Introduction
Diabetic neuropathy (DN) is one of the major
complications associated with diabetes. A previous clinical study
indicated that DN affects 15–25% of type 1 and 30–40% of type 2
diabetic patients (1). The symptoms
of DN include burning sensations, hyperalgesia, allodynia and
dysesthesia, which may substantially impact the patient quality of
life and emotional well-being (2). A
number of disorders have been associated with DN, including
vascular (3), metabolic (4) and neurotrophic abnormalities (5). These dysfunctions may lead to
electrophysiological alterations, abnormal sensory perception and
nerve fiber damage (6). Furthermore,
DN is characterized by neuronal degeneration and modifications in
the activity of nerve growth factor (NGF) and insulin-like growth
factor (IGF) (7).
Oxidative stress has been suggested as a potential
pathophysiological mechanism underlying DN and its involvement in
the development of other diabetic complications (8). Sciatic nerve dysfunction and reduced
endoneurial blood flow have been observed in diabetic rats
following exposure to oxidative stress (9,10).
Furthermore, hyperglycemia has been demonstrated to activate lipid
peroxidation (LPO) and induce the overproduction of reactive oxygen
species (ROS) in the sciatic nerve (11). Thus, diabetes-induced oxidative
stress may also damage the sciatic nerve similar to damage induced
by oxidative stress in other tissues.
Gymnema sylvestre (Gs), from the
Asclepiadaceae family, has been used as a traditional medicine in
southern India, tropical Africa and Australia for almost 2,000
years (12,13). Previous studies have indicated that
Gs may possess certain benefits in improving urination, stomach
ulceration and diabetes (14–16). The
hyperglycemia-induced elevation in urea, uric acid and creatinine
levels and the associated decline in the glomerular filtration rate
were significantly inhibited following the Gs treatment in diabetic
rats (15). Furthermore, previous
studies have indicated that the leaves of Gs contain gymnemic
acids, which are a group of triterpenoid saponins (13,17),
alkaloids, acidic glycosides and anthroquinones (18). Prior studies demonstrated that an
extract from Gs leaves exhibited antioxidative and LPO-inhibiting
activity in an experimental model of gastric ulceration and colitis
(19,20). As the pathogenesis of DN is
characterized by increased levels of ROS and an associated
inflammatory response, we hypothesized that the antioxidative
effect of Gs may function as an anti-inflammatory therapy to
ameliorate nerve tissue damage. Therefore, in the present study,
the antioxidative and anti-inflammatory potential of Gs leaf
extract was investigated in an streptozotocin (STZ)-induced model
of DN in the sciatic nerves of diabetic male Wistar rats.
Materials and methods
Animals
A population of 24 male, 8-week old Wistar albino
rats, weighing 270–290 g, was obtained from the Experimental Rat
Care Center of the College of Pharmacy at King Saud University
(Riyadh, Saudi Arabia). The rats were housed under controlled
conditions (25°C and a 12-h light/dark cycle) and had free access
to water and Purina rat chow (Grain Silos and Flour Mills
Organization, Riyadh, Saudi Arabia). All experimental procedures
and protocols, including anesthesia, were in accordance with the
National Institutes of Health (NIH) Guide for the Care and Use of
Laboratory Animals, Institute for Laboratory Animal Research (NIH
Publications no. 80-23, 1996). In addition, ethical approval (no.
235-EACC-2014) was granted by the Ethical Committee of the
Experimental Animal Care Center of the College of Pharmacy at King
Saud University.
Plant extract
ʻDiagluʼ capsules containing an ethanol extract of
dried Gs leaves were purchased from Mepaco-Medifood (Cairo, Egypt).
The extract was standardized with 25% gymneric acids as the major
constituent, in addition to anthroquinones and their derivatives as
described by the manufacturer. In a previous study, a phytochemical
analysis was conducted in our laboratory (19) and approximately the same amount of
gymneric acids were detected in the extract. The dried powder was
suspended in 0.25% carboxymethyl cellulose solution (Loba Chemie
Pvt., Ltd., Mumbai, India) and administered orally by gavage in
either a low dose (50 mg/kg/day) or high dose (100 mg/kg/day). In a
previous study, a phytochemical analysis of Gs dried ethanolic
leaves extract was conducted and the phytochemical constituents
present in the extract were reported (19).
Diabetes induction
Diabetes was chemically induced by an
intraperitoneal injection of freshly prepared STZ (60 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA) in 0.1 mol/l citrate-buffered
solution (pH 4.5). A group of vehicle control rats were injected
with an equal volume of 0.1 mol/l citrate buffer. Four days
following the STZ injection, diabetes induction was confirmed by
measuring the fasting blood glucose levels in a tail vein blood
sample using an Accu-Chek Compact Plus glucose meter system (Roche
Diagnostics, Meylan, France). Rats with a glucose level of ≥250
mg/dl were classified as diabetic.
Experimental design
Animals were divided into normal control and
diabetic rats and further allocated into four groups (n=6 per
group), which included the control, diabetic, diabetic rats treated
with 50 mg/kg/day Gs (GS50 group) and diabetic rats treated with
100 mg/kg/day Gs (GS100 group). Doses of 50 and 100 mg/kg/day were
selected for this experiment based on previous studies (19,20).
Treatment was initially administered orally by gavage two weeks
following the STZ injection, and was continued for five consecutive
weeks. Subsequent to the treatment, the rats were fasted overnight
then blood samples were collected via cardiac puncture, under
anesthetic (diethyl ether; Sigma-Aldrich). The blood samples were
centrifuged at 1800 × g for 10 min at room temperature (Hettich
EBA-20; Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany) and
the serum was collected and stored at −20°C until required for
further analysis. The sciatic nerves were dissected immediately and
a small cross section from each group was fixed in 10% formaldehyde
solution for histopathological evaluation. The remaining sciatic
nerve tissues were stored at −75°C for biochemical analysis.
Mechanical hyperalgesia
(Randall-Selitto method)
The mean right and left paw pressure thresholds were
determined using an MK-20D paw pressure analgesia meter (Muromachi
Kikai Co. Ltd., Tokyo, Japan). The pressure linear increase rate
was fixed at 10 mmHg/sec and the cut-off pressure was set at 500
mmHg in order to avoid tissue injury. Pressure was applied to the
center of the hind paw, and when the rats displayed pain by
withdrawal of the paw, the applied paw pressure was recorded by the
analgesia meter and expressed in mmHg. Three tests were performed
for each rat, separated by at least 10 min, and the mean value was
recorded.
Tail flick test
Acute nociception was induced using a Socrel model
DS 20 tail flick apparatus (Apelex, Bagneux, France). In brief, the
tail flick latency of the restrained rats was measured by focusing
an intensity-controlled beam of light on the distal 2 cm of the rat
tail. Next, the period required for the rat to remove the tail from
this thermal stimulus was recorded. In order to avoid manual error,
two or three recordings were measured at an interval of 15 min for
each rat, and the mean value was calculated for statistical
analysis.
Serum analysis
Serum levels of glucose and insulin were determined
using commercially available diagnostic kits (Randox Laboratories
Ltd., Crumlin, County Antrim, UK) and the levels of TNF-α, IL-1β
and IL-6 were estimated using enzyme-linked immunosorbent assay
(ELISA) kits (R&D Systems, Inc., Minneapolis, MN, USA).
