Introduction
Diabetes mellitus is a common disease in older
individuals, affecting ∼20% of the population aged >65 years
(1,2). It has been reported that diabetes has a
close association with the reduced performance in numerous domains
of cognitive function (1,3–5). A
clinical study by Arvanitakis et al (1) has indicated that diabetes may be
associated with an increased risk of Alzheimer's disease
development and may eventually affect cognitive function; however,
the underlying mechanisms of diabetes-induced cognitive dysfunction
have not been fully elucidated.
Numerous studies have focused on diabetes-induced
cognitive impairment, while the majority have focused on fat
metabolism and vascular dementia (6–8). Jafari
Anarkooli et al (9)
demonstrated that Bcl-2 family gene expression and caspase-3
activity were altered in the streptozotocin (STZ)-induced diabetic
rat hippocampus. Furthermore, Revsin et al (10) found that a glucocorticoid receptor
antagonist normalized hippocampal alterations and cognitive
impairment in STZ-induced diabetic mice. Our previous study
(11), however, found that
intraperitoneal injection of a single dose of 60 mg/kg STZ could
cause cognitive dysfunction and increase inflammatory cytokine
levels and oxidative activity in the rat hippocampus. Notably,
leptin levels in the rat hippocampus significantly decreased.
Leptin, which is a hormone-like protein secreted
from fat cells, plays a critical role in regulating food intake and
energy metabolism (12,13). It has been reported that leptin is a
potential cognitive enhancer (14).
Several studies have shown that leptin is the upstream activator of
the adenosine monophosphate-activated protein kinase (AMPK) pathway
(15,16). Yi et al (17) observed that acute and chronic
exercise could indirectly activate the leptin-AMPK signaling
pathway, while a study by Namkoong et al (18) showed that leptin and insulin
deficiencies in diabetes led to increased hypothalamic AMPK
activity.
The aim of the present study was to investigate the
effect of leptin on cognitive dysfunction in STZ-induced diabetic
rats and to explore whether AMPK activation was involved in any
potential therapeutic effects of leptin. An AMPK antagonist,
Compound C, was selected to investigate the potential involvement
of AMPK in the leptin-induced therapeutic effect in cognitive
impairment.
Materials and methods
Animals
Male Wistar rats weighing 220–300 g were purchased
from the Shanghai Animal Center (Shanghai, China). Six rats were
housed per cage with food and water available ad libitum and
maintained under a 12-h light/dark cycle (lights on at 7:00 a.m.).
Animal care was approved by the Animal Use and Protection Committee
of Soochow University (Changzhou, China). Thirty-six rats were
randomly divided into three groups (n=12 each). The rats were
intraperitoneally pretreated with either a single injection of
saline or STZ (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 60
mg/kg. One month later, the rats were either
intracerebroventricularly injected with saline or 10 µg leptin
(Sigma-Aldrich) dissolved in 5 µl Tris-HCl. For the second
experiment, a further 36 rats were randomly divided into three
groups (n=12 each). The rats were intraperitoneally pretreated with
either a single injection of saline or STZ at a dose of 60 mg/kg
according to the first protocol. One month later, the rats were
either intracerebroventricularly injected with saline, 10 µg leptin
or 10 µg leptin plus intraperitoneal injection of 1 mg/kg Compound
C (Tocris Bioscience, Bristol, UK).
Morris water maze test
According to our previous study (11), cognitive function was assessed using
the Morris water maze test system between 9:00 a.m. and 3:00 p.m.
The maze was 80 cm deep and 100 cm in diameter and was filled with
water to a depth of 30 cm. The maze was divided into four quadrants
of equal size on the monitor screen of a computer, and the water
temperature was maintained at 23–24°C. The swimming paths of the
rats were recorded by a video camera and analyzed by VideoMot
software (Huaibei Zhenghua Biologic Apparatus Facilities Co., Ltd.,
Huaibei, China). The trials were conducted for four consecutive
days in order to observe the escape latency of the rats and the
time spent in each quadrant. The escape latency and the proportion
of time spent in the target quadrant were recorded.
