Introduction
Bladder cancer is the second most common tumor of
the urogenital system in the USA, with 70% of patients diagnosed
with superficial tumors and 30% presenting with muscle-invasive
disease (1); ∼386,300 new cases of
bladder carcinoma are diagnosed around the world every year,
accounting for almost 150,200 mortalities (2). Currently, although surgical therapies,
such as transurethral electroresection of bladder tumors used
mainly to treat superficial bladder cancer and radical cystectomy
used to muscle-invasive bladder cancer, combined with adjuvant
chemotherapy after surgery have made great progress in the
treatment of bladder cancer, the rate of recurrence remains high
(3). Moreover, chemotherapy has a
high incidence of side-effects. Therefore, the research and
development of highly efficient and minimally toxic new drugs is
sorely required.
The human epidermal growth factor receptor (EGFR)
family comprises four members: EGFR, human EGFR (HER)-2, HER-3 and
HER-4 (4), which play crucial roles
in cell proliferation and survival via activation of the EGFR
signaling network (5). The
overexpression of EGFR and HER-2 is associated with higher EGFR
pathway signaling activity, increased proliferation of cancer cells
and reduced apoptosis (6). Afatinib
is the EGFR family blocker with the highest potential, and is a
highly selective, irreversible inhibitor of EGFR and HER-2
(7). Although preclinical and
clinical studies have indicated that afatinib has antitumor
activity and clinical efficacy in non-small cell lung carcinoma,
head and neck squamous cell carcinoma and breast cancer (8), there are few studies investigating its
inhibitory effect on human bladder carcinoma cells (9–11). Based
on the fact that it has been demonstrated that the overexpression
of EGFR and HER-2 is present in bladder carcinoma (12–14), it
is hypothesized that afatinib may be feasible and effective to use
in the treatment of bladder cancer by targeting both EGFR and
HER-2.
In this study, the inhibitory effects of afatinib
were investigated on the T24 bladder cancer cell line. Whether
afatinib inhibits proliferation and invasion and promotes apoptosis
of the T24 bladder cancer cell line by inhibiting the EGFR
signaling network was also investigated.
Materials and methods
Cell culture
The T24 human bladder cancer cell line was obtained
from Shanghai Institute of Biochemistry and Cellular Biology
Chinese Academy of Sciences (Shanghai, China), and was cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco Life Technologies,
Carlsbad, CA, USA) with 10% fetal bovine serum, 100 U/ml penicillin
and 100 U/ml streptomycin in a humidified atmosphere with 5%
CO2 at 37°C.
MTT assay
The MTT assay was used to estimate the proliferation
of the T24 cells. The cell concentration was adjusted to
5×104 cells/ml and cells were grown in a 96-well plate,
at 190 µl/well. After 24 h incubation at 37°C in a 5%
CO2 incubator, the T24 cells were treated with afatinib
at various concentrations (0, 1, 5, 10 and 20 µmol/l) for 12, 24
and 48 h. At each time-point, 20 µl 5 mg/ml MTT was added to each
well, and cells were incubated for an additional 4 h. Then, the
supernatant was removed, and 150 µl DMSO was added to every well.
The absorbance was measured at 490 nm. Cell survival rate (%) =
(treatment group absorbance/control group absorbance × 100). Each
assay was repeated three times.
Flow cytometric analysis of
apoptosis
Following treatment with various concentrations of
afatinib for 24 h, cells were harvested by trypsinization and
washed with phosphate-buffered saline three times, followed by
resuspending in binding buffer at a concentration of
2×105 cells/ml. Subsequently, 5 µl annexin-V-fluorescein
isothiocyanate (FITC) and 5 µl propidium iodide (PI) were added to
the suspension and the cells were incubated at room temperature in
the dark for 10 min. The apoptotic cells were then measured on a
FACScalibur Flow Cytometer (BD Biosciences, San Jose, CA, USA).
Invasion assay
Cell invasion ability was assessed using a Transwell
chamber (Corning Costar; Corning Incorporated Tewksbury, MA, USA)
with an 8.0-µm pore polycarbonate membrane filter that was
precoated with Matrigel (BD Biosciences) diluted at the ratio of
1:5. Cells treated with various concentrations of afatinib were
harvested and seeded in the upper chamber at a density of
5×105 cells/ml with serum-free DMEM, and the lower
chambers were filled with culture DMEM supplemented with 10% fetal
bovine serum. After reculturing at 37°C in a 5% CO2
atmosphere for 24 h, the Transwell chambers were inverted and
stained with hematoxylin. The cell invasion ability was assessed by
counting the number of cells that had migrated to the lower side of
the membrane. Cells in five visual fields (magnification, ×400)
selected randomly were counted in each Transwell chamber.