Estimation of the levels of
thiobarbituric acid reactive substances (TBARS) in the sciatic
nerve
Levels of the LPO product, malondialdehyde (MDA),
were estimated using an assay kit for TBARS (ZeptoMetrix
Corporation, Buffalo, NY, USA) in the sciatic nerve tissues. In
brief, 100 µl tissue homogenate was mixed with 2.5 ml reaction
buffer and heated for 1 h at 95°C. Following cooling, the
absorbance of the supernatant was measured at 532 nm by a
Pharmacia-LKB UVM II spectrophotometer (GE Healthcare Life
Sciences, Marlborough, MA, USA). The LPO products were expressed in
terms of nmol MDA/mg protein.
Estimation of reduced glutathione
(GSH) levels in the sciatic nerve
GSH levels in the sciatic nerve were determined
using the procedure previously described by Sedlak and Lindsay
(21). Briefly, 0.5 ml cold EDTA
tissue homogenate (0.02 mol/l) was added to 0.2 mol/l Tris buffer
(pH 8.2) and 0.1 ml 5,5-dithiobis-(2-nitrobenzoic acid) (0.01
mol/l; BDH Chemicals Ltd., Poole, England). Samples were
centrifuged at 1350 × g for 10 min at room temperature (Hettich
EBA-20). The absorbance of the clear supernatant was measured at
412 nm using a Pharmacia-LKB UVM II spectrophotometer (GE
Healthcare Life Sciences).
Estimation of the activity levels of
superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase
(GPx) and glutathione reductase (GR) in the sciatic nerve
Enzymatic activity levels of SOD in the
post-mitochondrial supernatant of the sciatic nerve homogenate were
measured following the method described by Kono (22). Superoxide anions generate
hydroxylamine hydrochloride by oxidation, mediating the reduction
of nitroblue tetrazolium to form blue formazan, which was
subsequently measured at 560 nm under aerobic conditions. SOD
inhibits the nitroblue tetrazolium reduction, and the extent of
this inhibition was used as a measure of SOD activity and expressed
as U/mg protein. The CAT enzymatic activity was measured according
to the method described by Aebi et al (23). In brief, the post-mitochondrial
supernatant of the sciatic nerve homogenate was mixed with 50
mmol/l phosphate buffer (pH 7.0) and 20 mmol/l
H2O2. The enzymatic activity of CAT was
subsequently determined based on the reduction in absorbance at 240
nm and expressed in U/mg protein. The enzymatic activities of GR
and GPx were determined using commercially available colorimetric
kits (WuXi PharmaTech Cayman, Inc., New York, NY, USA).
Estimation of the levels of IL-1β,
IL-6, TNF-α, NO, NGF and IGF in the sciatic nerve
Levels of IL-1β, IL-6, TNF-α, NGF and IGF in the
sciatic nerve were determined using ELISA kits (R&D Systems,
Inc.), and the results were recorded as pg/mg protein. The NO
concentrations in the sciatic nerve tissues were estimated via the
Griess test, using a commercially available kit (R&D Systems,
Inc.).
Western blot analysis
In order to determine the NGF protein expression
levels, the sciatic nerve tissues were homogenized in a buffer
containing 10 mM HEPES (pH 7.4), 100 mM NaCl, 1 mM
Na3VO4, 10 mM sodium pyrophosphate, 10 mM
NaF, 2 mM EDTA, 1 mM PMSF, 1% Triton X-100, 0.2% SDS and a protease
inhibitor cocktail. The samples were centrifuged at 15,000 × g for
10 min, the supernatants were collected and the protein
concentrations were estimated using a protein assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Protein samples were boiled
in Laemmlis sample buffer (Bio-Rad Laboratories, Inc.) for 5 min,
after which 50 µg protein was separated by 10% SDS-polyacrylamide
gel electrophoresis and transferred onto nitrocellulose membranes
(Bio-Rad Laboratories, Inc.). The membranes were blocked for 90 min
at room temperature with 5% non-fat milk in Tris-buffered saline
containing 0.1% Tween-20 (TBS-T). Next, the membranes were
incubated overnight with a rabbit anti-NGF antibody (1 µg/ml;
#Ab6199, Abcam, Cambridge, UK). Following overnight incubation with
the primary antibody, the membranes were washed three times with
TBS-T (5 min each) and incubated with a respective secondary
horseradish peroxidase-conjugated antibody (1:2,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 90 min at room
temperature. Subsequently, the membranes were washed four times
with TBS-T (5 min each) and the immunoreactivity of the bands was
visualized on a C-DiGit blot scanner (LI-COR Biosciences, Lincoln,
NE, USA) using enhanced chemiluminescence with a western blotting
luminol reagent (1:1; Santa Cruz Biotechnology, Inc.). Protein
bands were quantified by densitometric analysis using Image-Lab
software, version 2.0.1 (Bio-Rad Laboratories, Inc.). For an
internal control, the membranes were washed and incubated with a
monoclonal mouse β-actin antibody (1:2,000; Santa Cruz
Biotechnology, Inc.), according to the aforementioned method.
Histopathological assessment of the
sciatic nerve
Cross sections of the sciatic nerve tissue were
fixed in 10% formaldehyde solution, embedded into paraffin wax
blocks and cut into 5 µm slices using a microtome (American Optical
Rotary Mirotome, Middleton, WI, USA). The samples were stained with
hematoxylin and eosin, mounted and observed microscopically by a
histopathologist in a blinded fashion using a Leica DM5500 B (Leica
Biosystems Melbourne Pty Ltd., Melbourne, Australia). A microscopic
description for all the samples was graded accordingly as mild,
moderate or severe neuropathy for the sciatic nerves.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean, and statistical analysis was conducted using one-way
analysis of variance followed by the Student-Newman-Keuls post hoc
test. P≤0.05 was considered to indicate a statistically significant
difference. All statistical analyses were conducted using GraphPad
Prism 5 and Instat statistical software (GraphPad Software, Inc.,
La Jolla, CA, USA).
Results
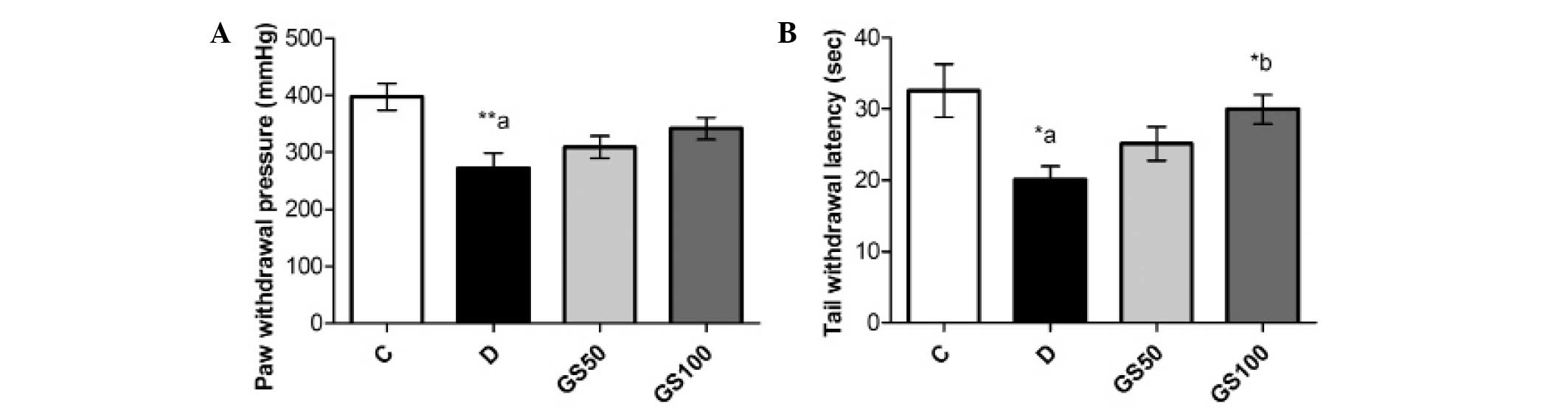
Effects of Gs on mechanical and
thermal pain threshold in diabetic rats
The paw pressure analgesia and tail flick tests
indicated a significant reduction in the pain threshold of the
diabetic rats when compared with the control rats. Administration
of the higher dose of Gs (100 mg/kg/day) to the diabetic rats for
five consecutive weeks resulted in a significant (P<0.05)
improvement in the tail withdrawal latency time (Fig. 1).
