Introduction
Prosthetic joint infection (PJI) occurs in 1–12% of
surgical cases and is one of the most common complications
associated with total hip arthroplasty (THA) and total knee
arthroplasty (TKA), often leading to revision. PJI has been
reported to be the most common cause of early failure and is
associated with several adverse outcomes (1,2).
Infection recurrence following re-implantation is associated with
significant morbidity (3,4).
Although various techniques can be used for the
diagnosis of PJI, including preoperative laboratory tests,
radiological examination, nuclear medicine detection,
intraoperative culture and histopathology (5–8), no
gold-standard test for the diagnosis of PJI has been established,
and the limited sensitivity and specificity of the tests that are
available make the differentiation between PJI and other causes of
prosthetic failure, such as metal allergy or aseptic loosening,
challenging (2,9). PJI continues to cause difficulties for
orthopedic surgeons, particularly when the clinical signs and
regular serum inflammatory markers are not fully indicative of
infection (10).
With no single serological test available, doctors
evaluate and diagnose PJI predominantly through a combination of
symptom evaluation, physical examination and results of joint
aspirates, even tissue samples (11). Gram staining (GS) is a widely used
test that is easily available. GS is also commonly used in the
diagnosis of PJI, particularly in developing countries, as a result
of its low cost, rapid turnaround time and ease of use; however,
its value remains controversial due to conflicting results on the
effectiveness of GS in the diagnosis of PJI (5,12–28). The
present meta-analysis was therefore performed as one of a series of
meta-analyses (6,7,29) in
order to evaluate the detection validity of GS for the diagnosis of
PJI and to provide evidence-based advice to clinicians.
Materials and methods
The protocol used in the present meta-analysis was
conducted as described in our previous studies (6,7,29) and based on recommendations in the
methodological guidelines for conducting systematic reviews
studying diagnostic accuracy (30)
and the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses statement (31).
Search strategy
Searches of the MEDLINE, EMBASE and OVID databases
were conducted for articles published between January 1990 and
December 2013. All searches were performed using the medical
subject headings ‘joint prosthesis’, ‘prosthesis infection’,
‘septic loosening’, ‘aseptic loosening’, ‘replacement’ and
‘arthroplasty’ and the free text words ‘gram’, ‘stain’,
‘intraoperative’ and ‘synovial fluid’. The reference lists of
eligible studies and review articles were also examined.
Selection of studies
The abstracts of the studies were read by two
investigators, and a standardized data extraction form was used to
identify potentially eligible articles. Subsequent full-text
analysis determined whether the studies were eligible for
inclusion. Disagreements were resolved by discussion with a third
investigator.
The inclusion criteria for the analysis were as
follows: i) Collection of data on GS in combination with an
accurate diagnosis of PJI based on visible purulence in the joint
aspirate or at the surgical site, evidence of communication between
the prosthesis and a sinus tract (fistula), histopathological or
periprosthetic tissue findings of acute inflammation, or
microbiological cultures simultaneously obtained from at least two
periprosthetic tissue samples (the reference standard); ii) studies
with sufficient data to enable the true-positive (TP),
false-negative (FN), false-positive (FP) and true-negative (TN)
values to be determined; and iii) inclusion of ≥10 patients.
Discrepancies were resolved through discussion with the other
investigators and consultation of the original articles.
Data extraction and assessment of
study quality
Relevant data regarding the study designs and
results were extracted independently by two investigators using a
standardized form. Blinding to the journal name, the authors' names
and affiliations and the year of the study publication was not
performed, as a previous study has shown such a step to be
unnecessary (32). Disagreements
between the reviewers were resolved through consultation with
another reviewer, who evaluated all discrepancies. The opinion of
the majority was used for the analysis. The methodological quality
of the studies included in the meta-analysis was independently
assessed by two reviewers using the Quality Assessment of
Diagnostic Accuracy Studies (QUADAS) tool (33), which has been specifically developed
for use in systematic reviews assessing diagnostic accuracy.
Validity analyses were performed through the use of
a standardized form to extract the following items from each study:
A description of the study participants, the authors' names,
country in which the study was performed, number of patients and
infected patients, mean age, study design, patient enrolment,
sample type, surgical site, type of blinding conducted and
characteristics of the reference standard used.
Statistical analysis
For each study, a two-by-two contingency table,
consisting of TP, FP, FN and TN results according to the GS values
and the reference standard, was constructed. The sensitivity
[TP/(TP + FN)], specificity [TN/(FP + TN)] and the diagnostic odds
ratios (DORs) [(TP × TN)/(FP × FN)] were calculated. To evaluate
the efficacy of GS assays in the diagnosis of PJI, the sensitivity,
specificity, positive likelihood ratio (PLR), negative likelihood
ratio (NLR), DOR, post-test probability and area under the receiver
operating characteristic curve (AUC) were calculated (34).
Heterogeneity was evaluated using the likelihood
ratio I2 index and χ2 tests (35). The I2 index demonstrates
the percentage of total variation across the studies as a result of
heterogeneity. An I2 value of >50% indicates that
there is more heterogeneity among the studies than would be
expected solely by chance. For the likelihood ratio χ2
test, heterogeneity among studies was shown by P-values of
<0.05. When heterogeneity was observed, the primary
meta-analysis was conducted using a random effects model to
generate a summary estimate for the test sensitivity with 95%
confidence intervals (CI). Meta-regression analyses were performed
to evaluate potential heterogeneity, and a Deeks' funnel plot
asymmetry test was utilized to assess potential publication bias
(36). The different study
characteristics, including infected arthroplasties, specimens
processed, publication year, reference standard, study design,
patient enrolment and type of blinding performed, were evaluated
through subgroup analyses. All the statistical analyses were
performed using STATA version 12 software (StataCorp, College
Station, TX, USA). P<0.05 was considered to be significant.