Western blot analysis
Cells were harvested after 24 h of treatment with
afatinib at various concentrations (0, 1, 5, 10 and 20 µmol/l).
Proteins from each sample were separated using 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to a nitrocellulose membrane. Then, the proteins were
incubated with primary antibody in blocking buffer, followed by
incubation with secondary antibody, for 1 h each at room
temperature. The primary antibodies and dilutions used were as
follows: Rabbit Bcl-2 monoclonal antibody, rabbit Bax monoclonal
antibody, rabbit phosphorylated (p)-Akt polyclonal antibody, rabbit
t-Akt polyclonal antibody, rabbit p-extracellular-signal-regulated
kinase (ERK) 1/2 monoclonal antibody, rabbit total (t)-ERK1/2
monoclonal antibody, rabbit matrix metalloproteinase (MMP)-2
polyclonal antibody, rabbit MMP-9 polyclonal antibody and rabbit
β-actin polyclonal antibody (all Wuhan Boster Biological
Technology, Ltd., Wuhan, China; 1:1,000). The secondary antibodies
were horseradish peroxidase-conjugated goat anti-rabbit IgG (Wuhan
Boster Biological Technology, Ltd.; 1:10,000). β-actin was examined
on the same membrane and used as a loading control. The relative
levels of the target protein were represented as the optical
density (OD).
Statistical analysis
All data were processed using the Statistical
Package for Social Sciences (SPSS for Windows, version 17.0; SPSS,
Inc., Chicago, IL, USA); monofactorial analysis of variance was
used for analysis. Data are represented as the mean ± standard
deviation. A P-value of <0.05 is considered to indicate a
statistically significant difference.
Results
Afatinib inhibits the proliferation of
T24 cells
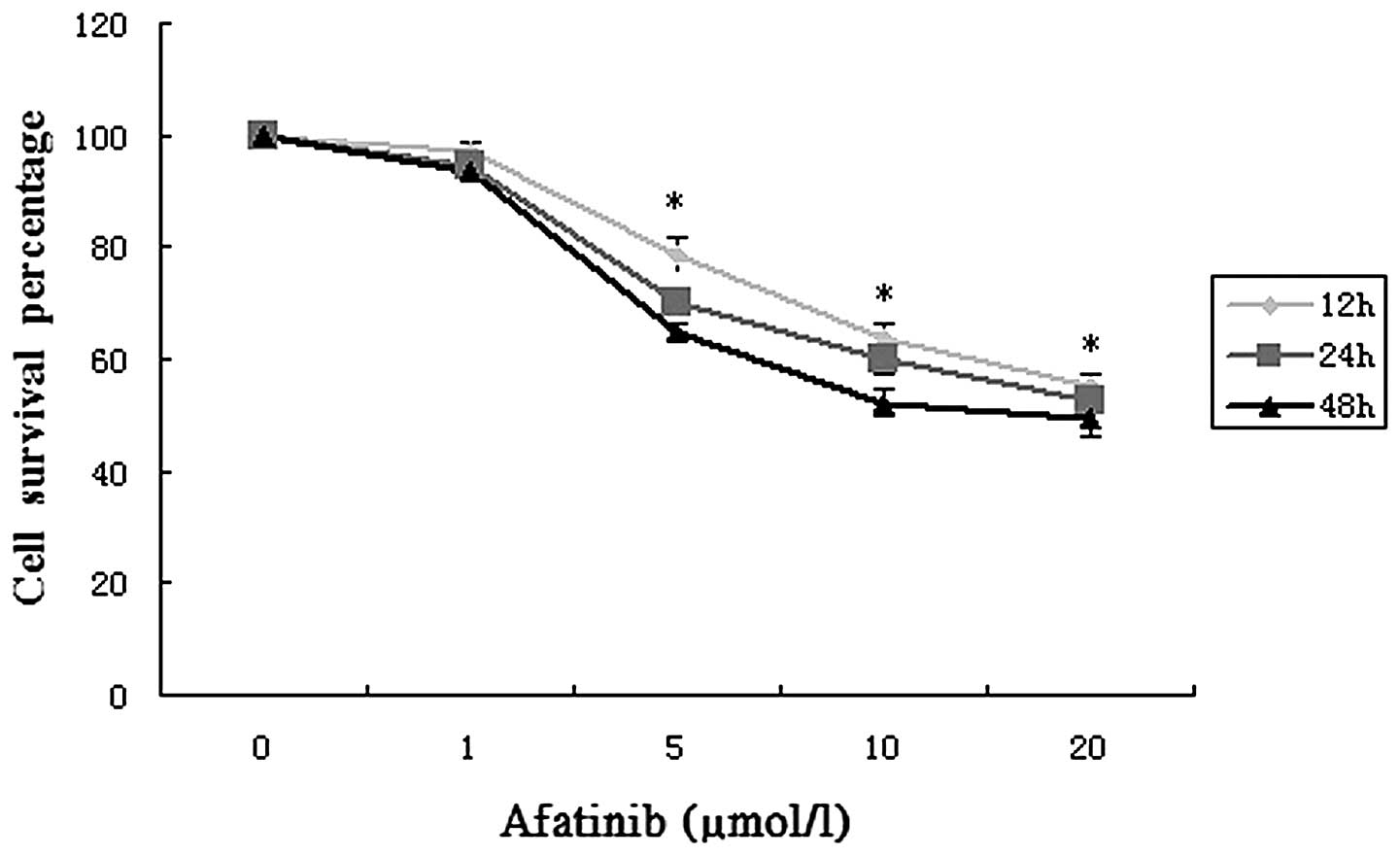
Using an MTT assay, the cytotoxicity of afatinib in
T24 cells was evaluated and is shown in Fig. 1. When the treatment concentration was
1 µmol/l, the viability of the cells changed very little. With
increases in the treatment time and concentration, an evident
reduction in cell viability occurred, particularly at
concentrations of 5–20 µmol/l. These data indicate that afatinib
exerts a significant inhibitory effect on T24 cells and that the
inhibition of cell viability by afatinib was dose- and
time-dependent.
Afatinib induces apoptosis in T24
cells
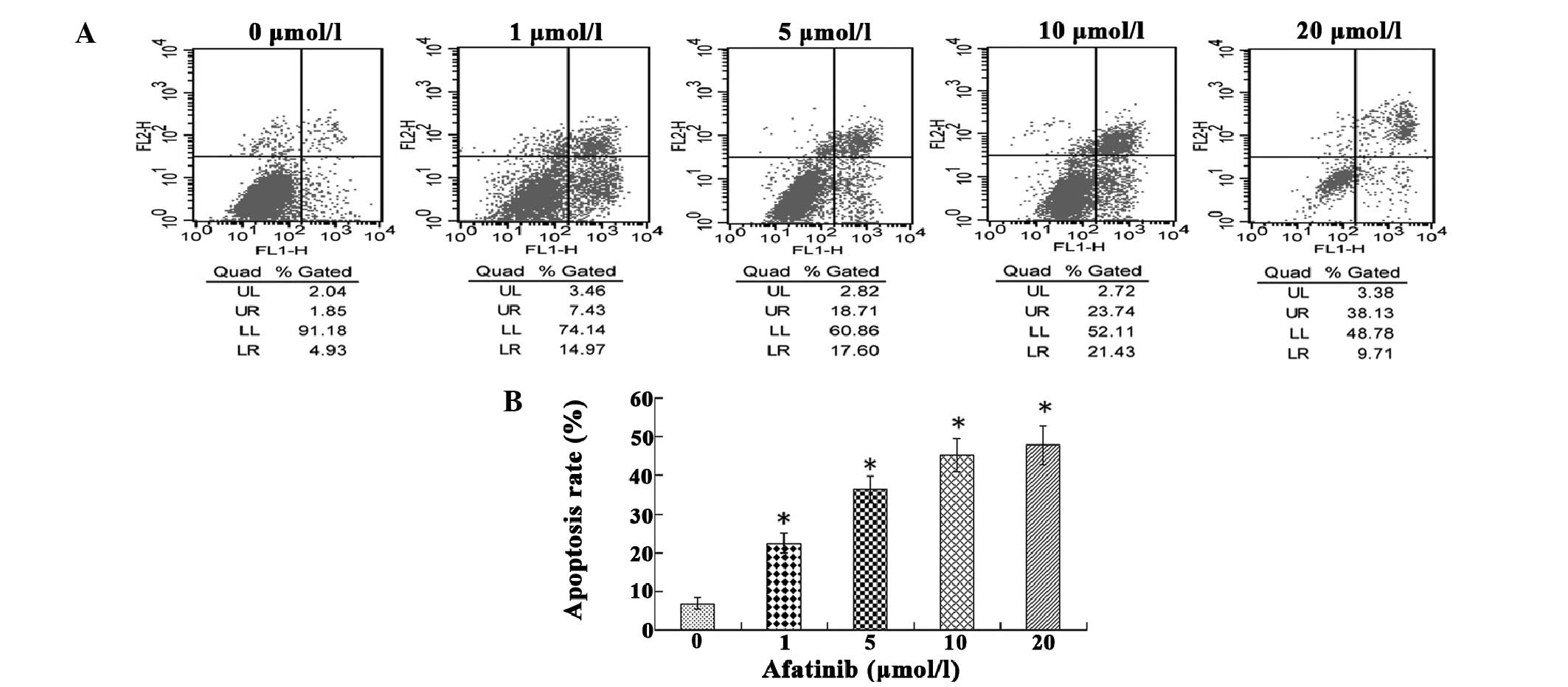