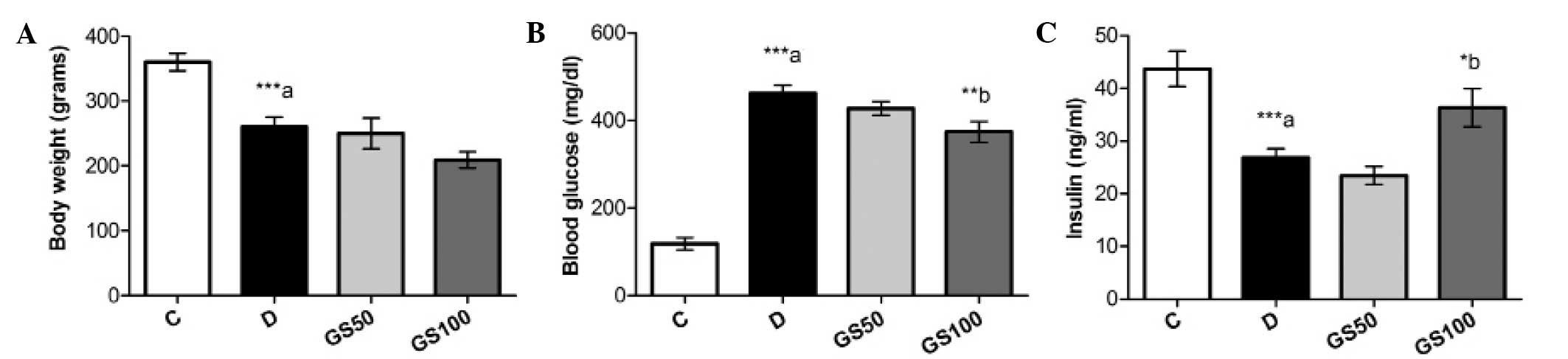
Effects of Gs on body weight, blood
glucose and insulin levels in diabetic rats
The mean body weight of the diabetic rats was
significantly (P<0.001) reduced and this symptom was not
ameliorated following treatment with Gs. However, the increased
blood glucose levels present in the diabetic rats were
significantly (P<0.01) mitigated by treatment with the higher
dose of Gs (100 mg/kg/day). Furthermore, while insulin levels were
markedly (P<0.001) reduced in the diabetic rats, a significant
(P<0.05) increase was observed in the levels of insulin in the
GS100 group rats when compared with the untreated diabetic rats
(Fig. 2).
Effects of Gs on levels of
proinflammatory cytokines in diabetic rats
Serum levels of proinflammatory cytokines, including
TNF-α, IL-1β and IL-6, were significantly (P<0.01) increased in
the diabetic rats, as compared with the control group rats. These
inflammatory mediators were markedly inhibited in the GS100 group
rats when compared with the untreated diabetic rats. However,
treatment with the lower dose of Gs (50 mg/kg/day) resulted in the
significant (P<0.05) inhibition of IL-6 levels only (Fig. 3).
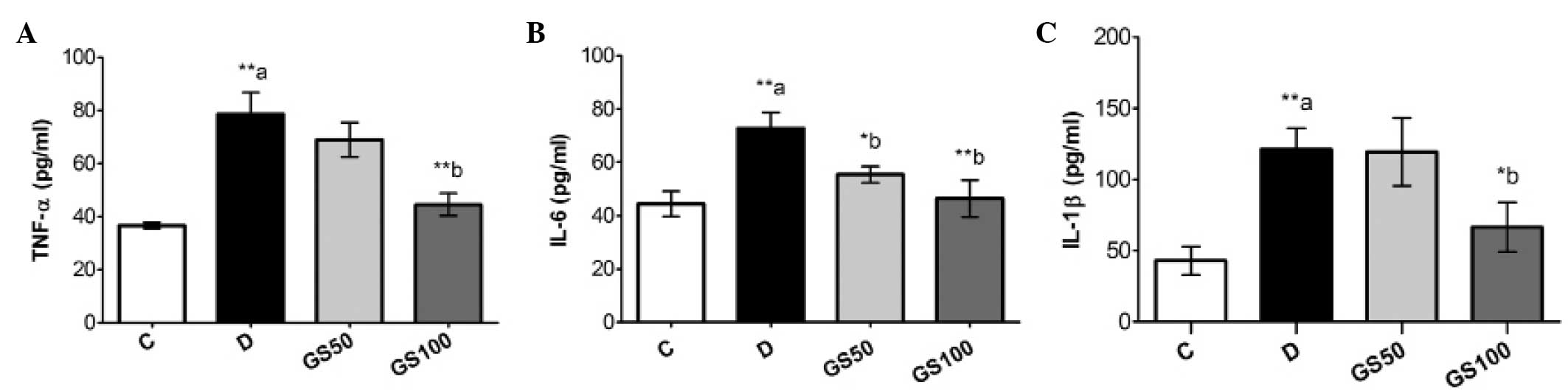 | Figure 3.Effects of Gs leaf extract on the
levels of (A) TNF-α, (B) IL-6 and (C) IL-1β in the serum of
diabetic rats. Data are expressed as the mean ± standard error of
the mean (n=6) and were analyzed using one-way analysis of variance
followed by the Student-Newman-Keuls post hoc test.
**aP<0.01, vs. C group; *bP<0.05 and
**bP<0.01, vs. D group. C, control group; D, diabetic
group; GS50, rats received 50 mg/kg/day Gs extract; GS100, rats
received 100 mg/kg/day Gs extract; TNF, tumor necrosis factor; IL,
interleukin; Gs, Gymnema sylvestre. |
Significant (P<0.01) elevations were observed in
the levels of inflammatory biomarkers, including TNF-α, IL-1β, IL-6
and NO, in the sciatic nerves of the diabetic rats. However, the
GS100 group rats exhibited significantly attenuated elevated levels
of NO, IL-1β (P<0.05), IL-6 and TNF-α (P<0.01) in the sciatic
nerves when compared with the untreated diabetic rats (Fig. 4).