Results
Study selection
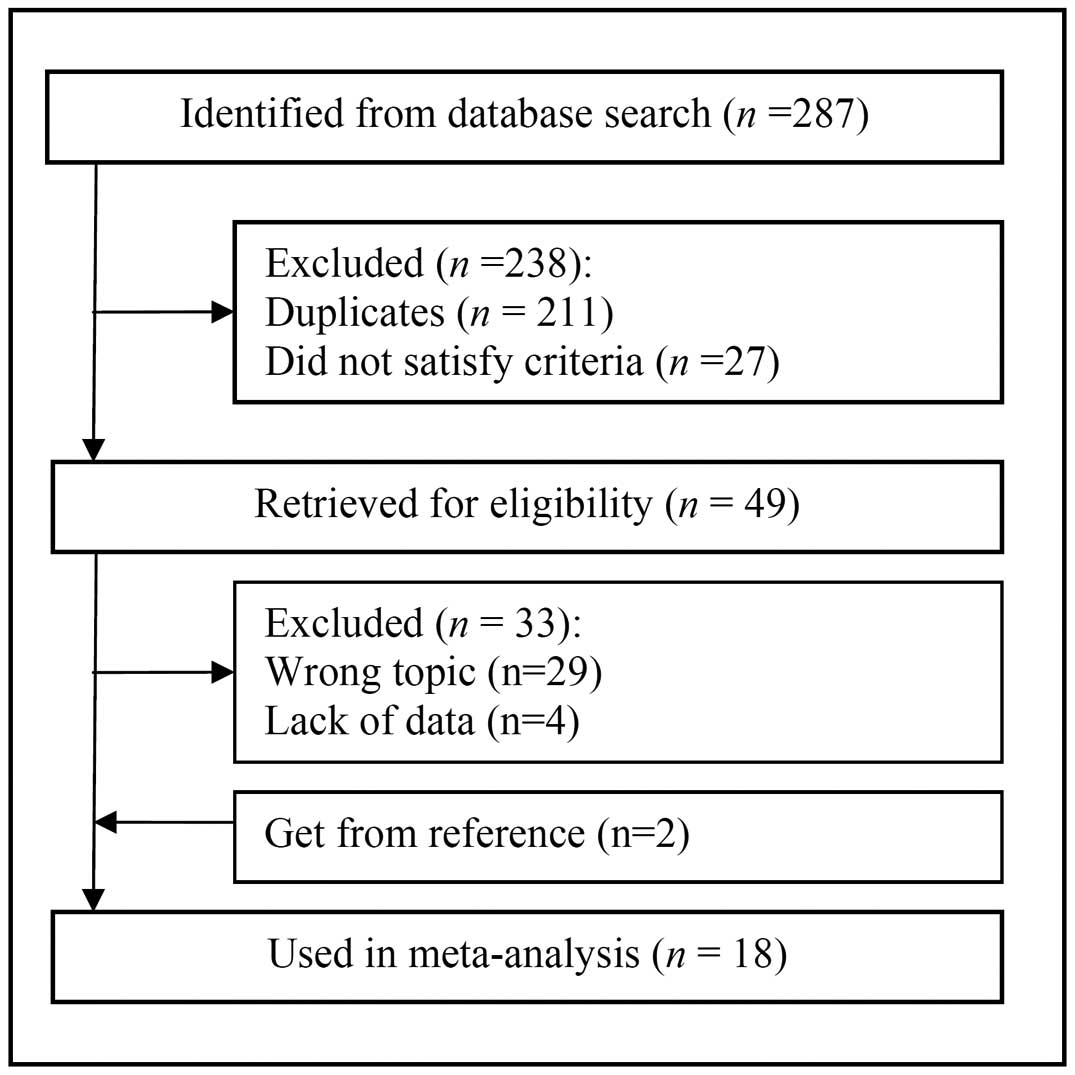
The database search yielded 287 primary studies. Of
these, 238 were excluded following reviews of the title and
abstract, and 33 were excluded following review of the full
article. Two additional studies were obtained from one of
manuscripts evaluated (12). In
summary, 18 articles involving a total of 4,647 patients fulfilled
all the inclusion criteria and were considered in the analysis
(5,12–28)
(Fig. 1). A general consensus was
reached among the investigators with regard to the included studies
(Cohen's unweighted κ=0.93).
Study description and quality
Eighteen studies in which GS was performed were
identified; all of these studies met the eligibility criteria.
Table I lists the included studies
and describes the baseline patient characteristics. The studies
were conducted in six different countries (12 in the United States,
two in Canada and one study each in China, Finland, France and the
United Kingdom). The median number of patients in each study was
169 (range, 33-1,004), and the median age of the participants was
66 years (range, 62–72.8 years). A total of six studies
prospectively enrolled patients (5,12,21,22,24,25),
and 12 studies were retrospective database reviews (13–20,23,26–28).
Patient recruitment was consecutive in five studies (17,21,22,25,27) and
was not documented in the remaining 13 studies (5,12–16,18–20,23,24,26,28).
Ten studies detected PJI on the hip and knee (5,12,18,19–21,23,24,26,27);
three, on the hip (16,22,28);
three, on the knee (13,17,25);
one, on the elbow (14); and two, on
the shoulder (14,21). All the eligible studies scored >9
points using the QUADAS quality assessment tool, indicating that
they were of moderate quality.
 | Table I.Characteristics of the 18 studies in
a meta-analysis of the diagnosis of prosthetic joint infection
using Gram staining. |
Table I.
Characteristics of the 18 studies in
a meta-analysis of the diagnosis of prosthetic joint infection
using Gram staining.