To examine whether afatinib was able to induce
apoptosis in T24 cells, flow cytometry was used to assess the cell
apoptosis rate. As shown in Fig. 2,
when compared with the untreated control, afatinib treatment
resulted in apoptosis. When T24 cells were treated with afatinib
for 24 h, it was observed that increasing the concentration of
afatinib from 1 to 10 µmol/l increased the proportion of early
apoptotic cells from 14.97 to 21.43%, respectively. However,
increasing the concentration of afatinib to 20 µmol/l resulted in a
reduction in the proportion of early apoptotic cells from 21.43 (at
10 µmol/l) to 9.71%. Between 1 and 20 µmol/l, the proportion of
late apoptotic cells increased from 7.43 to 38.13% in a
dose-dependent manner.
Afatinib inhibits the invasiveness of
T24 cells
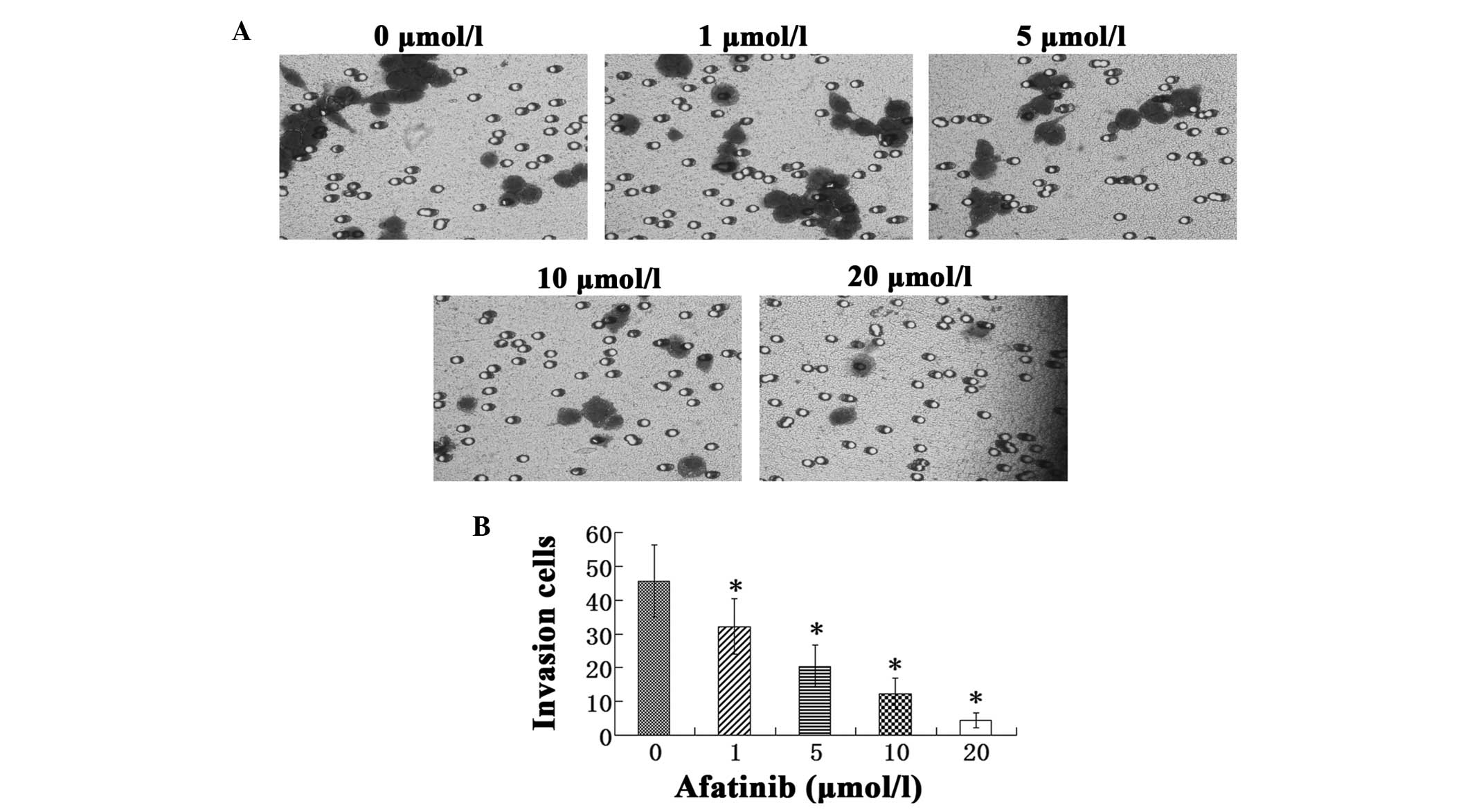
The Transwell cell invasion assay indicated that
afatinib treatment significantly inhibited the invasive behavior of
T24 cells in a dose-dependent manner (P<0.05). The number of
invasive cells in the afatinib-treated groups was observed to be
gradually reduced as the concentration of afatinib was increased
from 1 to 20 µmol/l (Fig. 3).