Leptin and tumor necrosis factor-α
(TNF-α) measurement
Leptin and TNF-α levels were determined using ELISA
kits. According to the manufacturer's instructions (Wuhan Huamei
Bioengineering Co., Ltd., Wuhan, China), microtiter plates were
coated for overnight incubation with the samples diluted 1:2 in
sample diluent. The plates were then washed three times with sample
diluent, and the primary antibody (monoclonal anti-leptin or
-TNF-α), diluted 1:1,000 in sample diluent, was added to each well
and incubated for 3 h at room temperature. Subsequent to washing, a
peroxidase-conjugated anti-rabbit antibody (diluted 1:10,000;
Nanjing Sunshine Biotechnology Co., Ltd.) was added to each well
and incubated at room temperature for 1 h. Streptavidin enzyme,
substrate and stop solution were then added, and the leptin and
TNF-α levels were determined by measuring the absorbance at 450 nm.
In addition, total protein was measured using the Lowry method with
bovine serum albumin as a standard.
Amyloid-β (Aβ) measurement
The animals were sacrificed immediately by
decapitation and the protein concentrations were determined using a
bicinchoninic acid assay kit (cat. no. P0012S; Beyotime Institute
of Biotechnology, Haimen, China). The samples were then centrifuged
at 3,000 × g at 4°C for 30 min to obtain the supernatants. The
proteins were separated using SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. Subsequent to blocking with 5%
non-fat milk, the membranes were incubated with rabbit anti-Aβ
primary antibody (1:200; Sigma-Aldrich). The membranes were then
incubated for 1 h at room temperature with anti-rabbit horseradish
peroxidase-conjugated immunoglobulin G secondary antibody
(1:20,000; CWBIO, Beijing, China). The labeled protein was detected
using enhanced chemiluminescence reagents (GE Healthcare Life