| First author, year
(ref.) | Country | No. of Patient | No of
infections | Age (years) | Study design | Patient
enrollment | Infected
arthroplasties | Specimen | Blinding | Reference
standard |
|---|
| Zywiel, 2011
(13) | United States | 347 | 156 | 62 | Retrospective | NA | Knee | Tissue swab | Yes | IOF, H, M |
| Schindler, 2011
(14) | France | 62 | 48 | 68 | Retrospective | NA | Knee, hip,
shoulder, elbow | NA | NA | IOF, H, M |
| Oethinger, 2011
(15) | United States | 269 | 84 | NA | Retrospective | NA | NA | Synovial fluid and
tissue | Yes | M |
| Johnson, 2010
(16) | United States | 202 | 82 | NA | Retrospective | NA | Hip | Tissue swab | NA | IOF, H, M |
| Morgan, 2009
(17) | United States | 921 | 247 | NA | Retrospective | Consecutive | Knee | Synovial fluid and
tissue | NA | IOF, H, M, L |
| Ghanem, 2009
(18) | United States | 1004 | 321 | 66 | Retrospective | NA | Knee, hip | Tissue | NA | IOF, H, M |
| Trampuz, 2007
(5) | United States | 331 | 79 | 68 | Prospective | NA | Knee, hip | Sonicate fluid | NA | IOF, H, M |
| Parvizi, 2006
(12) | United States | 70 | 39 | 68 | Prospective | NA | Knee, hip | Synovial fluid and
tissue | NA | IOF, M, L |
| Ko, 2005 (19) | China | 40 | 9 | 72 | Retrospective | NA | Knee, hip | Tissue | Yes | M |
| Banit, 2002
(21) | United States | 121 | 21 | 64 | Prospective | Consecutive | Knee, hip,
shoulder | Tissue swab | NA | M |
| Virolainen, 2002
(20) | Finland | 68 | 21 | 72.8a/65.1b | Retrospective | NA | Knee, hip | Tissue | NA | M |
| Spangehl, 1999
(22) | Canada | 178 | 35 | NA | Prospective | Consecutive | Hip | Tissue | Yes | IOF, H, M, L |
| Della Valle, 1999
(23) | United States | 413 | 68 | 62.8 | Retrospective | NA | Knee, hip | Tissue | Yes | IOF, H, M, |
| Atkins, 1998
(24) | United Kingdom | 297 | 41 | 70 | Prospective | NA | Knee, hip | Synovial fluid and
tissue | Yes | H |
| Barrack, 1997
(25) | United States | 67 | 20 | 65 | Prospective | Consecutive | Knee | Synovial fluid | NA | H, M |
| Chimento, 1996
(26) | United States | 169 | 32 | NA | Retrospective | NA | Knee, hip | Tissue | Yes | H, M |
| Feldman, 1995
(27) | United States | 33 | 9 | 62 | Retrospective | Consecutive | Knee, hip | Synovial fluid | NA | M |
| Kraemer, 1993
(28) | Canada | 55 | 13 | 60 | Retrospective | NA | Hip | Tissue | NA | M |
Diagnostic accuracy
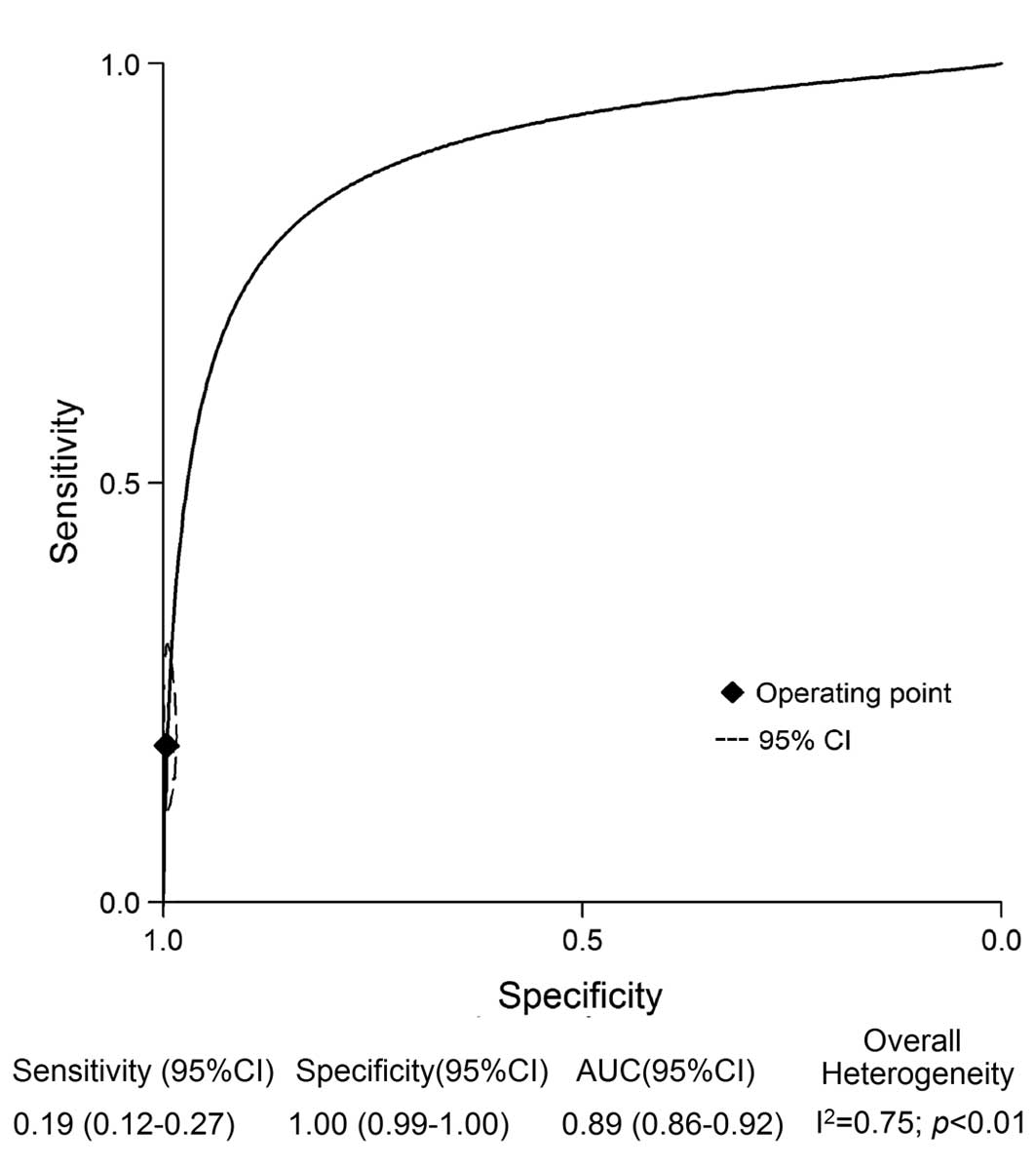
The pooled sensitivity, specificity, DOR and AUC
values obtained from the random effects model are shown in Fig. 2. The pooled sensitivity and
specificity values for the detection of PJI using GS were 0.19 (95%
CI, 0.12–0.27) and 1.00 (95% CI, 0.99–1.00), respectively. The
pooled DOR for GS was 51 (95% CI, 18–140), and the pooled AUC was
0.89 (95% CI, 0.86–0.91). The inconsistency index indicated
significant heterogeneity with respect to GS (I2=75%,
P<0.01); as a result, meta-regression and subgroup analyses were
performed to evaluate the potential sources of heterogeneity in the
GS studies (Table II). The analyses
of the sensitivity and specificity for the detection of PJI using
GS indicated no effect with regard to the type of infected
arthroplasty (knee versus hip versus knee plus hip), the
publication year (prior to 2006 versus 2006 or later), the
reference standard (simple versus multiple), the study design
(perspective versus retrospective) or patient enrollment
(consecutive versus not available). The sensitivities of GS for
infected arthroplasty of the knee, hip and knee plus hip were 0.14
(95% CI, 0.08–0.23), 0.14 (95% CI, 0.09–0.20) and 0.19 (95% CI,
0.11–0.32), respectively, whereas the specificities were 1.00 (95%
CI, 0.99–1.00), 0.99 (95% CI, 0.97–1.00) and 0.99 (95% CI,
0.98–1.00), respectively. The analysis also indicated that
specimens from synovial fluid had a higher sensitivity than those
from tissue swabs, tissue and synovial fluid plus tissue (0.30 vs.
