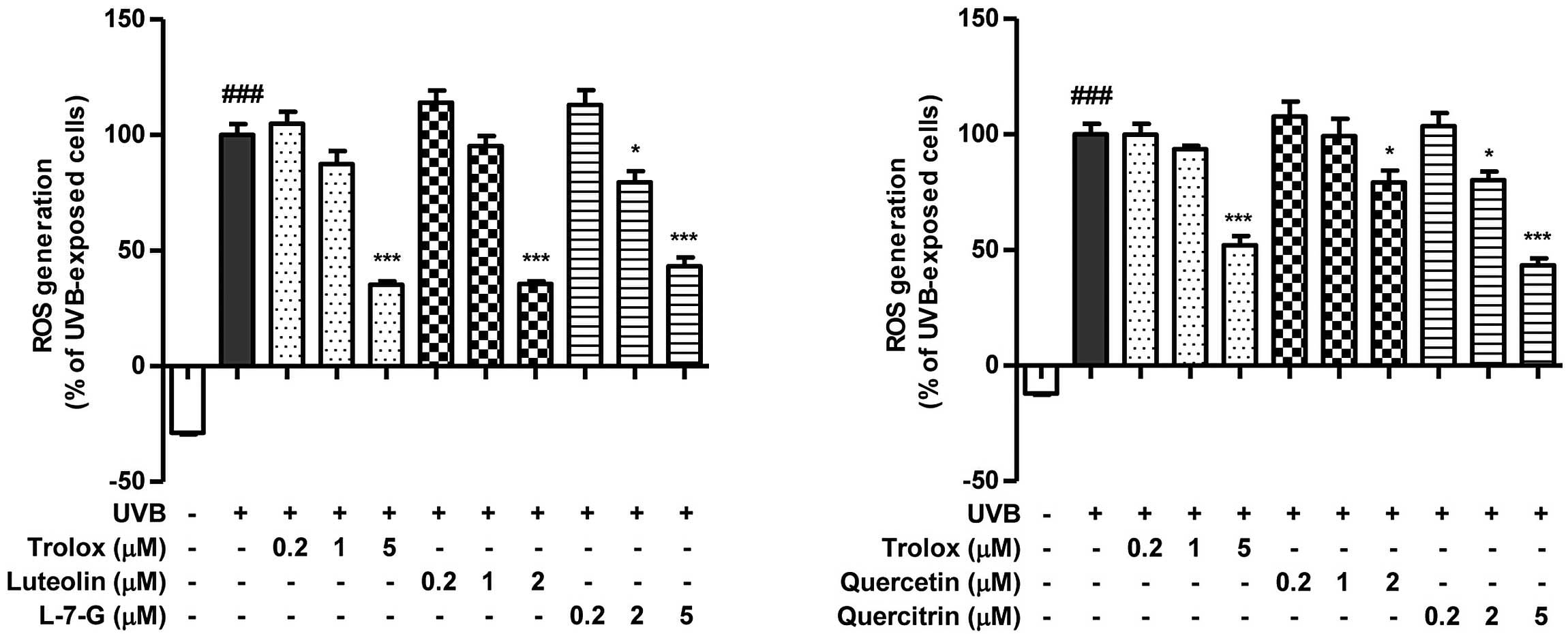 | Figure 5.Effects of active constituents from
Humulus japonicus on UVB-induced ROS generation in Hs27
cells. Cells were treated with the indicated concentration of
Trolox, luteolin, luteolin 7-glucoside, quercetin or quercitrin for
1 h, followed by treatment with 10 J/m2 UVB. ROS
generation was assessed by measuring the fluorescence intensity of
2′,7′-dichlorodihydrofluorescein diacetate following UVB exposure.
Results are expressed as the mean ± standard error.
###P<0.001, vs. untreated control; *P<0.05 and
***P<0.001, vs. UVB-treated control. L-7-G, luteolin
7-glucoside; ROS, reactive oxygen species; UVB, ultraviolet B;
Trolox, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. |
Discussion
The present study aimed to investigate the effects
of HJE on yeast lifespan. Furthermore, the effect of HJE on the
expression levels of SIRT1 and AMPK, which are involved in lifespan
modulation, was determined. In addition, as oxidative stress is a
major contributing factor in the aging process, the antioxidative
capacities of HJE and its active constituents were assessed.