Effects of afatinib on the expression
of proteins associated with tumor malignancy
To further investigate the probable mechanism of the
afatinib-mediated biological behavior, the levels of Bcl-2, Bax,
Akt, ERK1/2, MMP-2 and MMP-9 proteins were determined by western
blotting. It was observed that with increasing afatinib
concentration, Bcl-2, p-ERK1/2, p-Akt, MMP-2 and MMP-9 expression
levels were significantly decreased, whereas t-ERK1/2 and t-Akt
expression levels remained essentially unchanged, and Bax
expression levels were greatly increased. In addition, the ratio of
Bcl-2/Bax decreased evidently with increasing afatinib
concentration (P<0.05; Fig.
4).
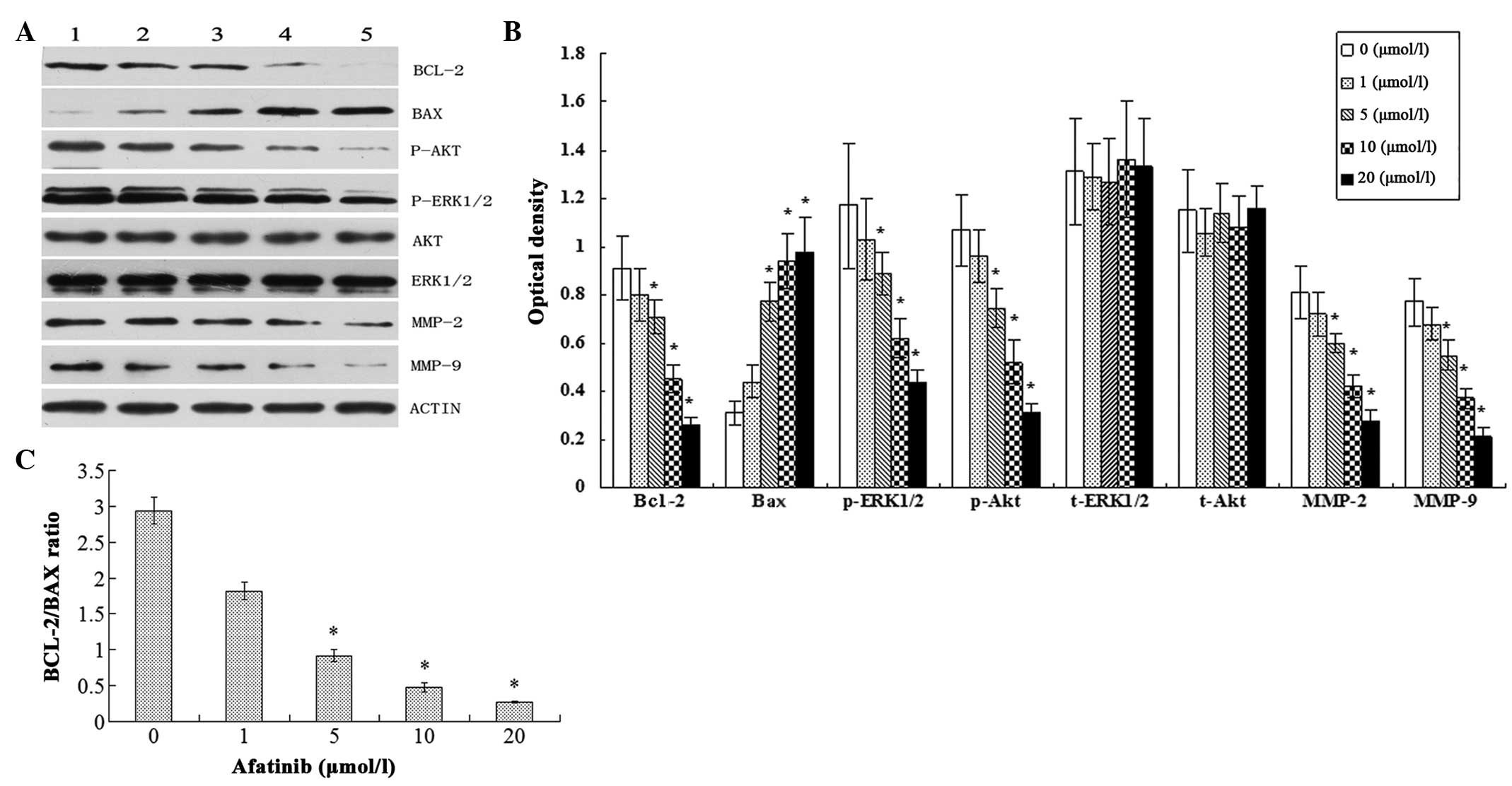 | Figure 4.Expression of proteins in cells
treated with different concentrations of afatinib for 24 h was
analyzed by western blotting. (A) Bcl-2, Bax, Akt, ERK1/2, MMP-2
and MMP-9 expression in T24 cells was measured by immunoblotting.
Lanes 1–5 represent different concentrations of afatinib; lane 1, 0
µmol/l; lane 2, 1 µmol/l; lane 3, 5 µmol/l; lane 4, 10 µmol/l; and
lane 5 20 µmol/l. (B) Exhibits the quantitation of tumor
malignancy-related proteins; the relative protein levels were
normalized to β-actin for comparison. (C) Bcl-2 and Bax expression
levels were normalized to β-actin and are presented as the
Bax/Bcl-2 ratio (mean ± standard deviation). The data shown here
are from a representative experiment repeated three times.
*P<0.05 compared with the control (0 µmol/l afatinib) group.
ERK, extracellular-signal-regulated kinase; MMP, matrix
metalloproteinase. |
Discussion
The present study investigated the effect on bladder
cancer cells of afatinib, which is currently being evaluated for
use in the treatment of various types of cancer. The biological
behavior of afatinib against the T24 bladder cancer cell line was
