Introduction
Advancements in transgenic technology have resulted
in genetically-modified mice being widely employed as model
organisms in mechanistic studies of cardiovascular disease
(1,2). The development of accurate and
non-invasive techniques for the quantitative and phenotypic
characterization of cardiac function in healthy myocardium is
crucial for obtaining the full benefits of these models.
Echocardiography is an effective approach for quantitatively and
accurately evaluating cardiac function and morphology (3,4). Despite
these advantages, conventional echocardiography, such as M-mode
assessment, is offset by the poor sensitivity for detecting subtle
cardiac dysfunction (5). Therefore,
there is a constant and pressing requirement for novel
echocardiography techniques to enhance the assessment of cardiac
function. The deformation (strain) imaging is one of these advanced
techniques, which enables the improved quantification of global and
regional cardiac function. It captures segmental displacement and
velocity of the left ventricle in multiple planes over the cardiac
circle and can be acquired from tissue Doppler or 2-dimensional
speckle tracking echocardiography (STE) (6). In the STE method, the speckles
resulting from acoustic interference phenomena are used to track
the movement of myocardium (7). Due
to the benefits of high reproducibility and angle-independency over
color tissue Doppler imagining, STE has been widely applied
(8). Prior studies have demonstrated
that anesthetic agents may serve a significant function in the
evaluation of echocardiographic parameters in mice (9). Isoflurane (ISF) is a volatile
anesthetic that has been identified as a potential anesthetic agent
for use in experimental studies involving mice, as it facilitates
rapid conduction and easy control of the depth of anesthesia during
conventional echocardiography (10–13).
Due to the novelty of the STE technique, few studies
have examined the effects of anesthesia on the parameters of STE.
Previous studies have focused primarily on investigating the
effects of anesthesia on the echocardiographic analysis of healthy
subjects (9,14). As a result, the effects of anesthesia
on cardiac function under pathological conditions remain
unknown.
Therefore, the present study aimed to investigate
the effects of an anesthetic agent on STE parameters in mice with
hypertrophic and failing myocardium, in addition to healthy mice.
Furthermore, the effects of anesthesia on conventional
echocardiographic parameters were assessed in mice with healthy,
hypertrophic and failing myocardium.
Materials and methods
Animals
The current study was approved by the Institutional
Animal Care and Use Committee of Tongji Hospital, Huazhong
University of Science and Technology (Wuhan, China). Adult C57BL/6
male mice (age, 8–10 weeks; weight, 20–24 g) were acquired from the
Wuhan University Centre for Animal Experiments (Wuhan, China). Mice
were maintained in an air-conditioned environment with a 12 h
alternating light and dark cycle and received a standard rodent
diet and water ad libitum.
Experimental protocol
In total, 45 mice were divided at random into the
sham (n=15), mild thoracic aortic banding (TAB; n=15) and severe
TAB (n=15) groups. The mice were anesthetized with ketamine (100
mg/kg; Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China) and
xylazine (5 mg/kg; Lloyd Inc., Shenandoah, IA, USA), administered
intraperitoneally. A 3-mm left-sided thoracotomy was made at the
second intercostal space. The transverse aortic arch was exposed
and ligated (7-0 Prolene suture; Shanghai Medical Suture Needle
Factory, Shanghai, China) using a 25 or 27G needle, between the
right innominate and left common carotid arteries, to induce the
mild and severe TAB, respectively. The needle was then removed
rapidly, leaving a discrete region of stenosis. The chest incision
was subsequently closed and the left-side pneumothorax was
evacuated. The sham group underwent an identical procedure, but
without the ligation of the aortic arch. At week 8, each group was
examined using conventional echocardiography and STE. During the
echocardiographic examinations, varying doses of ISF (Lunan
Pharmaceutical Group Corporation, Shandong, China) were
administered, and heart rate (HR) was used to determine the depth
of the anesthesia. The three states of anesthesia that were induced
in the mice were as follows: Awake (0–0.5% ISF administered
immediately prior to the mice waking up; HR, >520 bpm); light
anesthesia (LA; ISF, 0.5–1%; HR, 460–520 bpm); and deep anesthesia
(DA; ISF, 1–2%; HR, 390–460 bpm).