Sciences, Little Chalfont, UK) and the band intensity was analyzed
using ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Malondialdehyde (MDA) measurement
According to our previous study (11), the samples were mixed with 1 ml 10%
trichloroacetic acid and 1 ml 0.67% thiobarbituric acid, and were
then heated in a boiling water bath for 30 min. MDA equivalents
were determined in the tissue and submitochondrial particles of the
rat brain using a spectrophotometer at an absorbance of 532 nm.
Blood glucose measurement
The rats were anesthetized with 10% chloral hydrate
(0.4 ml/100 g, intraperitoneal) and blood samples were then
collected from the carotid artery. Blood glucose levels were
determined with a blood glucometer (OneTouch® UltraEasy®; Johnson
& Johnson, Inc., New Brunswick, NJ, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed by one-way analysis of
variance, and post hoc analyses were conducted using Fisher's least
significant difference tests. All statistical analyses were carried
out using SPSS version 17.0 software (SPSS, Inc., Chicago, IL,
USA). In the Morris water maze test, the percentage of time spent
in the target quadrant was evaluated using χ2 tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of STZ and/or leptin on the
escape latency of the rats and the proportion of time spent in the
target quadrant in the Morris water maze test
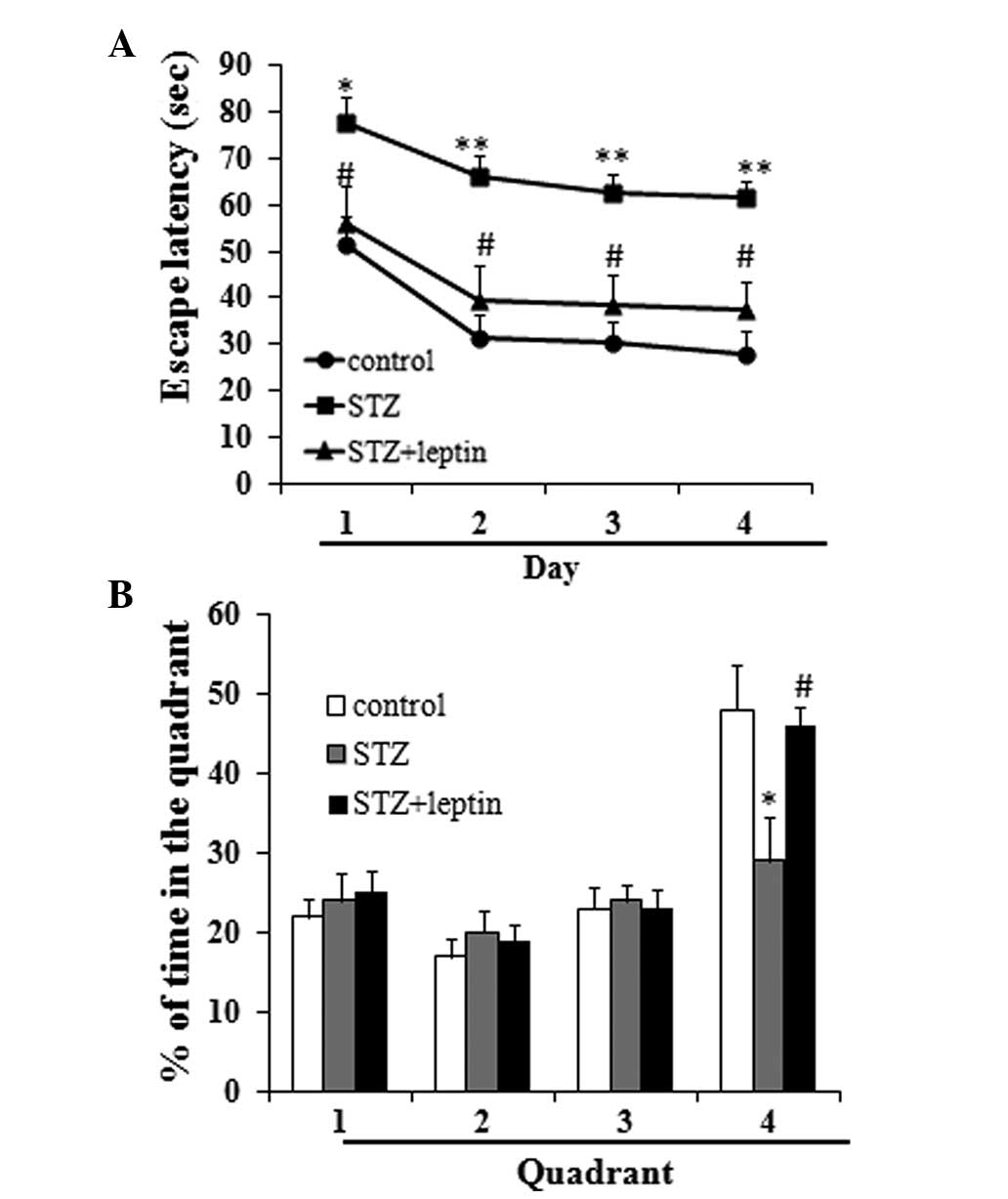
The results of the Morris water maze test
demonstrated that STZ administration significantly increased the
escape latency as compared with the control group, and decreased
the percentage of time spent in the fourth quadrant (P<0.05 or
0.01). Compared with the STZ group, however, rats in the STZ plus
leptin group exhibited significantly decreased escape latencies,
and an increased percentage of time spent in the fourth quadrant
(P<0.05) (Fig. 1).