confirmed to involve the inhibition of proliferation and invasion,
the induction of apoptosis and suppression of the
phosphoinositide-3-kinase (PI3K)/Akt and mitogen-activated protein
kinase (MAPK)/ERK pathways.
Initially, the anti-proliferative effect of afatinib
on T24 human bladder cancer cells was investigated at various
concentrations (0, 1, 5, 10 and 20 µmol/l) and exposure times (12,
24 and 48 h). The results indicated that afatinib exerted a
significant inhibitory effect on T24 cell proliferation, and that
the inhibition of cell viability by afatinib was dose- and
time-dependent.
Apoptosis is an essential physiological process in
the induction of cell death (15).
Inhibition of apoptosis is a characteristic of tumorigenesis. To
confirm whether afatinib promotes T24 cell apoptosis, flow
cytometry was used to assess the cell apoptosis rate, and it was
found that the cell apoptosis rate increased as the concentration
of afatinib increased. The association between afatinib and the
Bcl-2 protein family was further investigated. The Bcl-2 protein
family comprises pro-apoptotic (Bax) and anti-apoptotic (Bcl-2)
proteins that modulate permeabilization of the mitochondrial outer
membrane and caspase activation in the control of apoptosis
(16). The present study indicates
that afatinib treatment upregulates the pro-apoptotic protein Bax
and downregulates the anti-apoptotic protein Bcl-2. In additional,
it has been indicated that the ratio of Bcl-2/Bax protein
expression plays a pivotal role in activating apoptotic signals
(17). Therefore, the ratio of
Bcl-2/Bax was examined; it clearly decreased as the concentration
of afatinib increased, suggesting that the occurrence of T24 cell
apoptosis is associated with the involvement of Bcl-2 family
proteins. These results indicate that afatinib induces apoptosis in
T24 bladder cancer cells.