To the best of our knowledge, the present study is
the first to propose that HJE may be able to increase the life span
and delay the detrimental health effects associated with aging.
Previous studies have hypothesized that medicinal plants, such as
Lithospermum erythrorhizon, Panax ginseng, Ginkgo
biloba and Rhodiola rosea, may exert beneficial effects
on cellular senescence and longevity, which are associated with the
aging process (31–34). Although H. japonicus has been
described and used as a traditional remedy in Korea and China,
limited information is available with regard to the underlying
biological activities, active phytochemicals and action mechanisms
of this plant.
In the present study, HJE was observed to activate
AMPK in human fibroblast cells. AMPK has been demonstrated to serve
a key function in the process of aging and the determination of
lifespan (35). Overexpression of
AMPK has been associated with prolonged lifespans in
Caenorhabditis elegans and the Drosophila fruit fly
(26,36). In addition, Greer et al
demonstrated that the presence of AMPK is essential for lifespan
extension by CR in C. elegans via phosphorylation of the
FOXO transcription factor (37).
Notably, AMPK has been reported to phosphorylate FOXO3 in mammalian
cells, indicating that the modulation of FOXO by AMPK may be
conserved among species (38). Thus,
these studies indicate that the activation of AMPK is involved in
the extension of lifespan (36,35–38). The
results of the present study are consistent with those of previous
studies, which have demonstrated that small molecules, such as
chicoric acid and metformin, are able to prolong lifespan in worms
via modulation of AMPK expression (39,40).
Furthermore, previous studies have reported that quercitrin,
quercetin and luteolin, the active constituents of HJE, activate
AMPK, which indicates that these active constituents may contribute
to the effect of HJE on AMPK expression levels (41–43).
An additional possible mechanism for the
life-extending effect of HJE involves SIRT1. The function of
sirtuins in lifespan modulation in yeast was recognized over a
decade ago; however, the capacity of sirtuins to extend lifespans
in other organisms remains controversial. There are seven sirtuin
homologs (SIRT1–7) in mammals, of which SIRT1 is the most
extensively studied. In mammals, the anti-aging mechanism
underlying CR has been shown to involve the activation of SIRT1 in
numerous tissues (44). Thus,
increased expression of SIRT1 in mice results in phenotypes that
resemble the lifespan-extending effects of CR (45). Since SIRT1 performs a key function in
lifespan modulation, the protein has attracted increasing attention
as a potential drug target for delaying the onset of aging and
extending the lifespan. For example, the polyphenol, resveratrol,
which has been identified in red wine and grapes, targets SIRT1 and
exerts a beneficial effect on lifespan (5,6).
Furthermore, reduced levels of SIRT1 have been observed in aged
mouse heart tissue (28). Therefore,
it is possible that the lifespan-extending effect of HJE is
mediated by SIRT1 regulation. However, the effect exerted by HJE on
the lifespans of higher order organisms is yet to be fully
elucidated.
A recognized mechanism underlying the aging process
is the accumulation of oxidative damage; a hypothesis that has been
widely accepted (46). Thus, an
antioxidative effect may result in lifespan extension. For example,
the ability of resveratrol to function as an antioxidant is a
possible alternative mechanism for its lifespan-extending effect,
other than the activation of sirtuins (47). Results from the present study and
previous studies indicate that HJE and its active flavonoid
constituents exhibit antioxidative activity (10,12,13).
Notably, a discrepancy in the antioxidative activity of these
active constituents was observed between cell-free and cell culture
systems. The aglycone forms of the flavonoids (luteolin and
quercetin) exerted more potent ROS scavenging activities in Hs27
cells when compared with their sugar-conjugated forms (luteolin
7-glucoside and quercitrin). The results a previous study
demonstrated that the antioxidant activity of flavonoids is
determined by the position, number and state of the hydroxyl groups
located on the benzene ring, and that the glycosylation of these
hydroxyl groups results in a reduction in antioxidative capacity
(48). Thus, the role of the sugar
moiety in the antioxidative activity of flavonoids remains
controversial, and depends on the type and location of the sugar
group. Although luteolin exerted the strongest ROS scavenging
capacity, quercetin is the most extensively studied molecule among
the active constituents of H. japonicus. Cheng et al
reported that the antioxidative activity of quercetin in C.