Anesthesia and preparation of
echocardiography
The mice were anesthetized by ISF inhalation, which
was administered via a Vevo Compact Dual Anesthesia System
(VisualSonics, Inc., Toronto, ON, Canada). Anesthesia induction was
performed for 3 min with 2% ISF/98% O2. Following the
failure of the righting reflex, the mice were placed on a heating
pad in the supine position in order to maintain normothermia. The
dose of ISF was altered as echocardiography was performed under
awake, LA and DA conditions. Under each condition, the
concentration of ISF was adjusted to maintain the immobility and
sedation of the mice. Anesthetic was administered via a nose cone
at a flow rate of 1–1.5 l/min. To produce an electrocardiogram
(ECG), copper electrodes on the heating pad were covered with ECG
gel (Parker Laboratories, Inc., Fairfield, NJ, USA). The paws of
the mice were taped to the electrodes and the ECG was recorded
simultaneously. The walls of the chest were shaved and ultrasound
gel (Parker Laboratories, Inc.) was applied liberally to the
thoracic region to optimize the visibility of the left ventricular
(LV) chamber and the wall motion.
Conventional echocardiography and
parameters
Conventional echocardiography was performed using a
high-resolution Vevo 2100 System with a 30 MHz MS400 linear array
transducer (VisualSonics, Inc.). B-Mode and M-mode images were
obtained in short axis view at the papillary level and in
parasternal long-axis view, with the aortic arch and endocardium in
optimal view. The image depth, width and gain settings were
adjusted during the data acquisition period. The frame rate
remained at >200 Hz in all B-mode and M-mode images in order to
optimize image quality.
M-mode tracings were used to digitally measure the
LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD)
and HR. Based on these measurements, the LV end-systolic volume
(LVESV) was calculated as [7.0/(2.4 + LVESD)] × LVESD. The LV
end-diastolic volume (LVEDV) was calculated as [7.0/(2.4 + LVEDD)]
× LVEDD. The ejection fraction (EF) was calculated as
[(LVEDV-LVESV)/LVEDV] × 100%. The fraction shortening (FS) was
calculated as [(LVEDD-LVESD)/LVEDD] × 100%. The stroke volume (SV)
was calculated as LVEDV-LVESV. The LV mass was calculated as 1.053
× [(LVEDD + LVPWTd + LVAWTd)3-LVEDD3], in
which LVPWTd denotes LV posterior wall thickness in diastole and
LVAWTd denotes LV anterior wall thickness in diastole. The
corrected LV mass was the LV mass × 0.8. All measurements were
presented as the mean of three adjacent cardiac cycles.
Strain analysis
Myocardium strain analyses were performed using
VevoStrain software, version 1.4.0 (VisualSonics, Inc.). Images