Metastasis is the most lethal behavior of cancer,
and invasion is a crucial and characteristic process of cancer
metastasis. The present invasion assay demonstrated that afatinib
treatment significantly inhibited the invasive behavior of T24
cells in a dose-dependent manner. The method by which afatinib
inhibits the invasion of T24 bladder cancer cells requires
consideration. The overexpression of MMPs leads to the degradation
of extracellular matrix, which is essential for metastasis
(18). Furthermore, extracellular
matrix degradation as a result of MMP activity contributes to the
migration of bladder cancer cells and their extensive permeation of
the bladder parenchym (19,20). Previous studies have established that
gelatinases (MMP-2 and MMP-9) play an important role in promoting
the invasion and metastasis of cancer cells by degrading diffusely
the basal membrane type IV collagen (21,22).
Higher levels of MMP-2 and MMP-9 have been found in bladder cancer
tissue samples compared with normal bladder samples (23,24).
Kanayama et al (25)
indicated that the mRNA expression of MMP-2 and MMP-9 in muscular
invasive bladder cancers was significantly higher than that in
noninvasive tumors. The results of the present study suggest that
afatinib treatment downregulated the expression of MMP-2 and MMP-9.
Therefore, the present study indicates that afatinib is able to
inhibit the invasion of T24 bladder cancer cells.
The PI3K/Akt (lipid kinase PI3K) and MAPK/ERK
pathways, two important intracellular mediators of the EGFR
signaling network, play an important role in the transmission of
cell signals to the cell nucleus, where they control the expression
of genes that regulate cell migration, proliferation,
differentiation, apoptosis and cell invasion (26). It has been well established that
aberration of the EGFR network plays a crucial role in the
development of cancers, including non-small cell lung cancer,
breast cancer and head and neck squamous cell carcinoma (27). Hyperactivation of EGFR pathway
signaling leads to the overexpression of EGFR/HER-2, which
increases the proliferation of cancer cells and reduced apoptosis
(6). Akt, a serine-threonine kinase
that is a major effector of PI3K, regulating cancer cellular
growth, apoptosis and proliferation (28). Akt is widely activated in many
cancers including bladder cancer (29). Data from the present study
demonstrated that afatinib treatment resulted in significant
inhibition of p-Akt in the T24 bladder cancer cell line. As Akt is
a downstream target of PI3K, the observed inhibition of Akt
phosphorylation indicated that afatinib treatment could lead to
downregulation of the PI3K/Akt signaling pathway. The MAPK/ERK
signaling pathway is a receptor tyrosine kinase mediated signaling
pathway that regulates numerous biological processes such as
angiogenesis, survival, proliferation, migration and the cell cycle
by impacting the downstream activity of ERK (30–32).
Constitutive activation of the MAPK/ERK signaling pathway leads to
aberrant cellular proliferation, repressive apoptosis and the
development of drug resistance (32,33). The
observations of the present study demonstrated that afatinib
treatment resulted in significant inhibition of p-ERK1/2 in the T24
bladder cancer cell line, while no significant difference existed
in t-ERK1/2 levels. As ERK1/2 is a downstream target of the
MAPK/ERK signaling pathway, the observed inhibition of ERK1/2
phosphorylation indicated that afatinib treatment inhibited the
MAPK/ERK signaling pathway. In this study, the reason why the
PI3K/Akt and MAPK/ERK pathways are inhibited by afatinib treatment
is not clear. It is hypothesized that this may be attributed to
afatinib being a highly selective, irreversible inhibitor of EGFR
and HER-2. In a future study, the correlation between afatinib and
EGFR/HER-2 expression levels in bladder cancer should be
investigated. Based on the above analysis, the results of the
present study suggest that afatinib may inhibit proliferation and
invasion of the T24 bladder cancer cell line and promote its
apoptosis by inhibiting the PI3K/Akt and MAPK/ERK signaling
pathways. Additional studies are required to further explore and
elucidate the mechanism by which afatinib affects intracellular
signal transduction in the T24 bladder cancer cell line.
In conclusion, the findings of the present study
demonstrate that afatinib can inhibit the proliferation and
invasion of T24 cells and induce their apoptosis by inhibiting the
EGFR signaling network. However, further studies of the specific
molecular mechanism involved in the afatinib-induced anticancer
activity in bladder cancer are required.