0.14, 0.14 and 0.16, respectively; P<0.05). Specimens from
synovial fluid also yielded the highest DOR values (242 vs. 151, 22
and 36, respectively; P<0.05). By contrast, the highest AUC of
0.96 (95% CI, 0.94–0.97) (P>0.05) was noted in the tissue
specimens. The application of blinding to a study affected the
accuracy of the GS assay; low sensitivity (0.09 vs. 0.28,
P<0.05) and DOR (10 vs. 737, P<0.05) were apparent when the
pathologist was unaware of the clinical results.
 | Table II.Accuracy estimates from subgroup
analyses. |
Table II.
Accuracy estimates from subgroup
analyses.
| Factor | No. of studies | No. of
patients | Sensitivity (95%
CI) | Specificity (95%
CI) | AUC (95% CI) | Positive likelihood
ratio (95% CI) | Negative likelihood
ratio (95% CI) | DOR |
|---|
| Infected
arthroplasties |
| Knee
and hip | 9 | 2425 | 0.19
(0.11–0.32) | 0.99
(0.98–1.00) | 0.95
(0.93–0.97) | 38.1
(10.0–145.4) | 0.81
(0.71–0.93) | 47 (11–194) |
|
Knee | 3 | 1335 | 0.14
(0.08–0.23) | 1.00
(0.99–1.00) | 0.98
(0.96–0.99) | 50.1
(12.6–198.6) | 0.87
(0.79–0.95) | 58 (14–244) |
|
Hip | 4 | 435 | 0.14
(0.09–0.20) | 0.99
(0.97–1.00) | 0.47
(0.43–0.52) | 21.4
(4.8–95.1) | 0.87
(0.81–0.92) | 25 (5–112) |
| Specimens
processed |
| Tissue
swab | 3 | 670 | 0.14
(0.07–0.24) | 1.00
(0.97–1.00) | 0.86
(0.83–0.89) | 130.9
(3.9–4391.4) | 0.86
(0.78–0.95) | 151 (4–5351) |
|
Synovial fluid and tissue | 3 | 1487 | 0.14
(0.10–0.20) | 0.99
(0.99–1.00) | 0.73
(0.69–0.77) | 18.9
(7.7–45.9) | 0.86
(0.82–0.91) | 22 (9–56) |
|
Synovial fluid | 4 | 501 | 0.30
(0.17–0.48) | 1.00
(0.88–1.00) | 0.77
(0.73–0.80) | 170.3
(2.1–13590.0) | 0.70
(0.56–0.88) | 242 (3–19836) |
|
Tissue | 5 | 1927 | 0.16
(0.08–0.29) | 0.99
(0.98–1.00) | 0.96
(0.94–0.97) | 30.9
(7.4–129.7) | 0.85
(0.75–0.96) | 36 (8–163) |
| Publication
year |
| Prior
to 2006 | 10 | 1441 | 0.14
(0.08–0.22) | 0.99
(0.98–0.99) | 0.99
(0.97–0.99) | 13.6
(6.7–27.6) | 0.87
(0.81–0.94) | 16 (7–33) |
| 2006
or later | 8 | 3206 | 0.25
(0.15–0.39) | 1.00
(0.98–1.00) | 0.87
(0.84–0.89) | 61.4