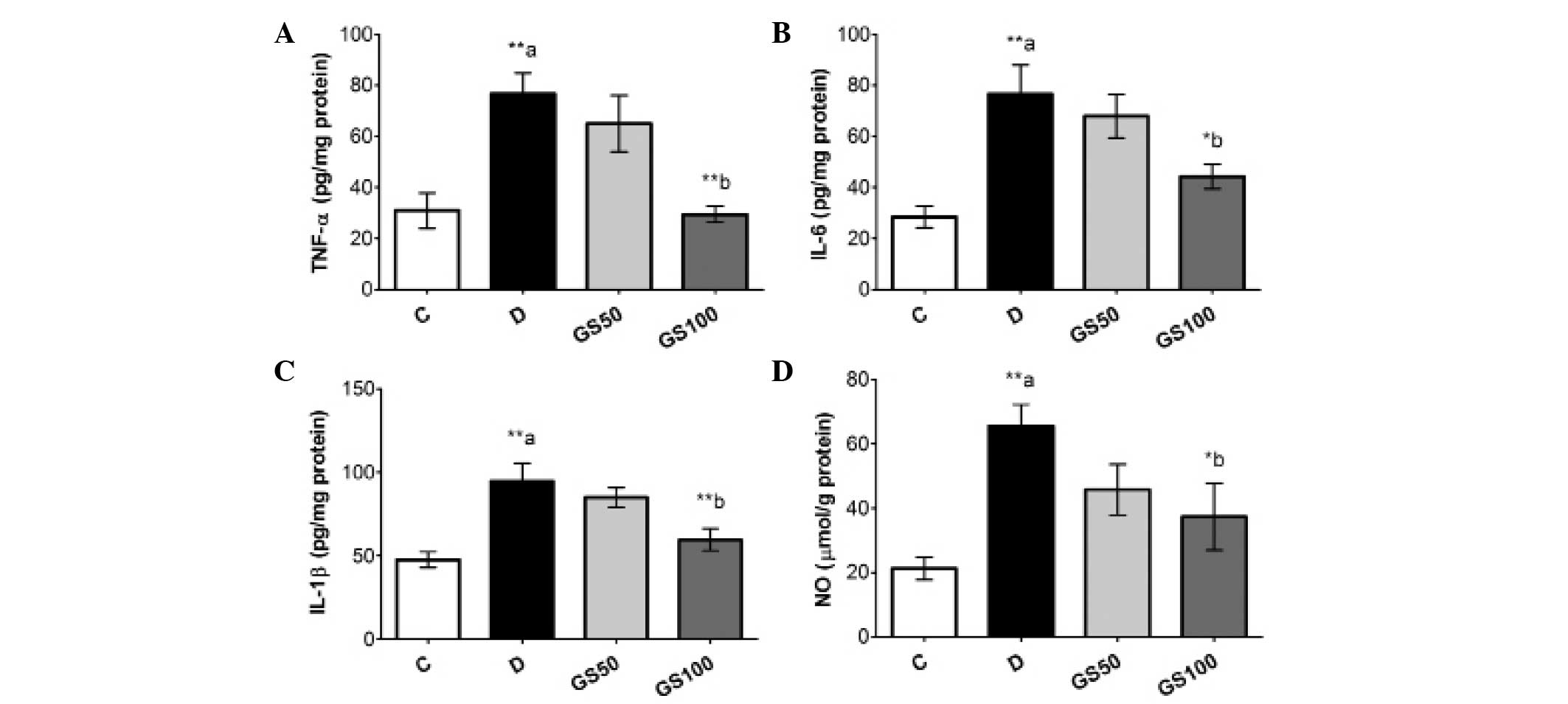 | Figure 4.Effects of Gs leaf extract on the
levels of (A) TNF-α, (B) IL-6, (C) IL-1β and (D) NO in the sciatic
nerves of diabetic rats. Data are expressed as the mean ± standard
error of the mean (n=6) and were analyzed using one-way analysis of
variance followed by the Student-Newman-Keuls post hoc test.
**aP<0.01, vs. C group; *bP<0.05 and
**bP<0.01, vs. D group. C, control group; D, diabetic
group; GS50, rats received 50 mg/kg/day Gs extract; GS100, rats
received 100 mg/kg/day Gs extract; TNF, tumor necrosis factor; IL,
interleukin; NO, nitric oxide; Gs, Gymnema sylvestre. |
Effects of Gs on LPO and oxidative
stress bio-markers in diabetic rats
The induction of diabetes in the rats resulted in
significantly (P<0.001) elevated levels of TBARS in the sciatic
nerve tissue. Furthermore, the diabetic rats exhibited reduced
levels of GSH (P<0.05) and lower enzymatic activity levels of
SOD (P<0.01), CAT (P<0.05), GPx (P<0.01) and GR
(P<0.05) when compared with the control rats. The GS50 and GS100
group rats exhibited significant inhibition of the diabetes-induced
elevation of TBARS in the sciatic nerve, at P<0.05 and
P<0.01, respectively. However, enhanced levels of GSH
(P<0.05), as compared with the untreated diabetic rats, were
observed only in the GS100 group rats. Furthermore, the inhibited
antioxidant enzymatic activity levels of SOD, CAT, GPx and GR in
the sciatic nerve were markedly improved (P<0.05) in the GS100
group rats (Fig. 5).
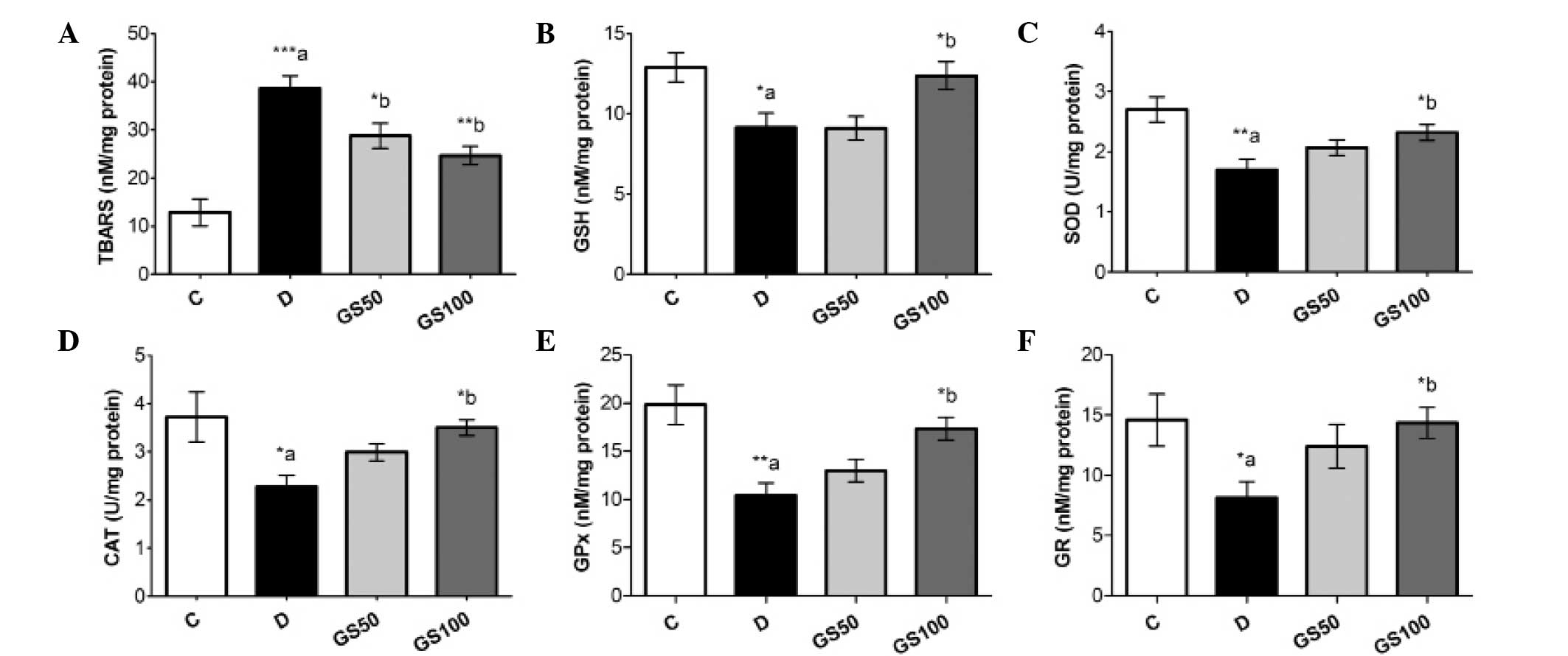 | Figure 5.Effects of Gs leaf extract on the
levels of (A) TBARS and (B) GSH, and the enzymatic activity levels
of (C) SOD, (D) CAT, (E) GPx and (F) GR in the sciatic nerves of
diabetic rats. Data are expressed as the mean ± standard error of
the mean (n=6) and were analyzed using one-way analysis of variance
followed by the Student-Newman-Keuls post hoc test.