References
|
1
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwak C, Ku JH, Park JY, Lee E, Lee SE and
Lee C: Initial tumor stage and grade as a predictive factor for
recurrence in patients with stage T1 grade 3 bladder cancer. J
Urol. 171:149–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Berezov A, Wang Q, et al: ErbB
receptors: from oncogenes to targeted cancer therapies. J Clin
Invest. 117:2051–2058. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li D, Ambrogio L, Shimamura T, et al:
BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in
preclinical lung cancer models. Oncogene. 27:4702–4711. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harbeck N, Solca F and Gauler TC:
Preclinical and clinical development of afatinib: a focus on breast
cancer and squamous cell carcinoma of the head and neck. Future
Oncol. 10:21–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai YC, Yeh CH, Tzen KY, Ho PY, Tuan TF,
Pu YS, Cheng AL and Cheng JC: Targeting epidermal growth factor
receptor/human epidermal growth factor receptor 2 signalling
pathway by a dual receptor tyrosine kinase inhibitor afatinib for
radiosensitisation in murine bladder carcinoma. Eur J Cancer.
49:1458–1466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quesnelle KM and Grandis JR: Dual kinase
inhibition of EGFR and HER2 overcomes resistance to cetuximab in a
novel in vivo model of acquired cetuximab resistance. Clinical
cancer research. 17:5935–5944. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greulich H, Kaplan B, Mertins P, Chen TH,
Tanaka KE, Yun CH, Zhang X, Lee SH, Cho J, Ambrogio L, et al:
Functional analysis of receptor tyrosine kinase mutations in lung
cancer identifies oncogenic extracellular domain mutations of
ERBB2. Proc Natl Acad Sci USA. 109:14476–14481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cardillo MR, Castagna G, Memeo L, De
Bernardinis E and Di Silverio F: Epidermal growth factor receptor,
MUC-1 and MUC-2 in bladder cancer. J Exp Clin Cancer Res.
19:225–233. 2000.PubMed/NCBI
|
|
13
|
Caner V, Turk NS, Duzcan F, et al: No
strong association between HER-2/neu protein overexpression and
gene amplification in high-grade invasive urothelial carcinomas.
Pathol Oncol Res. 14:261–266. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kiyoshima K, Oda Y, Kinukawa N, Naito S
and Tsuneyoshi M: Overexpression of laminin-5 gamma2 chain and its
prognostic significance in urothelial carcinoma of urinary bladder:
association with expression of cyclooxygenase 2, epidermal growth
factor receptor [corrected] and human epidermal growth factor
receptor [corrected] 2. Hum Pathol. 36:522–530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee DH, Ha JH, Kim Y, et al: A conserved
mechanism for binding of p53 DNA-binding domain and anti-apoptotic
Bcl-2 family proteins. Mol Cells. 37:264–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi YD, Cho NH, Ahn HS, Cho KS, Cho SY
and Yang WJ: Matrix metalloproteinase expression in the recurrence
of superficial low grade bladder transitional cell carcinoma. J
Urol. 177:1174–1178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Urinary gelatinase activities (matrix metalloproteinases
2 and 9) in human bladder tumors. Oncol Rep. 15:1321–1326.
2006.PubMed/NCBI
|
|
21
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanayama H, Yokota K, Kurokawa Y, Murakami
Y, Nishitani M and Kagawa S: Prognostic values of matrix
metalloproteinase-2 and tissue inhibitor of metalloproteinase-2
expression in bladder cancer. Cancer. 82:1359–1366. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davies B, Waxman J, Wasan H, et al: Levels
of matrix metalloproteases in bladder cancer correlate with tumor
grade and invasion. Cancer Res. 53:5365–5369. 1993.PubMed/NCBI
|
|
25
|
Kanayama H: Matrix metalloproteinases and
bladder cancer. J Med Invest. 48:31–43. 2001.PubMed/NCBI
|
|
26
|
Brzezianska E and Pastuszak-Lewandoska D:
A minireview: the role of MAPK/ERK and PI3K/Akt pathways in thyroid
follicular cell-derived neoplasm. Front Biosci (Landmark Ed).
16:422–439. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37 (Suppl 4):S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engelman JA: Targeting PI3K signalling in
cancer: opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu X, Obata T, Khan Q, Highshaw RA, De
Vere White R and Sweeney C: The phosphatidylinositol-3 kinase
pathway regulates bladder cancer cell invasion. BJU Int.
93:143–150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akinleye A, Furqan M, Mukhi N, Ravella P
and Liu D: MEK and the inhibitors: From bench to bedside. J Hematol
Oncol. 6:272013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Friday BB and Adjei AA: Advances in
targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase
cascade with MEK inhibitors for cancer therapy. Clin Cancer Res.
14:342–346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McCubrey JA, Steelman LS, Chappell WH, et
al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how
mutations can result in therapy resistance and how to overcome
resistance. Oncotarget. 3:1068–1111. 2012.PubMed/NCBI
|