(15.6–241.8) | 0.76
(0.64–0.89) | 81 (20–334) |
| Reference
standard |
|
Simple | 7 | 883 | 0.15
(0.09–0.25) | 0.99
(0.97–1.00) | 0.83
(0.80–0.86) | 21.9
(3.5–136.6) | 0.85
(0.78–0.94) | 26 (4–171) |
|
Multiple | 11 | 3764 | 0.20
(0.12–0.33) | 1.00
(0.99–1.00) | 0.87
(0.84–0.90) | 55.2
(16.4–186.2) | 0.80
(0.70–0.91) | 69 (20–239) |
| Blinding |
|
NA | 11 | 2934 | 0.28
(0.20–0.39) | 1.00
(0.99–1.00) | 0.73
(0.69–0.77) | 528.4
(35.5–7859.4) | 0.72
(0.63–0.82) | 737 (52–10434) |
|
Yes | 7 | 1713 | 0.09
(0.06–0.14) | 0.99
(0.98–0.99) | 0.83
(0.79–0.86) | 8.9 (4.6–17.2) | 0.92
(0.88–0.96) | 10 (5–19) |
| Study design |
|
Prospective | 6 | 1064 | 0.26
(0.19–0.33) | 0.99
(0.98–1.00) | 0.89
(0.86–0.91) | 34.6
(13.8–86.6) | 0.75
(0.68–0.83) | 46 (17–124) |
|
Retrospective | 12 | 3583 | 0.15
(0.08–0.26) | 1.00
(0.95–1.00) | 0.49
(0.44–0.53) | 41.1
(3.3–506.0) | 0.85
(0.77–0.95) | 48 (4–599) |
| Patient
enrollment |
|
Consecutive | 5 | 1320 | 0.25
(0.18–0.32) | 1.00
(0.98–1.00) | 0.47
(0.43–0.51) | 75.7
(11.8–485.8) | 0.76
(0.69–0.84) | 100 (15–679) |
| Not
provided | 13 | 3327 | 0.17
(0.10–0.28) | 0.99
(0.98–1.00) | 0.89
(0.86–0.91) | 33.6
(11.0–102.3) | 0.83
(0.75–0.93) | 40 (13–127) |
| Overall
studies | 18 | 4647 | 0.19
(0.12–0.27) | 1.00
(0.99–1.00) | 0.89
(0.86–0.91) | 41.6
(15.5–111.2) | 0.82
(0.75–0.89) |
51 (18–140) |
Evaluation of clinical utility
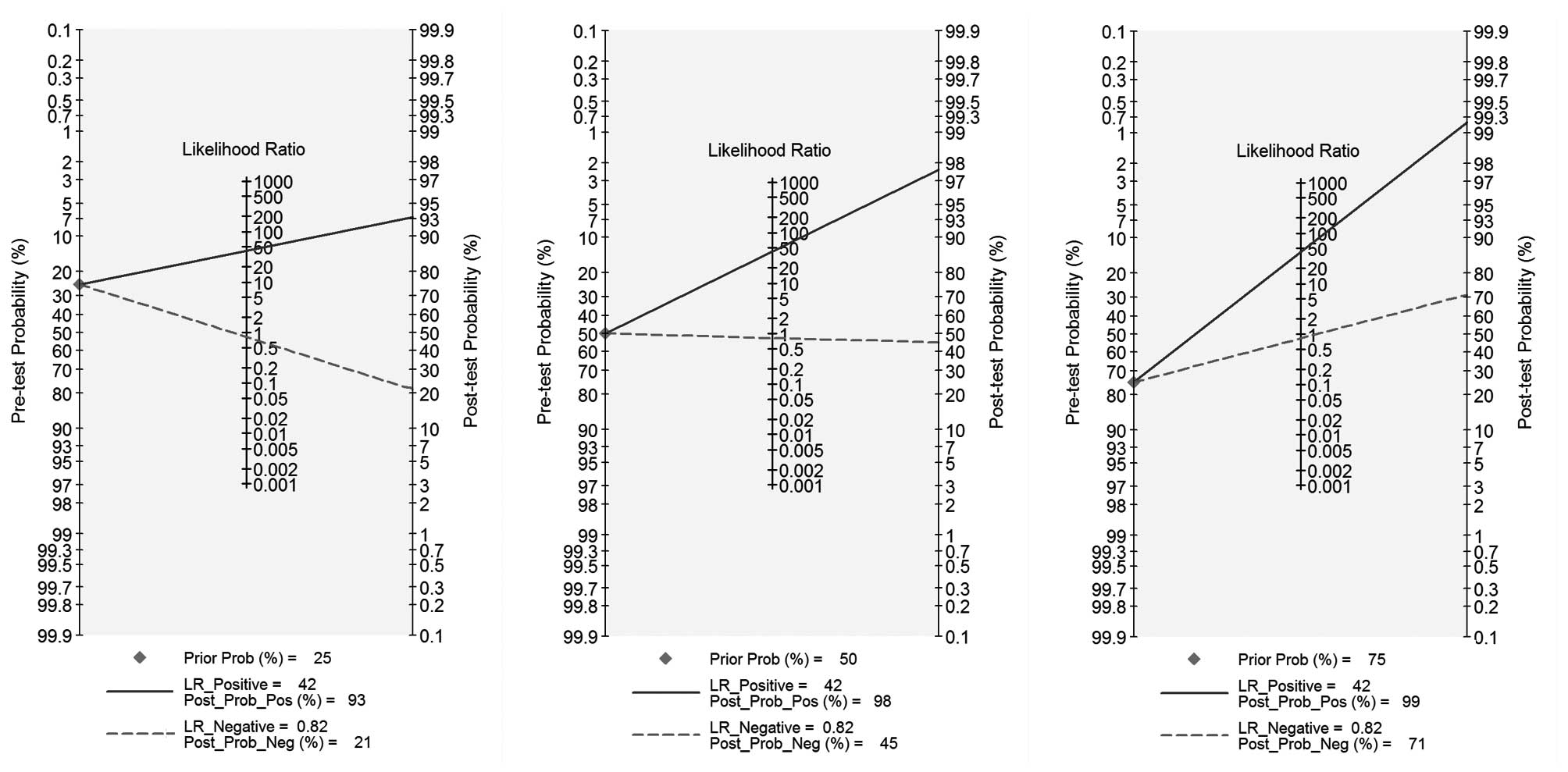
The PLR and NLR of GS for the diagnosis of PJI were
41.6 (95% CI, 15.5–111.2) and 0.82 (95% CI, 0.75–0.89),
respectively (Fig. 3). Likelihood
ratios were used to simulate clinical scenarios using 25, 50 and
75% pre-test probabilities of PJI. Subsequent post-test probability
was calculated and plotted on Fagan nomograms (Fig. 3). The post-test negative probability
of PJI was 82% for the 25% pre-test probability, which could be
considered sufficient to rule out PJI, and the post-test positive
probability was 99% for the 75% pre-test probability, which could
be considered sufficient for the diagnosis of PJI.