elegans was enhanced if the molecule was sugar-conjugated
(49). Furthermore, the authors
proposed that the antioxidant effect may be attributable to the
moiety promoted by the rhamnopyranoside, which is able to
facilitate flavonoid absorption, as described in the animal model
(49,50). However, Comalada et al
reported that the anti-inflammatory effect exerted by quercitrin
appeared to be mediated by the release of quercetin, which was
generated by glycoside cleavage in rat intestinal microbiota
(51). In accordance with these
observations, Jiang et al demonstrated that quercetin is a
major metabolite of quercitrin, and that the production of
quercetin was able to improve the absorption rate and
bioavailability of quercitrin in vivo (52). These results may explain the finding
that aglycone forms of HJE-derived flavonoids exhibit stronger
antioxidative capacities compared with those of the
sugar-conjugated forms in a cell culture system.
In conclusion, the results obtained in the present
study demonstrated that pretreatment with HJE enhanced the lifespan
of yeast. Furthermore, HJE was shown to exert antioxidant
activities in a cell-free system and in human fibroblast cells. In
addition, the active constituents of H. japonicus, namely
luteolin, luteolin 7-glucoside, quercetin and quercitrin, exhibited
antioxidative capacities, with luteolin exerting the most notable
ROS scavenging activity among the tested constituents. Thus, the
results of the present study indicate that HJE may have the
potential to be used as a source for the development of
pharmacological or nutraceutical interventions that delay the aging
process and extend longevity. However, further studies in animals
and humans are required to fully determine the potential of this
medicinal plant for improving human health.
Acknowledgements
The study was supported by grants from the Research
and Development Program of the Ministry of Trade, Industry and
Energy (MOTIE)/Korea Institute for Advancement of Technology (no.
N0000697; Establishment of Infrastructure for Anti Aging Industry
Support) and the Research and Development Program of MOTIE/Korea
Evaluation Institute of Technology (no. 10040391; Development of
Functional Food Materials and Device for Prevention of Aging
Associated Muscle Function Decrease). The authors thank the Aging
Tissue Bank (Busan, Korea) for providing research information.
References
|
1
|
López-Otín C, Blasco MA, Partridge L,
Serrano M and Kroemer G: The hallmarks of aging. Cell.
153:1194–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weindruch R, Walford RL, Fligiel S and
Guthrie D: The retardation of aging in mice by dietary restriction:
longevity, cancer, immunity and lifetime energy intake. J Nutr.
116:641–654. 1986.PubMed/NCBI
|
|
3
|
Mair W and Dillin A: Aging and survival:
the genetics of life span extension by dietary restriction. Annu
Rev Biochem. 77:727–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howitz KT, Bitterman KJ, Cohen HY, et al:
Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valenzano DR, Terzibasi E, Genade T,
Cattaneo A, Domenici L and Cellerino A: Resveratrol prolongs
lifespan and retards the onset of age-related markers in a
short-lived vertebrate. Curr Biol. 16:296–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baur JA, Pearson KJ, Price NL, et al:
Resveratrol improves health and survival of mice on a high-calorie
diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCarty MF: Chronic activation of
AMP-activated kinase as a strategy for slowing aging. Med
Hypotheses. 63:334–339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu BC, Yang MC, Lee KH, Kim KH, Choi SU
and Lee KR: Two new phenolic constituents of Humulus japonicus and
their cytotoxicity test in vitro. Arch Pharm Res. 30:1471–1475.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hong M, Son E, Lee S, et al:
Anti-mycobacterial effects of the extract of Humulus japonicus.
Han'guk Sikp'um Kwahakhoe chi. 46:94–99. 2014.[(In Korean)].
|
|
10
|
Park SW, Woo CJ, Chung SK and Chung KT:
Antimicrobial and antioxidative activities of solvent fraction from
Humulus japonicus. Han'guk Sikp'um Kwahakhoe chi. 26:464–470.
1994.[(In Korean)].
|
|
11
|
Park SW, Kim SH and Chung SK:
Antimutagenic effects and isolation of flavonoids from Humulus
japonicus extract. Han'guk Sikp'um Kwahakhoe chi. 27:897–901.