that presented the clearest visualization of the endocardium and
epicardium border with the fewest artifacts were selected. All the
analyses were conducted by the same trained operator who was
blinded to the groups. Tracking points were placed in three
consecutive cardiac circles. The endocardium and epicardium were
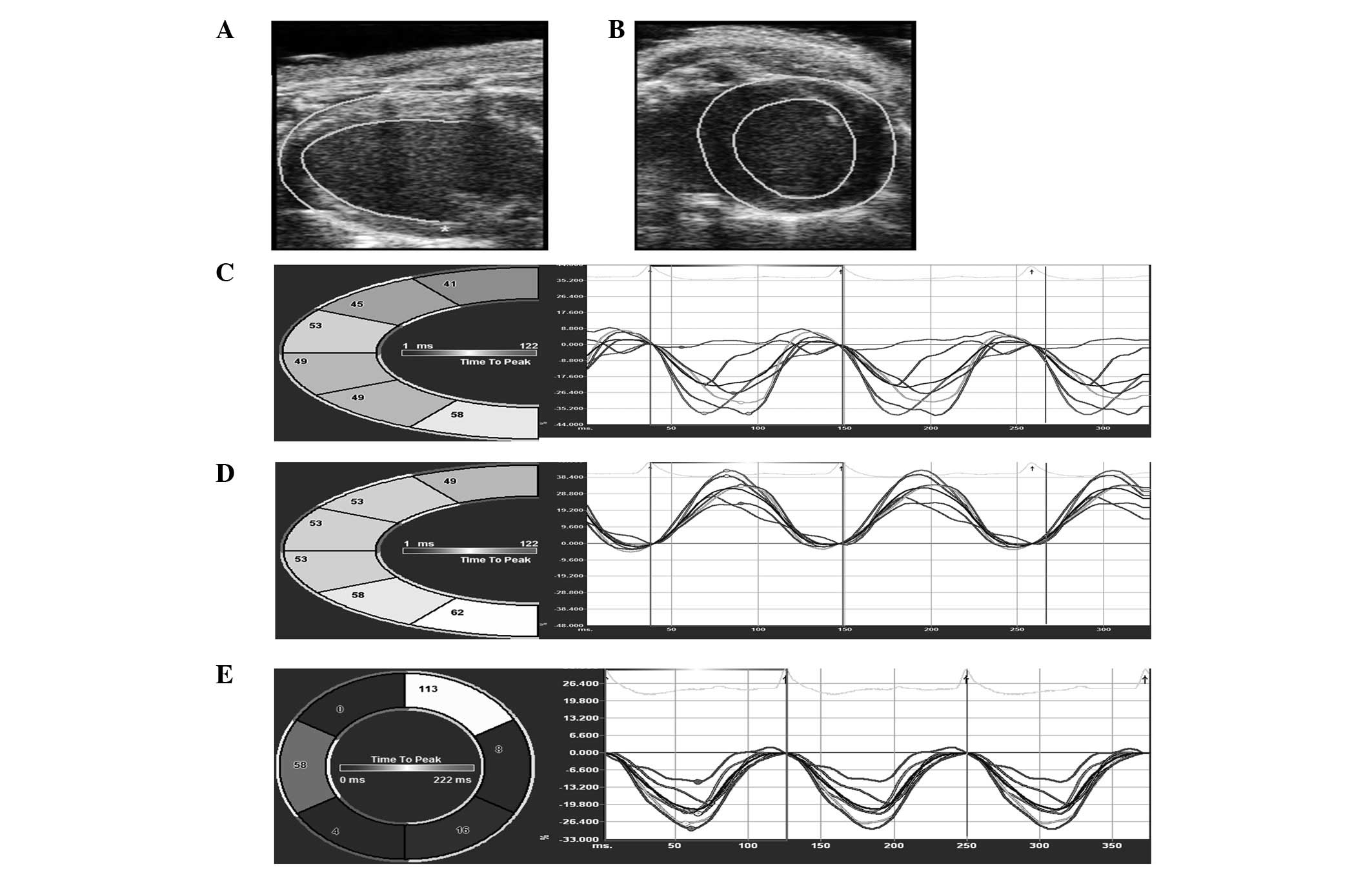
then traced semi-automatically (Fig. 1A
and B). The tracking points were adjusted manually to achieve
well-defined tracking. Images were analyzed frame-by-frame using
the VevoStrain software. The left ventricles were divided into six
segments. For the parasternal long-axis view, they were divided as
follows: Posterior apex, posterior base, posterior medium, anterior
base, anterior medium and anterior apex (Fig. 1A and C). For the short axis view,
they were divided into anterior free wall, lateral wall, posterior
wall, inferior free wall, posterior septum wall and anterior septum
wall. Strain values and the corresponding strain-rate curves were
acquired for each segment in longitudinal (Fig. 1C), radial (Fig. 1D) and circumferential planes
(Fig. 1E). The strain and
strain-rate of each segment were averaged in order to analyze
global function.
Intra- and inter-observer
variability
To assess the intra-observer variability, nine data
sets from each group were selected at random and re-analyzed by the
original investigator following an interval of several weeks.
Inter-observer variability was assessed by comparing the results of
two observers repeatedly analyzing the same data sets. Data were
evaluated as the difference between the two observations divided by
the mean and expressed as the percentage and the correlation
coefficient (15,16).
Statistical analysis
Data are expressed as the mean ± standard error. The
differences between the awake, LA and DA conditions were calculated
using one-way analysis of variance, while Bonferroni post hoc
analysis was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS software, version 17.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Classic hypertrophic responses were
observed in mice in the mild TAB group
The response included the preservation of EF and FS
during the hypertrophic stage and a significant increase in the LV
wall thickness and mass compared with the sham group (Table I). The STE parameters, including
longitudinal strain (LS), radial strain (RS) and corresponding
strain rate exhibited a small reduction compared with the sham
group. The circumferential strain (CS) and strain rate were
preserved in the mild TAB group compared with the sham group
(Table I), which may be attributed
to the compensation mechanism (15).