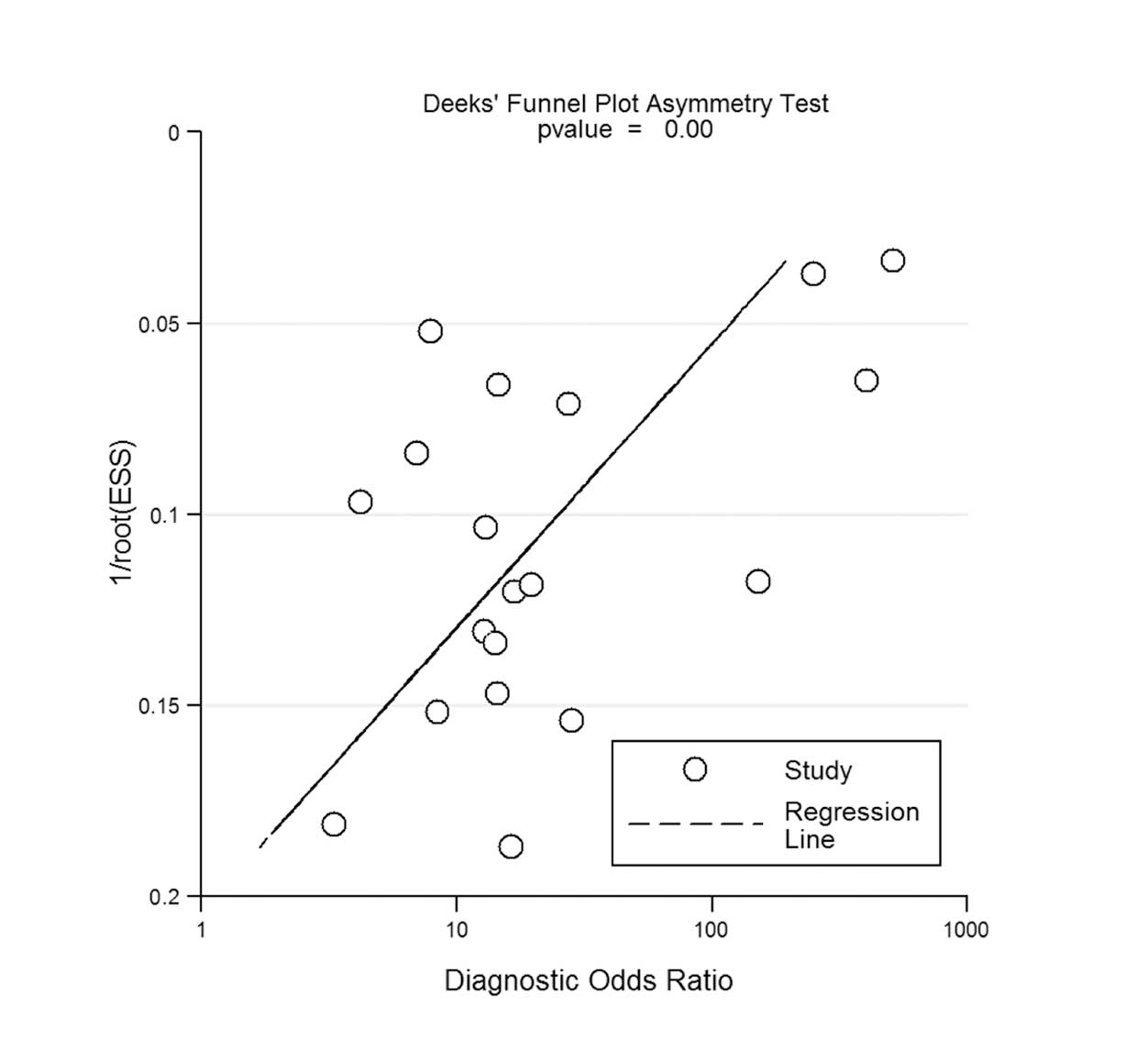
Assessment of publication bias
Potential publication bias was evaluated through the
creation of Deeks' funnel plots by plotting the logDOR of the
individual studies against their sample size. The funnel plots for
GS are presented in Fig. 4. The
regression test of asymmetry found evidence of a small-study effect
for GS (P<0.01).
Discussion
In the present meta-analysis of 18 articles with a
total of 4,647 patients, it was determined that GS could not be
used alone for the diagnosis of PJI among patients who underwent
THA or TKA, as the sensitivity and AUC, the NLR findings and the
low clinical scenario-negative post-test probabilities demonstrated
the poor clinical utility of GS to accurately diagnose PJI.
Although numerous preoperative and intraoperative
tests have been employed, the diagnosis of PJI following THA or TKA
remains a challenge. In contrast to aseptic loosening, the results
of revision total joint arthroplasty of prosthetic infection can
lead to high cost and even patient morbidity; despite this, none of
the currently available tests have perfect sensitivity and
specificity (1,2). Fluorodeoxyglucose positron emission
tomography (FDG-PET) and antigranulocyte scintigraphy with
99mTc-labeled monoclonal antibodies have been reported
to be effective imaging modalities for PJI diagnosis, and two
meta-analyses have demonstrated that the techniques exhibit
acceptable diagnostic capability, with sensitivities of 0.82 and
0.83 and specificities of 0.87 and 0.80, respectively (37,38).
There are, however, certain limitations to these diagnostic
techniques for PJI diagnosis, including high expense, complex
techniques and the requirement for skilled operators. Even the most
common preoperative laboratory tests that are used for the
diagnosis of PJI, such as white blood cell count (WBC), erythrocyte
sedimentation rate (ESR) and serum C-reactive protein (CRP) levels
(2,5,39),
demonstrate low suitability for the diagnosis of PJI (40). A meta-analysis revealed that the
accuracy of inflammation markers as indicators of PJI, as
represented by DORs, was 13.1 for CRP, 7.2 for ESR and 4.4 for WBC
(40).
GS, first described by Hans Christian Gram in 1884,
has been widely used for the evaluation of bacterial infection
through the results of staining (41). Whether the result is positive or
negative depends on the morphological characteristics of the
bacterium, such as the bacterial peptidoglycan layer and the outer
membrane. GS is still widely used by laboratories and clinics all
over the world, although there has been a significant development
in diagnostic technology. In bacterial pneumonia, sepsis and
bacteriuria, for example, the sensitivities and specificities are
>90% (42,43). In the diagnosis of PJI, particularly
in developing countries, GS is commonly used, possibly due to
certain desirable characteristics, including the rapid turnaround
time, convenience and cost-effectiveness; however, a number of
studies have reported sensitivities of between 0 and 50%, which
questions the application of GS in the diagnosis of PJI (17,18,23).
Despite the low and variable sensitivity, numerous institutions
continue to perform GS on all preoperative aspiration samples or
intraoperative wound swab samples sent for microbiological
analysis.
Consistent with the aforementioned reports (17,18,23),
guidelines by the Infectious Diseases Society of America (IDSA)
have recommended against using GS for the assessment of PJI
(39,44); however, several factors can influence
the final results, such as different criteria used to define a true
infection and specimens from different sites. More than three
positive criteria for the diagnosis of PJI were reported by Morgan
et al (17), in which a
number of true infections could be missed. In comparison, only one
positive intraoperative culture was sufficient in the study
investigating the diagnosis of PJI following TKA by Banit et
al (21), and the sensitivity
was found to be 44%, which is relatively high compared with other
reports (5,12–14,17,19,25–27). In
the present meta-analysis, no difference was found in the
sensitivity or specificity for the detection of PJI using GS
between THA or TKA, publication year, reference standard, study
design or patient enrolment; however, the type of specimen screened
and whether blind analysis was performed or not did have an effect.