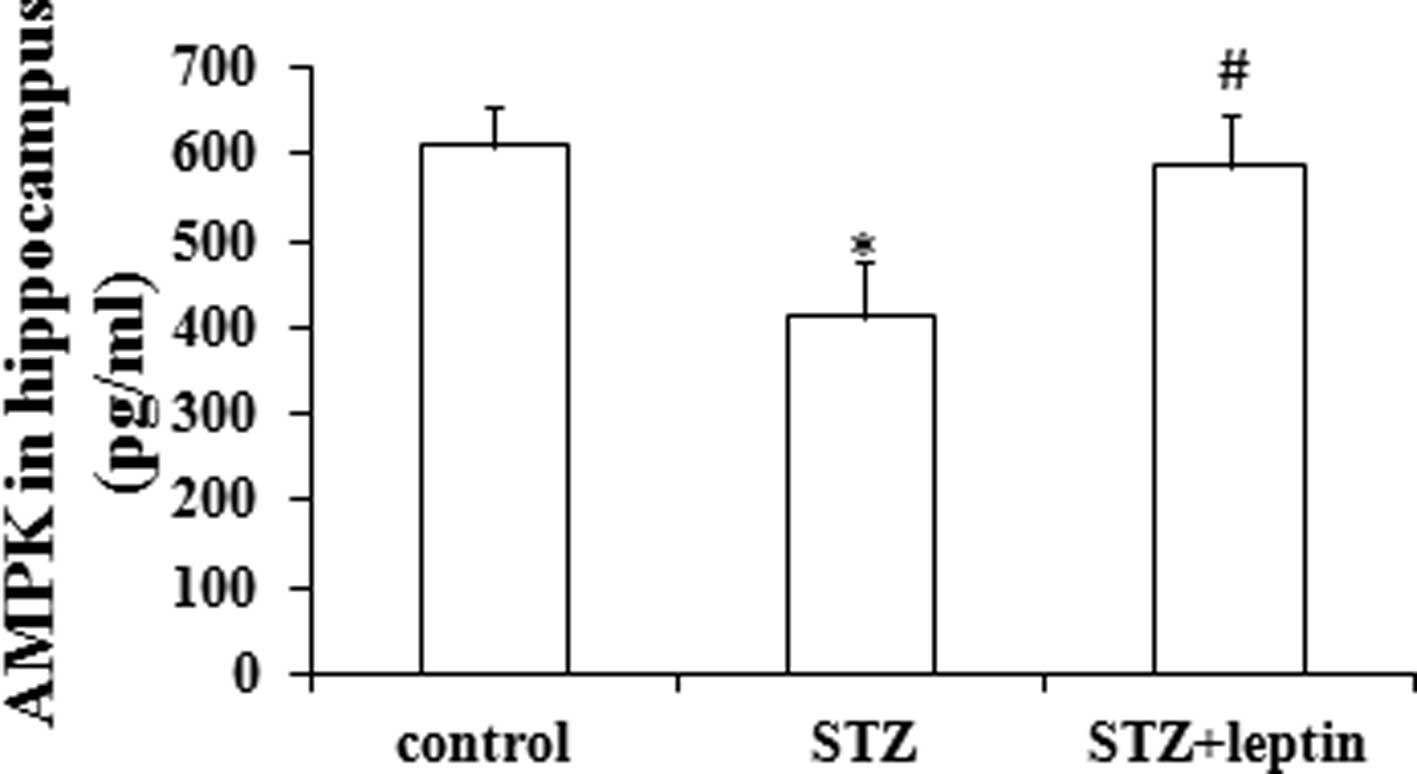
Effect of STZ and/or leptin on the
expression of AMPK in the rat hippocampus
A single injection of STZ significantly decreased
the protein expression of AMPK in the rat hippocampus (P<0.05).
By contrast, leptin administration could abrogate the STZ-induced
decrease in AMPK expression in the hippocampus (P<0.05)
(Fig. 2).