Furthermore, the severe TAB group mice exhibited a trend towards
heart failure (HF) following TAB (Table
I). In addition, severe TAB mice manifested typical clinical
features of HF syndrome, including lethargy, impaired mobility and
edema. During the post-banding period, 4/15 severe TAB mice
succumbed, possibly to acute decompensated HF and arrhythmia. Three
mice were excluded from the severe TAB group due to bradycardia.
Conventional echocardiography indicated that EF and FS were
significantly reduced in the severe TAB group (Table I). The STE parameters, including LS,
RS, CS and corresponding strain rate were significantly reduced in
the severe TAB group compared with the sham and mild TAB groups,
whilst an increase was observed in the LVEDV and LVESV values
(Table I). Fig. 2 exhibits marked differences in
cardiac contraction and LV wall chamber cavity sizes among the
sham, mild TAB and severe TAB groups.
 | Table I.Postoperative changes in
echocardiographic parameters following TAB. |
Table I.
Postoperative changes in
echocardiographic parameters following TAB.
| Parameter | Sham | Mild TAB | Severe TAB |
|---|
| Conventional
measures |
|
|
|
| Body
weight (g) | 23.27±0.74 | 27.06±0.35 | 25.44±0.79 |
| HR
(bpm) | 520±7a b | 512±6a | 477±9 |
| LVEDD
(mm) | 3.6±0.09a |
3.67±0.17a | 4.31±0.44 |
| LVESD
(mm) |
2.09±0.09a |
2.05±0.23a | 3.14±0.60 |
| LVWT
(mm) |
1.13±0.02b |
1.46±0.03a | 1.17±0.06 |
| LVEDV
(µl) |
55.23±2.87a |
60.96±6.60a | 100.57±21.88 |
| LVESV
(µl) |
15.10±1.35a |
22.71±6.79a | 70.72±23.05 |
| EF
(%) |
76.15±2.71a |
73.74±4.02a | 46.18±4.79 |
| FS
(%) |
46.75±2.47a |
41.36±3.61a | 22.10±3.92 |
| LV mass
(mg) |
105.52±5.59a b |
159.04±11.30a | 198.88±12.87 |
|
Corrected LV mass (mg) |
84.41±4.47a b |
133.27±10.21a | 161.10±10.30 |
| SV
(µl) |
40.13±2.08a |
42.06±2.67a | 29.85±6.25 |
| Strain
measures |
|
|
|
| Short axis |
|
|
|
| CS
(%) |
−28.91±0.51a |
−29.15±1.44a | −12.87±0.78 |
| CSR
(s−1) |
−10.46±0.45a |
−10.68±0.8a | −4.74±0.06 |
| Long axis |
|
|
|
| LS
(%) |
−26.83±1.8a b |
−20.01±1.43a | −15.4±0.94 |
| LSR
(s−1) |
−10.99±0.34a b |
−7.8±0.41a | −5.7±0.21 |
| RS
(%) |
34.22±1.01a b |
26.77±0.12a | 14.28±1.08 |
| RSR
(s−1) |
9.95±0.62a b |
7.45±0.22a | 4.58±0.32 |
Effects of anesthesia on conventional
echocardiography
To assess the effect of anesthesia on conventional
echocardiographic parameters, the values for these parameters in
the awake, LA and DA conditions were compared among the sham
(Table II), mild TAB (Table III) and severe TAB groups (Table IV). In all three groups the stepwise
increase of ISF dose appeared to gradually reduce HR under the
awake, LA and DA conditions (P<0.05). The conventional
echocardiographic parameters examined included LVEDD, LVESD,
LVPWTd, LVPWTs (LV posterior wall thickness in systole), LVAWTd,
LVAWTs (LV anterior wall thickness in systole), LVEDV, LVESV, EF,
FS, LV mass, corrected LV mass and SV. All of these parameters
remained stable under the LA and awake conditions in the sham and
mild TAB groups (P>0.05; Tables
II and III). However, an
increase in ISF concentration resulted in a significant suppression
of these conventional parameters under the DA condition compared
with the awake and LA conditions (P<0.05; Tables II and III). However, there were exceptions,
including the LV and corrected LV mass in the sham and mild TAB
groups, which did not change with the different ISF conditions.