Consistent with the IDSA guidelines (44) and a number of reports, these results
confirm that GS is a diagnostic method with low sensitivity and NLR
for the diagnosis of PJI (5,12–28).
Notably, the low sensitivity of intraoperative GS
has led to general discouragement regarding the use of the test for
revision arthroplasty; however, positive findings are generally
believed to have relatively high specificity. In the current
meta-analysis it is therefore suggested that GS could be of value
to help identify an organism to guide early antibiotic treatment in
cases of re-implantation with a preoperative diagnosis of
Gram-positive bacterial infection or gross purulence. In addition,
GS may be useful when used as an adjuvant tool.
There are a number of limitations to the present
study. Firstly, no established gold standard exists for the
diagnosis of PJI. In the current meta-analysis, several reference
standards were utilized among the studies, including clinical
manifestation (purulence or fistula), laboratory studies (acute
inflammation on histopathological examination or in blood tests)
and microbiological growth (in periprosthetic tissues or sonication
fluid culture). None of these techniques can be considered to be an
optimal reference standard for the diagnosis of PJI, and
misclassification bias, occurring due to an sub-optimal reference
standard, may influence the predicted diagnostic accuracy of a
tested method (37), generally
resulting in an underestimation of the diagnostic accuracy. A
second limitation in the current analysis was that the summary GS
results exhibited high levels of statistical heterogeneity. This
fact may diminish the strength of the conclusions that can be
extracted from this meta-analysis. Thirdly, it was not clear in all
of the studies whether a prospective study design was used,
although the inclusion of a prospective study design, such as a
covariate, compared with a bivariate model (prospective versus
retrospective design) was not shown to significantly affect
sensitivity or specificity. Finally, only a small number of studies
mentioned the administration of antibiotics or the duration between
the GS assessment and the confirmation of PJI; these factors may
have had an effect on diagnostic accuracy, as antibiotic
administration can increase the number of false negative
results.
In combination, the results of this diagnostic
accuracy meta-analysis indicate that GS in association with
revision arthroplasty has low sensitivity, and that GS is therefore
a poor choice for the diagnosis of PJI following knee and hip
arthroplasty. Based on these data, we recommend that GS as a
microbiological analysis should no longer be performed on the wound
samples obtained when PJI is suspected.
Acknowledgements
This study was supported by the Fund for Key
National Basic Research Program of China (grant no. 2012CB619101),
a major basic research grant from the Science and Technology
Commission of Shanghai Municipality (grant no. 11DJ1400303), a
scientific research grant from the National Natural Science
Foundation for the Youth of China (grant no. 81201364), a
scientific research grant for the Youth of Shanghai (grant no.
ZZjdyx 2097), a scientific research grant from Zhejiang National
Science Foundation (grant no. Y2110653) and an innovative research
grant from the Shanghai Municipal Education Commission (grant no.
13YZ031).
References
|
1
|
Clohisy JC, Calvert G, Tull F, McDonald D
and Maloney WJ: Reasons for revision hip surgery: a retrospective
review. Clin Orthop Relat Res. 188–192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Del Pozo JL and Patel R: Clinical
practice. Infection associated with prosthetic joints. N Engl J
Med. 361:787–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanssen AD, Trousdale RT and Osmon DR:
Patient outcome with reinfection following reimplantation for the
infected total knee arthroplasty. Clin Orthop Relat Res. 55–67.
1995.PubMed/NCBI
|
|
4
|
Mont MA, Waldman BJ and Hungerpord DS:
Evaluation of preoperative cultures before second-stage
reimplantation of a total knee prosthesis complicated by infection.
A comparison-group study. J Bone Joint Surg Am. 82-A:1552–1557.
2000.PubMed/NCBI
|
|
5
|
Trampuz A, Piper KE, Jacobson MJ, et al:
Sonication of removed hip and knee prostheses for diagnosis of
infection. N Engl J Med. 357:654–663. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu X, Zhai Z, Wu C, et al: Preoperative
aspiration culture for preoperative diagnosis of infection in total
hip or knee arthroplasty. J Clin Microbiol. 51:3830–3834. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu X, Zhai Z, Li H, et al: PCR-based
diagnosis of prosthetic joint infection. J Clin Microbiol.
51:2742–2746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu X, Zhai Z, Liu X, Li H, Wu C, Li Y, Li
H, Zhu Z, Qin A and Dai K: Evaluation of white cell count and
differential in synovial fluid for diagnosing infections after
total hip or knee arthroplasty. PLoS One. 9:e847512014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel R, Osmon DR and Hanssen AD: The
diagnosis of prosthetic joint infection: current techniques and
emerging technologies. Clin Orthop Relat Res. 55–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kraay MJ, Goldberg VM, Fitzgerald SJ and
Salata MJ: Cementless two-staged total hip arthroplasty for deep
periprosthetic infection. Clin Orthop Relat Res. 441:243–249. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leone JM and Hanssen AD: Management of
infection at the site of a total knee arthroplasty. J Bone Joint
Surg Am. 87:2335–2348. 2005.PubMed/NCBI
|
|
12
|
Parvizi J, Ghanem E, Menashe S, Barrack RL
and Bauer TW: Periprosthetic infection: what are the diagnostic
challenges? J Bone Joint Surg Am. 88 Suppl 4:138–147. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zywiel MG, Stroh DA, Johnson AJ, Marker DR
and Mont MA: Gram stains have limited application in the diagnosis
of infected total knee arthroplasty. Int J Infect Dis.
15:e702–e705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schindler M, Christofilopoulos P, Wyssa B,
et al: Poor performance of microbiological sampling in the
prediction of recurrent arthroplasty infection. Int Orthop.
35:647–654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oethinger M, Warner DK, Schindler SA,
Kobayashi H and Bauer TW: Diagnosing periprosthetic infection:
false-positive intraoperative Gram stains. Clin Orthop Relat Res.