*aP<0.05, **aP<0.01 and
***aP<0.001, vs. C group; *bP<0.05 and
**bP<0.01, vs. D group. C, control group; D, diabetic
group; GS50, rats received 50 mg/kg/day Gs extract; GS100, rats
received 100 mg/kg/day Gs extract; TBARS, thiobarbituric acid
reactive substance; GSH, glutathione; SOD, superoxide dismutase;
CAT, catalase; GPx, glutathione peroxidase; GR, glutathione
reductase; Gs, Gymnema sylvestre. |
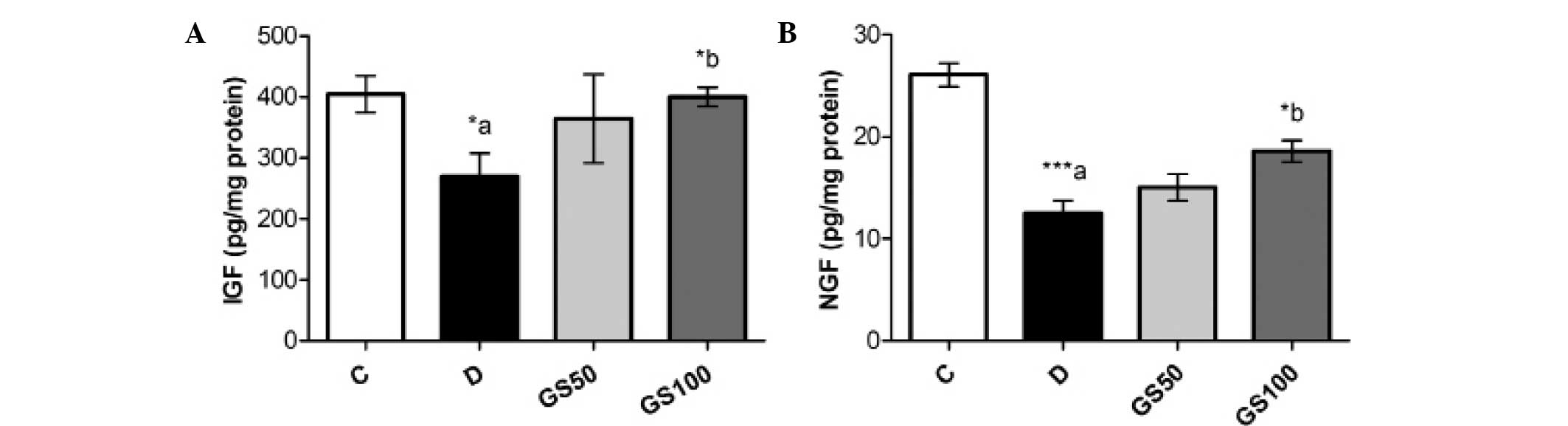
Effects of Gs on sciatic levels of IGF
and NGF in diabetic rats
Sciatic levels of IGF and NGF were significantly
(P<0.05 and P<0.01; respectively) reduced in the diabetic
rats when compared with the control rats. However, the GS100 group
rats exhibited significant (P<0.05) reductions in the levels of
IGF and NGF when compared with the untreated diabetic rats
(Fig. 6).
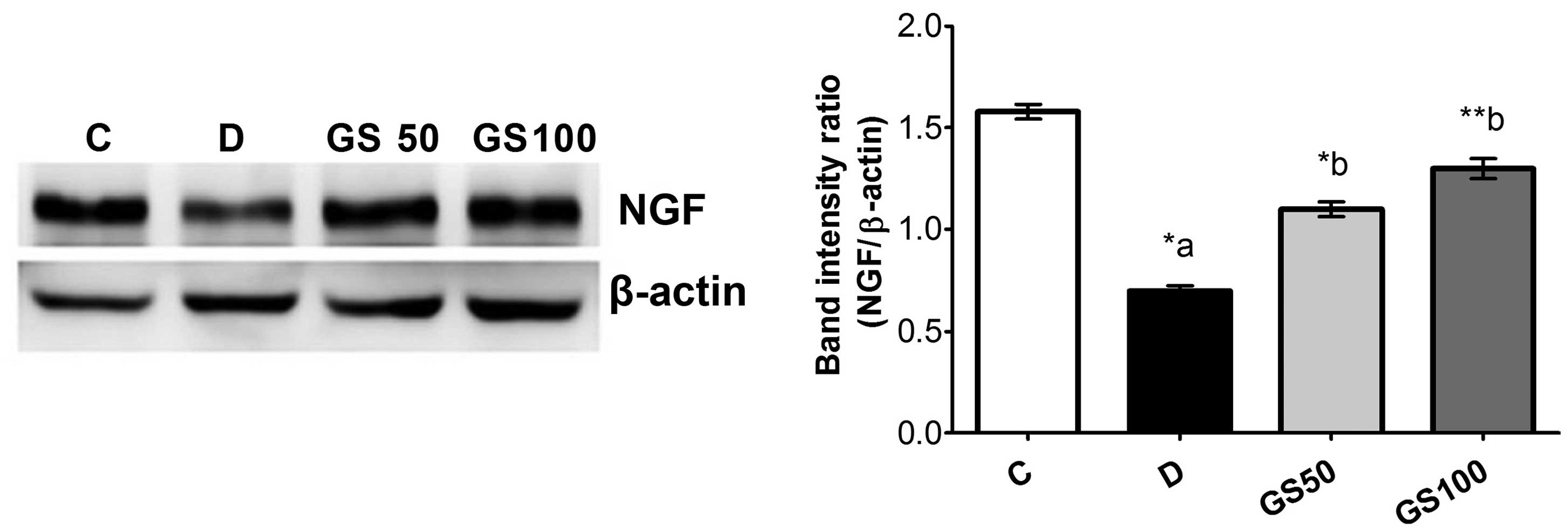
Furthermore, western blot analysis of NGF protein
expression in the sciatic nerve tissue samples revealed a
significant downregulation of NGF levels in the diabetic rats when
compared with the control group. However, NGF levels were
significantly increased in the GS50 and GS100 group rats (P<0.05
and P<0.01, respectively), as compared with the untreated
diabetic rats (Fig. 7).