1995.[(In Korean)].
|
|
12
|
Park SW, Chung SK and Park JC: Active
oxygen scavenging activity of luteolin-7-O-b-D-glucoside isolated
from Humulus japonicus. Han'guk Sikp'um Yŏngyang Kwahakhoe chi.
29:106–110. 2000.[(In Korean)].
|
|
13
|
Lee YR, Kim K, Lee SH, Kim MY, Park HJ and
Jeong HS: Antioxidant and antitumor activities of methanolic
extracts from Humulus japonicus. Han'guk Sikp'um Yŏngyang Hakhoe
chi. 25:357–361. 2012.[(In Korean)].
|
|
14
|
Hwang S, Jung H, Jang W, Jo M, Kim S and
Jee S: Anti-inflammatory effects of the MeOH extract of Humulus
japonicus in vitro. Han'bang An IIbi Inhu P'ibu Kwahakhoe chi.
22:71–91. 2009.[(In Korean)].
|
|
15
|
Aritomi M: Studies on the chemical
constituents in leaves of Humulus japonicus Siebold et Zuccarini.
Yakugaku Zasshi. 82:1331–1332. 1962.[(In Japanese)].
|
|
16
|
Naya Y and Kotake M: The constituents of
Hops. V. The volatile composition of Humulus japonicus Sieb. et
Zucc. Bull Chem Soc Jpn. 43:3594–3596. 1970. View Article : Google Scholar
|
|
17
|
Brachmann CB, Davies A, Cost GJ, et al:
Designer deletion strains derived from Saccharomyces cerevisiae
S288C: a useful set of strains and plasmids for PCR-mediated gene
disruption and other applications. Yeast. 14:115–132. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sherman F: Getting started with yeast.
Methods Enzymol. 350:3–41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alvers AL, Fishwick LK, Wood MS, et al:
Autophagy and amino acid homeostasis are required for chronological
longevity in Saccharomyces cerevisiae. Aging Cell. 8:353–369. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu JM, Lin PH, Yao Q and Chen C: Chemical
and molecular mechanisms of antioxidants: experimental approaches
and model systems. J Cell Mol Med. 14:840–860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Liang Y and Vanhoutte PM: SIRT1
and AMPK in regulating mammalian senescence: a critical review and
a working model. FEBS Lett. 585:986–994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitada M, Kume S, Takeda-Watanabe A, Tsuda
S, Kanasaki K and Koya D: Calorie restriction in overweight males
ameliorates obesity-related metabolic alterations and cellular
adaptations through anti-aging effects, possibly including AMPK and
SIRT1 activation. Biochim Biophys Acta. 1830:4820–4827. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salminen A and Kaarniranta K:
AMP-activated protein kinase (AMPK) controls the aging process via
an integrated signaling network. Ageing Res Rev. 11:230–241. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hardie DG, Ross FA and Hawley SA: AMPK: a
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biol. 13:251–262. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reznick RM, Zong H, Li J, et al:
Aging-associated reductions in AMP-activated protein kinase
activity and mitochondrial biogenesis. Cell Metab. 5:151–156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stenesen D, Suh JM, Seo J, et al:
Adenosine nucleotide biosynthesis and AMPK regulate adult life span
and mediate the longevity benefit of caloric restriction in flies.
Cell Metab. 17:101–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanno M, Kuno A, Yano T, et al: Induction
of manganese superoxide dismutase by nuclear translocation and
activation of SIRT1 promotes cell survival in chronic heart
failure. J Biol Chem. 285:8375–8382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tong C, Morrison A, Mattison S, et al:
Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart
during ischemic stress. FASEB J. 27:4332–4342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chainiaux F, Magalhaes JP, Eliaers F,
Remacle J and Toussaint O: UVB-induced premature senescence of
human diploid skin fibroblasts. Int J Biochem Cell Biol.
34:1331–1339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chung KW, Choi YJ, Park MH, et al:
Molecular insights into SIRT1 protection against UVB-induced skin
fibroblast senescence by suppression of oxidative stress and p53
acetylation. J Gerontol A Biol Sci Med Sci. Aug 27–2014.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoo HG, Lee BH, Kim W, et al: Lithospermum
erythrorhizon extract protects keratinocytes and fibroblasts
against oxidative stress. J Med Food. 17:1189–1196. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang E, Lee TH, Park SY, Yi TH and Kim
SY: Enzyme-modified Panax ginseng inhibits UVB-induced skin aging
through the regulation of procollagen type I and MMP-1 expression.