469:954–960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson AJ, Zywiel MG, Stroh DA, Marker DR
and Mont MA: Should gram stains have a role in diagnosing hip
arthroplasty infections? Clin Orthop Relat Res. 468:2387–2391.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morgan PM, Sharkey P, Ghanem E, et al: The
value of intraoperative Gram stain in revision total knee
arthroplasty. J Bone Joint Surg Am. 91:2124–2129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghanem E, Ketonis C, Restrepo C, Joshi A,
Barrack R and Parvizi J: Periprosthetic infection: where do we
stand with regard to Gram stain? Acta Orthop. 80:37–40. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ko PS, Ip D, Chow KP, Cheung F, Lee OB and
Lam JJ: The role of intraoperative frozen section in decision
making in revision hip and knee arthroplasties in a local community
hospital. J Arthroplasty. 20:189–195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Virolainen P, Lähteenmäki H, Hiltunen A,
Sipola E, Meurman O and Nelimarkka O: The reliability of diagnosis
of infection during revision arthroplasties. Scand J Surg.
91:178–181. 2002.PubMed/NCBI
|
|
21
|
Banit DM, Kaufer H and Hartford JM:
Intraoperative frozen section analysis in revision total joint
arthroplasty. Clin Orthop Relat Res. 230–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spangehl MJ, Masterson E, Masri BA,
O'Connell JX and Duncan CP: The role of intraoperative gram stain
in the diagnosis of infection during revision total hip
arthroplasty. J Arthroplasty. 14:952–956. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Della Valle CJ, Scher DM, Kim YH, et al:
The role of intraoperative Gram stain in revision total joint
arthroplasty. J Arthroplasty. 14:500–504. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Atkins BL, Athanasou N, Deeks JJ, et al:
Prospective evaluation of criteria for microbiological diagnosis of
prosthetic-joint infection at revision arthroplasty. The OSIRIS
Collaborative Study Group. J Clin Microbiol. 36:2932–2939.
1998.PubMed/NCBI
|
|
25
|
Barrack RL, Jennings RW, Wolfe MW and
Bertot AJ: The Coventry Award. The value of preoperative aspiration
before total knee revision. Clin Orthop Relat Res. 8–16.
1997.PubMed/NCBI
|
|
26
|
Chimento GF, Finger S and Barrack RL: Gram
stain detection of infection during revision arthroplasty. J Bone
Joint Surg Br. 78:838–839. 1996.PubMed/NCBI
|
|
27
|
Feldman DS, Lonner JH, Desai P and
Zuckerman JD: The role of intraoperative frozen sections in
revision total joint arthroplasty. J Bone Joint Surg Am.
77:1807–1813. 1995.PubMed/NCBI
|
|
28
|
Kraemer WJ, Saplys R, Waddell JP and
Morton J: Bone scan, gallium scan and hip aspiration in the
diagnosis of infected total hip arthroplasty. J Arthroplasty.
8:611–616. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qu X, Huang X, Yan W, Wu L and Dai K: A
meta-analysis of 8FDG-PET-CT, 18FDG-PET, MRI and bone
scintigraphy for diagnosis of bone metastases in patients with lung
cancer. Eur J Radiol. 81:1007–1015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Devillé WL, Buntinx F, Bouter LM, et al:
Conducting systematic reviews of diagnostic studies: didactic
guidelines. BMC Med Res Methodol. 2:92002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liberati A, Altman DG, Tetzlaff J, et al:
The PRISMA statement for reporting systematic reviews and
meta-analyses of studies that evaluate health care interventions:
explanation and elaboration. Ann Intern Med. 151:W65–W94. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berlin JA: Does blinding of readers affect
the results of meta-analyses? University of Pennsylvania
Meta-analysis Blinding Study Group. Lancet. 350:185–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Whiting P, Rutjes AW, Reitsma JB, Bossuyt
PM and Kleijnen J: The development of QUADAS: a tool for the
quality assessment of studies of diagnostic accuracy included in
systematic reviews. BMC Med Res Methodol. 3:252003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moses LE, Shapiro D and Littenberg B:
Combining independent studies of a diagnostic test into a summary
ROC curve: data-analytic approaches and some additional
considerations. Stat Med. 12:1293–1316. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huedo-Medina TB, Sánchez-Meca J,
Marín-Martínez F and Botella J: Assessing heterogeneity in
meta-analysis: q statistic or i2 index? Psychol Methods.
11:193–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Deeks JJ, Macaskill P and Irwig L: The
performance of tests of publication bias and other sample size
effects in systematic reviews of diagnostic test accuracy was
assessed. J Clin Epidemiol. 58:882–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pakos EE, Trikalinos TA, Fotopoulos AD and
Ioannidis JP: Prosthesis infection: diagnosis after total joint
arthroplasty with antigranulocyte scintigraphy with
99mTc-labeled monoclonal antibodies – a meta-analysis.
Radiology. 242:101–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwee TC, Kwee RM and Alavi A: FDG-PET for
diagnosing prosthetic joint infection: systematic review and
metaanalysis. Eur J Nucl Med Mol Imaging. 35:2122–2132. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parvizi J, Adeli B, Zmistowski B, Restrepo
C and Greenwald AS: Management of periprosthetic joint infection:
the current knowledge: AAOS exhibit selection. J Bone Joint Surg
Am. 94:e1042012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berbari E, Mabry T, Tsaras G, et al:
Inflammatory blood laboratory levels as markers of prosthetic joint
infection: a systematic review and meta-analysis. J Bone Joint Surg
Am. 92:2102–2109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Popescu A and Doyle RJ: The Gram stain
after more than a century. Biotech Histochem. 71:145–151. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Anevlavis S, Petroglou N, Tzavaras A, et
al: A prospective study of the diagnostic utility of sputum Gram
stain in pneumonia. J Infect. 59:83–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Søqaard M, Nørgaard M and Schønheyder HC:
First notification of positive blood cultures and the high accuracy
of the gram stain report. J Clin Microbiol. 45:1113–1117. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Osmon DR, Berbari EF, Berendt AR, et al:
Infectious Diseases Society of America: Executive summary:
diagnosis and management of prosthetic joint infection: clinical
practice guidelines by the Infectious Diseases Society of America.
Clin Infect Dis. 56:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|