Effects of Gs on the histological
features of sciatic nerve in diabetic rats
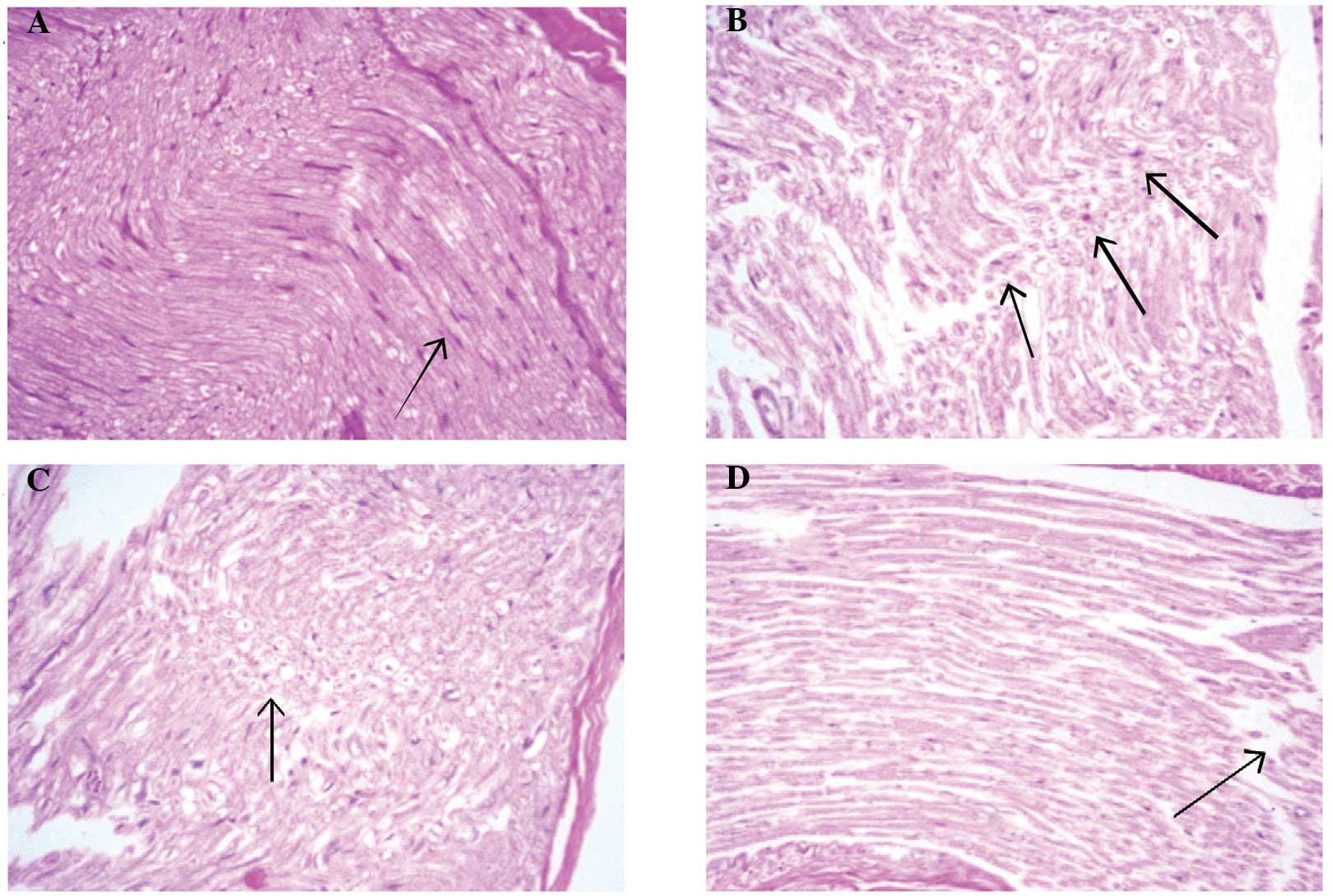
Histological analyses of sciatic nerve sections from
the control group rats revealed near-normal evenly distributed
sciatic nerve axons within the myelin sheath, with no indication of
degeneration, inflammatory infiltration or other abnormalities
(Fig. 8A). Histological sections
from the diabetic group rats exhibited focal peripheral axonal loss
and lipoid degeneration of the axons, with clusters of regenerative
thin myelin axons and few degenerated bodies. Thus, the
histological diagnosis was defined as mild degeneration and
regenerative neuropathy (Fig. 8B).
Sections from the GS50 group rats (Fig.
8C) presented partial focal peripheral axonal loss and
regenerating thin myelinated axons. Similarly to the untreated
diabetic rats, the histological diagnosis was mild degenerative and
regenerative neuropathy. However, histological analysis of the
sections from the GS100 group rats exhibited minimal axonal
degeneration, with no regenerative features. The histological
diagnosis was subsequently indicated as minor degenerative
neuropathy (Fig. 8D).
Discussion
The results of the present study indicate that Gs
exerts an ameliorative effect against experimentally induced DN in
Wistar rats. Gs was observed to induce an attenuation in the
diabetes-induced biochemical and histopathological alterations in
the sciatic nerves of rats. Thus, it was hypothesized that this
alleviation was a result of the capacity of Gs leaf extract to
inhibit STZ-induced hyperglycemia and reduce oxidative and
inflammatory injury of the peripheral nervous system.
DN and associated neuropathic pain are among the
most common complications of diabetes, occurring in ∼50% of
diabetic patients. In the present study, diabetic rats exhibited a
significant delay in paw and tail withdrawal latencies when
compared with the control rats. These observations indicated that
the diabetic rats had developed mechanical and thermal
hyperalgesia, which was consistent with the observations of
previous studies (24,25).
In the current study, a significant reduction in
serum glucose levels and an increase in insulin levels were
observed in the diabetic rats treated with Gs leaf extract (100
mg/kg) for a period of five weeks, as compared with the untreated
diabetic rats. This result is consistent with previous studies that
have indicated that Gs may possess antidiabetic properties
(26,27). Numerous mechanisms have been proposed
to explain the apparent antidiabetic activity of Gs extract.
Yoshioka reported that gymnemic acid, the primary active
constituent of Gs leaf extract, may be responsible for inhibiting
Na+-dependent active glucose transport in the small
intestine (28). An additional study
suggested that the levels of gastric inhibitory peptide may be
suppressed by Gs, producing an antidiabetic effect (29). Furthermore, Sahu et al
(30) reported that gymnemic acid
blocks the receptor in the intestinal absorptive external layers,
similar to oral taste buds, thereby preventing glucose
absorption.