Furthermore, no reduction was observed in the LVPWTd parameter in
the sham group under DA conditions. Analysis of the severe TAB
group indicated that all the conventional echocardiographic
parameters remained constant under awake, LA and DA conditions
(Table IV).
 | Table II.Conventional echocardiographic
parameters in the sham group mice under different states of
anesthesia. |
Table II.
Conventional echocardiographic
parameters in the sham group mice under different states of
anesthesia.
| Parameter | Awake | LA | DA |
|---|
| Weight (g) | 22.52±0.41 | 23.27±0.74 | 23.15±0.66 |
| HR (bpm) | 567±11a b | 520±5a | 407±10 |
| LVEDD (mm) |
3.51±0.07a |
3.6±0.09a | 4.01±0.10 |
| LVESD (mm) |
2.06±0.07a |
2.09±0.09a | 2.88±0.12 |
| LVPWTd (mm) | 0.82±0.02 | 0.81±0.03 | 0.77±0.028 |
| LVPWTs (mm) |
1.31±0.04a |
1.40±0.06a | 1.08±0.044 |
| LVAWTd (mm) |
0.80±0.03a |
0.85±0.03a | 0.69±0.04 |
| LVAWTs (mm) |
1.34±0.04a |
1.35±0.04a | 1.00±0.06 |
| LVEDV (µl) |
52.41±2.27a |
55.23±2.87a | 70.791±4.00 |
| LVESV (µl) |
14.79±1.18a |
15.10±1.35a | 40.32±3.23 |
| EF (%) |
72.53±1.52a |
76.15±2.71a | 54.34±2.55 |
| FS (%) |
41.29±1.30a |
46.75±2.47a | 28.09±1.60 |
| LV mass (mg) | 96.79±3.43 | 105.52±5.59 | 106.00±5.72 |
| Corrected LV mass
(mg) | 77.44±2.75 | 84.41±4.47 | 84.81±4.58 |
| SV (µl) |
37.62±1.57a |
40.13±2.08a | 27.48±1.93 |
 | Table III.Conventional echocardiographic
parameters in the mild TAB group mice under different states of
anesthesia. |
Table III.
Conventional echocardiographic
parameters in the mild TAB group mice under different states of
anesthesia.
| Parameter | Awake | LA | DA |
|---|
| Weight (g) | 27.23±0.21 | 27.06±0.35 | 26.47±0.77 |
| HR (bpm) | 563±4a b | 512±6a | 395±8 |
| LVEDD (mm) |
3.53±0.10a |
3.67±0.17a | 4.44±0.08 |
| LVESD (mm) |
1.88±0.13a |
2.05±0.23a | 3.46±0.14 |
| LVPWTd (mm) |
1.13±0.04a |
1.09±0.02a | 0.93±0.04 |
| LVPWTs (mm) |
1.67±0.06a |
1.59±0.09a | 1.12±0.07 |
| LVAWTd (mm) |
1.24±0.05a |
1.13±0.05a | 0.94±0.05 |
| LVAWTs (mm) |
1.88±0.07a |
1.74±0.08a | 1.27±0.08 |
| LVEDV (µl) |
52.85±3.48a |
60.96±6.60a | 89.70±3.75 |
| LVESV (µl) |
12.33±2.23a |
22.71±6.79a | 51.29±4.82 |
| EF (%) |
78.88±2.53a |
73.74±4.02a | 44.45±3.49 |
| FS (%) |
47.96±2.29a |
41.36±3.61a | 22.47±2.19 |
| LV mass (mg) | 172.86±9.33 | 159.04±11.30 | 175.02±7.72 |
| Corrected LV mass
(mg) | 138.37±7.47 | 133.27 ±10.21 | 140.04±6.18 |
| SV (µl) |
40.53±2.06a |
42.06±2.6a | 31.41±2.01 |
 | Table IV.Conventional echocardiographic
parameters in the severe TAB group mice under different states of
anesthesia. |
Table IV.