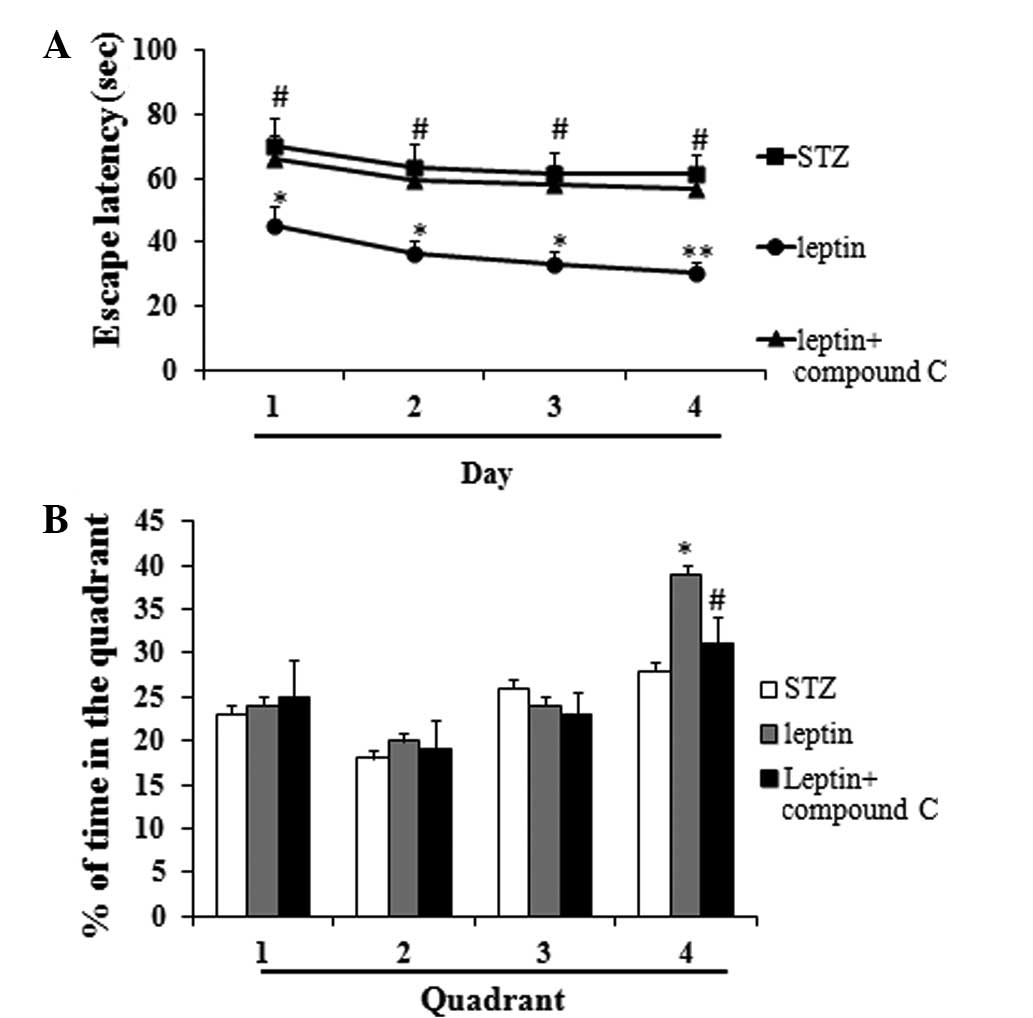
Effect of Compound C, an AMPK
inhibitor, on the escape latency of STZ-induced diabetic rats and
the percentage of time spent in the target quadrant in the Morris
water maze test
Compound C, which acts as an AMPK inhibitor,
significantly increased the escape latency as compared with the
leptin group, and decreased the percentage of time spent in the
fourth quadrant (P<0.05 or 0.01) (Fig. 3A and B).
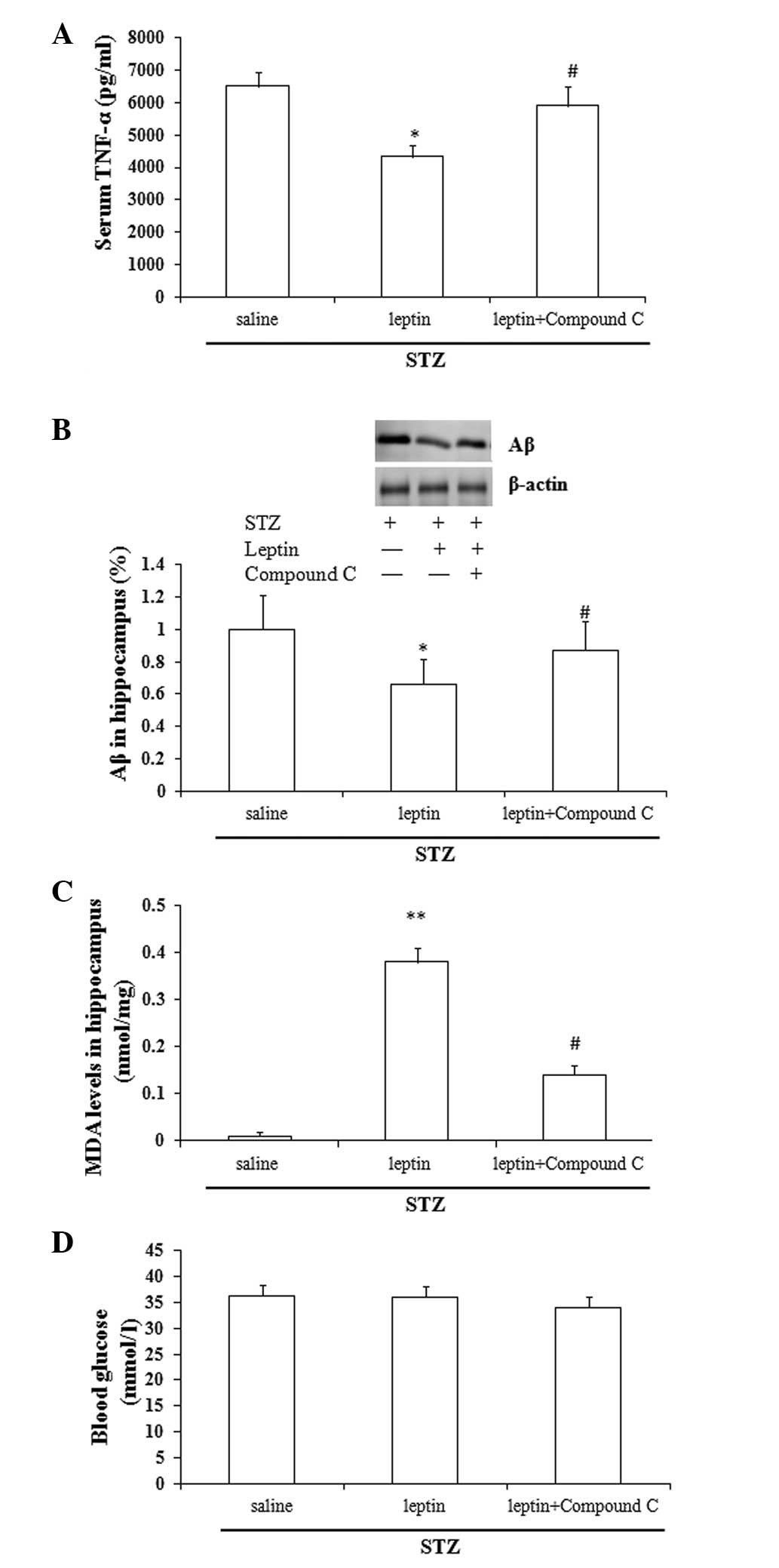
Effect of Compound C on the
leptin-induced changes in the expression of TNF-α, Aβ and MDA and
the blood glucose levels
Compared with the saline group, leptin
administration caused a significant decrease in the serum TNF-α and
Aβ levels in the rat hippocampus (P<0.05), and a significant
increase in the MDA levels (P<0.01). Compared with the leptin
group, Compound C abrogated the leptin-induced changes in the
expression of TNF-α, Aβ and MDA (P<0.05), but did not affect the
blood glucose levels (P>0.05) (Fig.
4A-D).
Discussion
In the present study, the results demonstrated that
leptin could attenuate STZ-induced cognitive impairment, which was
characterized by the poor performance of the rats in the Morris
water maze test. Compound C, an AMPK inhibitor, significantly
abrogated the protective effects of leptin against the STZ-induced
rat cognitive impairment. The findings of these results suggested,
therefore, that AMPK activation contributed to the underlying
mechanism of the therapeutic effect of leptin in STZ-induced
cognitive impairment.
Numerous studies have shown that the mechanism of
cognitive dysfunction in STZ-induced diabetic rats is associated
with the vascular lesions caused by diabetes (8,19).