Oxidative stress serves a vital function in the
pathogenesis of DN (9,31,32) and
is known to be induced by hyperglycemia following the autoxidation
of monosaccharides and proteins (33). In the present study, significant
increases in oxidative stress and LPO biomarkers were observed in
the sciatic nerve tissue of diabetic rats. This finding is
consistent with previous studies (11), which reported that hyperglycemia
resulted in significantly elevated levels of TBARS and reduced
levels of GSH in the sciatic nerve. Hyperglycemia is known to
reduce the activity of antioxidative enzymes in diabetic model
animals, which may involve non-enzymatic glycosylation (34). Similarly, the present study
demonstrated an association between DN and oxidative stress through
the inhibition of antioxidant enzymes in the sciatic nerve. Thus,
neural cells in the sciatic nerve may be more vulnerable to damage
as a result of hyperglycemia-induced oxidative stress. Gs treatment
of diabetic animals has been observed to protect the peripheral
neuronal cells from oxidative damage (35). Furthermore, Gs exhibited
antioxidative and LPO-inhibiting properties, as the levels of
endogenous antioxidant molecules and the activities of antioxidant
enzymes were significantly improved in the sciatic nerves of the
diabetic rats. The antidiabetic properties of Gs leaf extract
observed in the present study and previous studies may be
associated with its strong antioxidative properties (36). Furthermore, these antioxidant and
anti-LPO properties have been observed in a number of previous
in vivo and in vitro studies (37–39). Gs
contains a number of bioactive compounds, including oleanane-type
triterpenoid saponins (known as gymnemic acids) (13,17),
alkaloids, acidic glycosides and anthroquinones and their
derivatives (18). Thus, these
groups of molecules may be responsible for the apparent
antidiabetic and antioxidative properties exhibited by Gs extract.
Previous phytochemical analysis further confirmed the presence of
these compounds in the Gs extract (19).
Furthermore, the present study demonstrated an
association between the development of DN and elevated levels of
proinflammatory mediators, with proinflammatory cytokines detected
in the serum and sciatic nerves of the diabetic rats. Increased
levels of inflammatory molecules are considered to be produced as a
result of hyperglycemia and insulin resistance in diabetes
(40,41). In addition, the diabetes-associated
overexpression of inflammatory biomarkers is known to provoke
neural cell dysfunction and death (42,43).
Previous studies have indicated that Gs extract may exert a
protective effect against inflammation (44,45). In
the present study, treatment of diabetic rats with Gs extract
attenuated the elevated levels of cytokines in the serum and
sciatic tissues; however, these effects were more marked in the
GS100 group rats. Recently, Yasukawa et al (46) observed that treatment with Gs extract
suppressed inflammatory tumor promotion in a mouse model of
carcinogenesis. Thus, the anti-inflammatory properties of Gs may
ameliorate oxidative stress by blocking the nuclear factor (NF)-κB
pathway, which subsequently downregulates downstream target genes
of NF-κB, such as inducible nitric oxide synthase (iNOS). iNOS
catalyzes the oxidative stress-mediated production of NO, which is
a vital inflammatory mediator in the pathogenesis of DN. In the
present study, treatment with Gs extract produced a marked
reduction in the STZ-induced elevation of NO levels in the sciatic
tissue. This reduction indicates that Gs may inhibit iNOS activity
or the expression downstream of NF-κB. However, Gs may also have an
inhibitory effect on upstream NF-κB.
Neuronal growth factors, such as NGF and IGF, serve
a crucial function in the survival and maintenance of sympathetic
and sensory nerves. IGF is a neurological growth factor with a
structure similar to that of insulin, and exhibits anabolic effects
that inhibit neural cell apoptosis. Furthermore, IGF regulates DNA
synthesis and thus the growth and proliferation of nerve cells
(47). NGF performs a vital
neuroprotective role with the ability to potentiate axonal growth.
Pathological conditions that alter NGF expression may result in
neuronal dysfunction and death (48). In the present study, levels of IGF
and NGF were markedly reduced in the sciatic nerves of the diabetic
rats, indicating a loss of neural integrity and an increased rate
of nerve cell apoptosis. However, the reduced sciatic tissue levels
of IGF and NGF in the diabetic rats were corrected following
treatment with Gs. Additionally, histological features of sciatic
nerve sections from the Gs-treated groups presented a reduction in
degenerated bodies and a preservation of sciatic nerve
architecture, as compared with the untreated diabetic rats.
In conclusion, the results of the present study
demonstrate the ameliorative properties of Gs extract against
chemically-induced DN in rats via its antihyperglycemic,
antioxidative and anti-inflammatory properties. Furthermore,
morphological examinations indicate that the damage caused to the
sciatic nerve by STZ was markedly reduced following the
administration of Gs. Therefore, Gs extract application may be
useful for the treatment of neuropathy in patients with chronic
diabetes.
Acknowledgements
The authors thank the Deanship of Scientific
Research at King Saud University for funding the study through the
research group project no. RGP-VPP-266.
References
|
1
|
Callaghan BC, Little AA, Feldman EL and
Hughes RA: Enhanced glucose control for preventing and treating
diabetic neuropathy. Cochrane Database Syst Rev.
6:CD0075432012.PubMed/NCBI
|
|
2
|
Galer BS, Gianas A and Jensen MP: Painful
diabetic polyneuropathy: Epidemiology, pain description, and
quality of life. Diabetes Res Clin Pract. 47:123–128. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cameron NE and Cotter MA: Effects of
antioxidants on nerve and vascular dysfunction in experimental
diabetes. Diabetes Res Clin Pract. 45:137–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stevens MJ, Obrosova I, Cao X, Van Huysen
C and Greene DA: Effects of DL-alpha-lipoic acid on peripheral
nerve conduction, blood flow, energy metabolism, and oxidative
stress in experimental diabetic neuropathy. Diabetes. 49:1006–1015.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calcutt NA: Experimental models of painful
diabetic neuropathy. J Neurol Sci. 220:137–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sima AA, Zhang W, Xu G, et al: A
comparison of diabetic polyneuropathy in type II diabetic BBZDR/Wor
rats and in type I diabetic BB/Wor rats. Diabetologia. 43:786–793.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edwards JL, Vincent AM, Cheng HT and
Feldman EL: Diabetic neuropathy: Mechanisms to management.
Pharmacol Ther. 120:1–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ceriello A: Controlling oxidative stress
as a novel molecular approach to protecting the vascular wall in
diabetes. Curr Opin Lipidol. 17:510–518. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Figueroa-Romero C, Sadidi M and Feldman
EL: Mechanisms of disease: The oxidative stress theory of diabetic
neuropathy. Rev Endocr Metab Disord. 9:301–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zherebitskaya E, Akude E, Smith DR and
Fernyhough P: Development of selective axonopathy in adult sensory
neurons isolated from diabetic rats: Role of glucose-induced
oxidative stress. Diabetes. 58:1356–1364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cunha JM, Jolivalt CG, Ramos KM, Gregory
JA, Calcutt NA and Mizisin AP: Elevated lipid peroxidation and DNA
oxidation in nerve from diabetic rats: Effects of aldose reductase
inhibition, insulin, and neurotrophic factors. Metabolism.
57:873–881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeh GY, Eisenberg DM, Kaptchuk TJ and
Phillips RS: Systematic review of herbs and dietary supplements for
glycemic control in diabetes. Diabetes Care. 26:1277–1294. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanetkar P, Singhal R and Kamat M: Gymnema
sylvestre: A Memoir. J Clin Biochem Nutr. 41:77–81. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daisy P, Eliza J and Mohamed Farook KA: A
novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre
possessing normoglycemic and hypolipidemic activity on STZ-induced
diabetic rats. J Ethnopharmacol. 126:339–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramkumar KM, Ponmanickam P,
Velayuthaprabhu S, Archunan G and Rajaguru P: Protective effect of
Gymnema montanum against renal damage in experimental diabetic
rats. Food Chem Toxicol. 47:2516–2521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grover JK, Yadav S and Vats V: Medicinal
plants of India with anti-diabetic potential. J Ethnopharmacol.