Conventional echocardiographic
parameters in the severe TAB group mice under different states of
anesthesia.
| Parameter | Awake | LA | DA |
|---|
| Weight (g) | 27.16±1.27 | 25.44±0.79 | 24.35±0.47 |
| HR (bpm) | 523±5a b | 477±9a | 404±8 |
| LVEDD (mm) | 4.54±0.47 | 4.31±0.44 | 4.95±0.28 |
| LVESD (mm) | 3.60±0.61 | 3.14±0.60 | 4.09±0.43 |
| LVPWTd (mm) | 0.99±0.12 | 0.96±0.11 | 0.84±0.04 |
| LVPWTs (mm) | 1.29±0.17 | 1.40±0.17 | 1.04±0.04 |
| LVAWTd (mm) | 1.16±0.06 | 1.13±0.06 | 1.02±0.05 |
| LVAWTs (mm) | 1.46±0.11 | 1.49±0.10 | 1.32±0.11 |
| LVEDV (µl) | 101.30±23.73 | 100.57±21.88 | 103.03±14.44 |
| LVESV (µl) | 75.66±23.74 | 70.72±23.05 | 75.16±18.14 |
| EF (%) | 47.03±11.23 | 46.18±4.79 | 46.84±7.93 |
| FS (%) | 26.42±8.36 | 22.10±3.92 | 28.44±4.37 |
| LV mass (mg) | 209.36±12.13 | 198.88±12.87 | 204.42±20.13 |
| Corrected LV mass
(mg) | 167.48±9.71 | 161.10±10.30 | 163.53±16.11 |
| SV (µl) | 28.64±7.55 | 29.85±6.25 | 27.87±7.16 |
Effects of anesthesia on STE
The strain and corresponding strain rates were
acquired from mice under the awake, LA and DA states in order to
determine the effect of anesthesia on the results of STE
examination. No significant differences in LS, CS, RS or
corresponding strain rates were observed between the LA and awake
conditions in the three groups (P>0.05; Fig. 3). However, the STE parameters in all
three groups exhibited significant differences under DA conditions
compared with LA and awake conditions (P<0.05; Fig. 3).
Inter- and intra-observer
variability
The results of the analysis of inter- and
intra-observer variability are presented in Table V. The data indicated no significant
level of inter- and intra-observer variability and very high
correlation coefficients for the conventional and STE
parameters.
 | Table V.Intra- and inter-observer variability
for conventional and speckle tracking echocardiography. |
Table V.
Intra- and inter-observer variability
for conventional and speckle tracking echocardiography.
|
| Inter-observer | Intra-observer |
|---|
|
|
|
|
|---|
| Parameter | % Error | CC | % Error | CC |
|---|
| Conventional
measures |
|
|
|
|
| LVEDD
(mm) | 0.7±1.3 | 0.84 | 0.5±0.2 | 0.86 |
| LVESD
(mm) | 3.3±2.6 | 0.98 | 2.4±1.7 | 0.99 |
| LVPWTd
(mm) | 9.6±2.5 | 0.89 | 4.3±1.8 | 0.94 |
| LVPWTs
(mm) | 3.8±2.6 | 0.95 | 4.5±1.4 | 0.92 |
| LVAWTd
(mm) | 4.9±1.1 | 0.89 | 3.7±1.3 | 0.9 |
| LVAWTs
(mm) | 2.6±3.1 | 0.97 | 1.7±3.5 | 0.98 |
| LVEDV
(µl) | 1.6±3.0 | 0.92 | 1.4±3.2 | 0.91 |
| LVESV
(µl) | 4.6±0.9 | 0.80 | 3.5±0.7 | 0.82 |
| EF
(%) | 6.2±1.5 | 0.82 | 4.9±0.5 | 0.87 |
| FS
(%) | 5.7±1.2 | 0.87 | 7.8±0.6 | 0.89 |
| LV mass
(mg) | 8.2±4.6 | 0.83 | 5.6±1.7 | 0.87 |
|
Corrected LV mass (mg) | 8.2±4.6 | 0.83 | 4.4±6.2 | 0.91 |
| SV
(µl) | 0.7±1.8 | 0.82 | 0.9±1.4 | 0.85 |
| Strain
measures |
|
|
|
|
| Short axis |
|
|
|
|
| CS
(%) | 1.8±1.2 | 0.97 | 1.4±0.3 | 0.98 |
| CSR
(s−1) | 4.2±4.1 | 0.94 | 5.9±4.5 | 0.92 |
| Long axis |
|
|
|
|
| LS
(%) | 1.9±1.6 | 0.92 | 1.8±2.1 | 0.90 |
| LSR
(s−1) | 3.8±3.0 | 0.93 | 4.5±4.0 | 0.96 |
| RS
(%) | 2.5±1.6 | 0.90 | 3.8±1.9 | 0.91 |
| RSR
(s−1) | 3.2±2.6 | 0.97 | 2.3±1.9 | 0.99 |
Discussion
The present study demonstrated that under LA
conditions (HR range, 460–520 bpm; ISF range, 0.5–1%), conventional
echocardiography and STE may be applied in healthy, hypertrophic
and HF myocardium with a slight reduction of cardiac function.