Denver et al (12), however,
proposed that diabetes is simply a trigger in the pathogenesis of
diabetes-induced cognitive dysfunction, rather than the main
mechanism. Zhu et al (20)
suggested that oxidative stress, the inflammatory response and Aβ
formation comprised the primary mechanism in diabetes-induced
cognitive dysfunction, which was also consistent with the results
of our previous study (11). In the
present study, the results supported the above-mentioned hypothesis
that diabetes-induced cognitive dysfunction manifested as a result
of the abnormal expression of TNF-α, Aβ and MDA. Notably, the blood
glucose levels did not show any significant change following leptin
treatment. Although it has previously been demonstrated that leptin
acts to reduce blood glucose levels (21), the present study utilized an
injection of exogenous leptin, not endogenous; therefore, the
leptin was not able to rapidly activate the leptin receptors, which
prevented it from exerting its biological effects.
AMPK is an enzyme that plays a role in cellular
energy homeostasis. Numerous studies have highlighted the important
role of AMPK in the pathogenesis of Alzheimer's disease, which is
characterized by cognitive impairment (22,23). An
in vitro study by Greco et al (24) showed that leptin increased cellular
metabolism by activating AMPK to reduce Aβ expression in neurons. A
different study also performed by this group (25) demonstrated that leptin directly
regulated Aβ through the AMPK pathway. The results of the present
study showed that STZ administration significantly decreased the
AMPK levels in the rat hippocampus, while leptin abrogated this
effect. Notably, it was observed that Compound C, an AMPK
inhibitor, significantly reversed the effects of leptin, suggesting
that AMPK activation is likely to be involved in the mechanism
underlying the protective effect of leptin against cognitive
impairment.
The activation of oxidative stress has been
considered to be an important inducing factor for the incidence of
cognitive impairment. Praticò et al (26) demonstrated that increased oxidative
stress could be used as a predictor for the onset of cognitive
impairment. A different study suggested that oxidative activation
has harmful effects on the rat synapses in the cerebral cortex and
hippocampus, which may result in cognitive impairment (27). Notably, a clinical study found that
the successful treatment of patients with cognitive impairment
could restore the oxidative cytokine levels in the peripheral blood
of the patients to a normal levels (28). In the present study, it was observed
that an AMPK inhibitor could abrogate the effect of leptin on
cognitive impairment and change hippocampal MDA levels. This result
indirectly showed that the control of cognitive function by AMPK
signaling potentially occurs through the regulation of oxidative
stress. Similarly, the inflammatory response plays a critical role
in the incidence of diabetes-related cognitive impairment. The
present results showed that TNF-α levels decreased following leptin
treatment, while the AMPK inhibitor reversed this change. This
result was consistent with our expected hypothesis.
In conclusion, AMPK activation may contribute to the
therapeutic effect of leptin in cognitive impairment in an
STZ-induced rat model. It is likely that oxidative stress and the
inflammatory response are also involved.
References
|
1
|
Arvanitakis Z, Wilson RS, Bienias JL,
Evans DA and Bennett DA: Diabetes mellitus and risk of Alzheimer
disease and decline in cognitive function. Arch Neurol. 61:661–666.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engelgau MM, Geiss LS, Saaddine JB, et al:
The evolving diabetes burden in the United States. Ann Intern Med.
140:945–950. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van den Berg E, Reijmer YD, de Bresser J,
et al: Utrecht Diabetic Encephalopathy Study Group: A 4 year
follow-up study of cognitive functioning in patients with type 2
diabetes mellitus. Diabetologia. 53:58–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huber JD: Diabetes, cognitive function,
and the blood-brain barrier. Curr Pharm Des. 14:1594–1600. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Strachan MW, Price JF and Frier BM:
Diabetes, cognitive impairment, and dementia. BMJ. 336:62008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peila R, Rodriguez BL and Launer LJ:
Honolulu-Asia Aging Study: Type 2 diabetes, APOE gene and the risk
for dementia and related pathologies: The Honolulu-Asia Aging
Study. Diabetes. 51:1256–1262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Breteler MM: Vascular risk factors for
Alzheimer's disease: An epidemiologic perspective. Neurobiol Aging.
21:153–160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gispen WH and Biessels GJ: Cognition and
synaptic plasticity in diabetes mellitus. Trends Neurosci.