81:81–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye WC, Zhang QW, Liu X, Che CT and Zhao
SX: Oleanane saponins from Gymnema sylvestre. Phytochemistry.
53:893–899. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Surveswaran S, Cai YZ, Xing J, Corke H and
Sun M: Antioxidant properties and principal phenolic phytochemicals
of Indian medicinal plants from Asclepiadoideae and Periplocoideae.
Nat Prod Res. 24:206–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Rejaie SS, Abuohashish HM, Ahmed MM,
Aleisa AM and Alkhamees O: Possible biochemical effects following
inhibition of ethanol-induced gastric mucosa damage by Gymnema
sylvestre in male Wistar albino rats. Pharm Biol. 50:1542–1550.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aleisa AM, Al-Rejaie SS, Abuohashish HM,
Ola MS, Parmar MY and Ahmed MM: Pretreatment of Gymnema sylvestre
revealed the protection against acetic acid-induced ulcerative
colitis in rats. BMC Complement Altern Med. 14:492014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sedlak J and Lindsay RH: Estimation of
total, protein-bound, and nonprotein sulfhydryl groups in tissue
with Ellmans reagent. Anal Biochem. 25:192–205. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kono Y: Generation of superoxide radical
during autoxidation of hydroxylamine and an assay for superoxide
dismutase. Arch Biochem Biophys. 186:189–195. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aebi H: CatalaseMethods in Enzymatic
Analysis. Bergmeyer: New York: pp. 674–684. 1974
|
|
24
|
Kamboj SS, Vasishta RK and Sandhir R:
N-acetylcysteine inhibits hyperglycemia-induced oxidative stress
and apoptosis markers in diabetic neuropathy. J Neurochem.
112:77–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al Sharari SD, Al-Rejaie SS, Abuohashish
HM, Aleisa AM, Parmar MY and Ahmed MM: Ameliorative potential of
morin in streptozotocin-induced neuropathic pain in rats. Trop J
Pharm Res. 13:1429–1436. 2014. View Article : Google Scholar
|
|
26
|
El Shafey AAM, El-Ezabi MM, Seliem MME,
Ouda HHM and Ibrahim DS: Effect of Gymnema sylvestre R. Br. leaves
extract on certain physiological parameters of diabetic rats.
Journal of King Saud University – Science. 25:135–141. 2013.
View Article : Google Scholar
|
|
27
|
Verma N, Shakya VK and Saxena RC:
Antidiabetic activity of glycoside isolated from Gymnema sylvestre
in streptozotocin induced diabetic rats. Asian J Chem.
20:5033–5036. 2008.
|
|
28
|
Yoshioka S: Inhibitory effects of gymnemic
acid and an extract from the leaves of Ziziphus jujuba on glucose
absorption in the rat small intestine. J Yonago Med Assoc.
37:142–154. 1986.
|
|
29
|
Fushiki T, Kojima A, Imoto T, Inoue K and
Sugimoto E: An extract of Gymnema sylvestre leaves and purified
gymnemic acid inhibits glucose-stimulated gastric inhibitory
peptide secretion in rats. J Nutr. 122:2367–2373. 1992.PubMed/NCBI
|
|
30
|
Sahu NP, Mahato SB, Sarkar SK and Poddar
G: Triterpenoid saponins from Gymnema sylvestre. Phytochemistry.
41:1181–1185. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bolajoko EB, Mossanda KS, Adeniyi F,
Akinosun O, Fasanmade A and Moropane M: Antioxidant and oxidative
stress status in type 2 diabetes and diabetic foot ulcer. S Afr Med
J. 98:614–617. 2008.PubMed/NCBI
|
|
32
|
Julius U, Drel VR, Grässler J and Obrosova
IG: Nitrosylated proteins in monocytes as a new marker of
oxidative-nitrosative stress in diabetic subjects with
macroangiopathy. Exp Clin Endocrinol Diabetes. 117:72–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonnefont-Rousselot D: Glucose and
reactive oxygen species. Curr Opin Clin Nutr Metab Care. 5:561–568.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Halliwell B: Drug antioxidant effects. A
basis for drug selection? Drugs. 42:569–605. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Preuss HG, Jarrell ST, Scheckenbach R,
Lieberman S and Anderson RA: Comparative effects of chromium,
vanadium and Gymnema sylvestre on sugar-induced blood pressure
elevations in SHR. J Am Coll Nutr. 17:116–123. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang MH, Lee MS, Choi MK, Min KS and
Shibamoto T: Hypoglycemic activity of Gymnema sylvestre extracts on
oxidative stress and antioxidant status in diabetic rats. J Agric
Food Chem. 60:2517–2524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar V, Bhandari U, Tripathi CD and
Khanna G: Evaluation of antiobesity and cardioprotective effect of
Gymnema sylvestre extract in murine model. Indian J Pharmacol.
44:607–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharma K, Singh U, Vats S, Priyadarsini K,
Bhatia A and Kamal R: Evaluation of evidenced-based radioprotective
efficacy of Gymnema sylvestre leaves in mice brain. J Environ
Pathol Toxicol Oncol. 28:311–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rachh PR, Patel SR, Hirpara HV, et al: In
vitro evaluation of antioxidant activity of Gymnema sylvestre R.
BR. leaf extract. Rom J Biol. 54:141–148. 2009.
|
|
40
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Navarro-González JF and Mora-Fernández C:
The role of inflammatory cytokines in diabetic nephropathy. J Am
Soc Nephrol. 19:433–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li J, Wei GH, Huang H, Lan YP, Liu B, Liu
H, Zhang W and Zuo YX: Nerve injury-related autoimmunity activation
leads to chronic inflammation and chronic neuropathic pain.
Anesthesiology. 118:416–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Djordjevic A, Bursać B, Veličković N,
Vasiljević A and Matić G: The impact of different fructose loads on
insulin sensitivity, inflammation, and PSA-NCAM-mediated plasticity
in the hippocampus of fructose-fed male rats. Nutr Neurosci.
18:66–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Diwan PV, Margaret I and Ramakrishna S:
Influence of Gymnema sylvestre on inflammation.
Inflammopharmacology. 3:271–277. 1995. View Article : Google Scholar
|
|
45
|
Jitender KM, Manvi F, Alagawadi K and
Noolvi M: Evaluation of anti-inflammatory activity of Gymnema
sylvestre leaves extract in rats. IJGP. 2:114–115. 2008.
|
|
46
|
Yasukawa K, Okuda S and Nobushi Y:
Inhibitory effects of Gymnema (Gymnema sylvestre) leaves on tumour
promotion in two-stage mouse skin carcinogenesis. Evid Based
Complement Alternat Med. 2014:3286842014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Scarth JP: Modulation of the growth
hormone-insulin-like growth factor (GH-IGF) axis by pharmaceutical,
nutraceutical and environmental xenobiotics: An emerging role for
xenobiotic-metabolizing enzymes and the transcription factors
regulating their expression. A review. Xenobiotica. 36:119–218.
2006. View Article : Google Scholar
|
|
48
|
Freeman RS, Burch RL, Crowder RJ, Lomb DJ,
Schoell MC, Straub JA and Xie L: NGF deprivation-induced gene
expression: After ten years, where do we stand? Prog Brain Res.
146:111–126. 2004. View Article : Google Scholar : PubMed/NCBI
|