Food Funct. 5:265–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kampkötter A, Pielarski T, Rohrig R, et
al: The Ginkgo biloba extract EGb761 reduces stress sensitivity,
ROS accumulation and expression of catalase and glutathione
S-transferase 4 in Caenorhabditis elegans. Pharmacol Res.
55:139–147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gospodaryov DV, Yurkevych IS, Jafari M,
Lushchak VI and Lushchak OV: Lifespan extension and delay of
age-related functional decline caused by Rhodiola rosea depends on
dietary macronutrient balance. Longev Healthspan. 2:52013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burkewitz K, Zhang Y and Mair WB: AMPK at
the nexus of energetics and aging. Cell Metab. 20:10–25. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Apfeld J, OConnor G, McDonagh T, DiStefano
PS and Curtis R: The AMP-activated protein kinase AAK-2 links
energy levels and insulin-like signals to lifespan in C. Elegans.
Genes Dev. 18:3004–3009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Greer EL, Dowlatshahi D, Banko MR, et al:
An AMPK-FOXO pathway mediates longevity induced by a novel method
of dietary restriction in C. Elegans. Curr Biol. 17:1646–1656.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Greer EL, Oskoui PR, Banko MR, et al: The
energy sensor AMP-activated protein kinase directly regulates the
mammalian FOXO3 transcription factor. J Biol Chem. 282:30107–30119.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schlernitzauer A, Oiry C, Hamad R, et al:
Chicoric acid is an antioxidant molecule that stimulates AMP kinase
pathway in L6 myotubes and extends lifespan in Caenorhabditis
elegans. PLoS One. 8:e787882013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Onken B and Driscoll M: Metformin induces
a dietary restriction-like state and the oxidative stress response
to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS
One. 5:e87582010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yin Y, Li W, Son YO, et al: Quercitrin
protects skin from UVB-induced oxidative damage. Toxicol Appl
Pharmacol. 269:89–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eid HM, Martineau LC, Saleem A, et al:
Stimulation of AMP-activated protein kinase and enhancement of
basal glucose uptake in muscle cells by quercetin and quercetin
glycosides, active principles of the antidiabetic medicinal plant
Vaccinium vitis-idaea. Mol Nutr Food Res. 54:991–1003. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu JF, Ma Y, Wang Y, Du ZY, Shen JK and
Peng HL: Reduction of lipid accumulation in HepG2 cells by luteolin
is associated with activation of AMPK and mitigation of oxidative
stress. Phytother Res. 25:588–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cohen HY, Miller C, Bitterman KJ, et al:
Calorie restriction promotes mammalian cell survival by inducing
the SIRT1 deacetylase. Science. 305:390–392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bordone L, Cohen D, Robinson A, et al:
SIRT1 transgenic mice show phenotypes resembling calorie
restriction. Aging Cell. 6:759–767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lam YY, Peterson CM and Ravussin E:
Resveratrol vs. calorie restriction: data from rodents to humans.
Exp Gerontol. 48:1018–1024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Amić D, Davidović-Amić D, Beslo D, Rastija
V, Lucić B and Trinajstić N: SAR and QSAR of the antioxidant
activity of flavonoids. Curr Med Chem. 14:827–845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheng SC, Li WH, Shi YC, et al:
Antioxidant activity and delayed aging effects of hot water extract
from Chamaecyparis obtusa var. formosana leaves. J Agric Food Chem.
62:4159–4165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Heim KE, Tagliaferro AR and Bobilya DJ:
Flavonoid antioxidants: chemistry, metabolism and
structure-activity relationships. J Nutr Biochem. 13:572–584. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Comalada M, Camuesco D, Sierra S, et al:
In vivo quercitrin anti-inflammatory effect involves release of
quercetin, which inhibits inflammation through down-regulation of
the NF-kappaB pathway. Eur J Immunol. 35:584–592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiang S, Yang J, Qian D, et al: Rapid
screening and identification of metabolites of quercitrin produced
by the human intestinal bacteria using ultra performance liquid
chromatography/quadrupole-time-of-flight mass spectrometry. Arch
Pharm Res. 37:204–213. 2014. View Article : Google Scholar : PubMed/NCBI
|