23:542–549. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anarkooli Jafari I, Sankian M, Ahmadpour
S, Varasteh AR and Haghir H: Evaluation of Bcl-2 family gene
expression and Caspase-3 activity in hippocampus STZ-induced
diabetic rats. Exp Diabetes Res. 2008:6384672008.PubMed/NCBI
|
|
10
|
Revsin Y, Rekers NV, Louwe MC, et al:
Glucocorticoid receptor blockade normalizes hippocampal alterations
and cognitive impairment in streptozotocin-induced type 1 diabetes
mice. Neuropsychopharmacology. 34:747–758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang C, Zhu B, Ding J and Wang ZG:
Isoflurane anesthesia aggravates cognitive impairment in
streptozotocin-induced diabetic rats. Int J Clin Exp Med.
7:903–910. 2014.PubMed/NCBI
|
|
12
|
Denver RJ, Bonett RM and Boorse GC:
Evolution of leptin structure and function. Neuroendocrinology.
94:21–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh A, Wirtz M, Parker N, et al:
Leptin-mediated changes in hepatic mitochondrial metabolism,
structure and protein levels. Proc Natl Acad Sci USA.
106:13100–13105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harvey J, Shanley LJ, O'Malley D and
Irving AJ: Leptin: A potential cognitive enhancer? Biochem Soc
Trans. 33:1029–1032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Minokoshi Y, Kim YB, Peroni OD, et al:
Leptin stimulates fatty-acid oxidation by activating AMP-activated
protein kinase. Nature. 415:339–343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minokoshi Y and Kahn BB: Role of
AMP-activated protein kinase in leptin-induced fatty acid oxidation
in muscle. Biochem Soc Trans. 31:196–201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi X, Cao S, Chang B, et al: Effects of
acute exercise and chronic exercise on the liver leptin-AMPK-ACC
signaling pathway in rats with type 2 diabetes. J Diabetes Res.
2013:9464322013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Namkoong C, Kim MS, Jang PG, et al:
Enhanced hypothalamic AMP-activated protein kinase activity
contributes to hyperphagia in diabetic rats. Diabetes. 54:63–68.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sharma B and Singh N: Attenuation of
vascular dementia by sodium butyrate in streptozotocin diabetic
rats. Psychopharmacology (Berl). 215:677–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Raina AK, Lee HG, Casadesus G,
Smith MA and Perry G: Oxidative stress signalling in Alzheimer's
disease. Brain Res. 1000:32–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davis C, Mudd J and Hawkins M:
Neuroprotective effects of leptin in the context of obesity and
metabolic disorders. Neurobiol Dis. 72:61–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salminen A, Kaarniranta K, Haapasalo A,
Soininen H and Hiltunen M: AMP-activated protein kinase: A
potential player in Alzheimer's disease. J Neurochem. 118:460–474.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai Z, Yan LJ, Li K, Quazi SH and Zhao B:
Roles of AMP-activated protein kinase in Alzheimer's disease.
Neuromolecular Med. 14:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Greco SJ, Sarkar S, Johnston JM and
Tezapsidis N: Leptin regulates tau phosphorylation and amyloid
through AMPK in neuronal cells. Biochem Biophys Res Commun.
380:98–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greco SJ, Hamzelou A, Johnston JM, Smith
MA, Ashford JW and Tezapsidis N: Leptin boosts cellular metabolism
by activating AMPK and the sirtuins to reduce tau phosphorylation
and β-amyloid in neurons. Biochem Biophys Res Commun. 414:170–174.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Praticò D, Clark CM, Liun F, Rokach J, Lee
VY and Trojanowski JQ: Increase of brain oxidative stress in mild
cognitive impairment: a possible predictor of Alzheimer disease.
Arch Neurol. 59:972–976. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fukui K, Omoi NO, Hayasaka T, et al:
Cognitive impairment of rats caused by oxidative stress and aging
and its prevention by vitamin E. Ann NY Acad Sci. 959:275–284.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Padurariu M, Ciobica A, Hritcu L, Stoica
B, Bild W and Stefanescu C: Changes of some oxidative stress
markers in the serum of patients with mild cognitive impairment and
Alzheimer's disease. Neurosci Lett. 469:6–10. 2010. View Article : Google Scholar : PubMed/NCBI
|