Previous studies have reported that
echocardiographic parameters are affected by anesthetic agents and
that varying doses of anesthesia may influence the result of
echocardiographic measurement (16–18). In
the present study, a marked reduction was observed in the
conventional echocardiographic parameters of the sham group under
DA conditions. However, these appeared to remain constant under
awake and LA conditions. In agreement with this, conventional
echocardiographic parameters were highly repeatable in the present
study under LA conditions, despite a slight elevation in ISF dose
and HR.
Anesthesia is considered to be an influential factor
in the measurement of conventional echocardiographic parameters in
healthy myocardium. However, a limited number of studies have
investigated the effects of anesthesia on hypertrophic and failing
myocardium. In the present study, conventional echocardiographic
parameters in hypertrophic myocardium were reduced under DA
conditions, but preserved under LA and awake conditions. However,
the conventional echocardiographic parameters were unchanged in the
failing myocardium under LA, DA and awake conditions. This may be
attributable to the limited number of mice, which resulted in a
large variability in conventional echocardiographic parameters in
the severe TAB group compared with the sham and mild TAB
groups.
The underlying mechanism for the improved
reproducibility in mice under LA conditions may be attributed to
the high HR in the LA and awake conditions. Generally,
force-frequency dependence varied only minimally in high HR
(>550 bpm) but altered significantly in the low HR conditions
(<400 bpm) in murine hearts. Therefore, LV contractility
remained almost unaltered under high HR conditions, which explained
why echocardiographic parameters were constant under LA conditions
with high HR (18). The
aforementioned data supports the hypothesis that conventional
echocardiographic evaluation is clinically applicable under LA
conditions in mice, with reduced cardiac systolic depression and
perfect reproducibility. This protocol may be used in healthy or
diseased mice.
The present study demonstrated that an increase of
ISF dose and subsequent suppression of HR resulted in an evident
reduction of the strain parameters in mice under DA conditions, but
no alteration in mice in the LA and awake states. However, a
previous study by Weidemann et al (19) indicated that strain increased
following a reduction in HR. This contradiction may be explained by
the following mechanism: Strain is determined primarily by ejection
performance parameters such as SV (20,21).
Following a reduction in HR, the preload and ejection period
increased, which led to an increase in SV and strain. However,
under DA conditions, the increased administration of ISF reversed
this trend by significantly reducing cardiac contraction (22,23). Due
to the abnormal contraction observed in mice under DA conditions,
the SV was considerably suppressed, which resulted in a reduction
in strain.
In addition, the effect of anesthesia on strain rate
was analyzed. Increasing the ISF dose and thus reducing HR resulted
in a reduction in strain rate in mice under DA conditions. However,
strain rate remained constant in mice in the LA and awake states in
each group. However, previous studies involving rats and dogs have
indicated that strain rate is independent of HR (14,24).
This paradox may be explained by the following mechanism: With the
reduction of HR, the intrinsic contractility reduced due to the
negative Treppe effect (25).
However, as the preload and ejection period increased, so did the
intrinsic contractility, via the negative Frank-Starling mechanism
(26). The combination of these two
factors may have resulted in a balancing effect on the extent of
contractility. Therefore, the strain rate as an index of change of
contractility, remained the same as the HR fluctuated. However, as
the dose of ISF increased under DA conditions, the contractility
was suppressed significantly and the balancing effect on
contractility was prevented. This may explain the significant
reduction of strain rate under DA conditions.
There were a number of limitations to the present
study: Firstly, conscious mice were not included as a control, as
the elevation of sympathetic tone during conscious measurement
could not be fully eliminated, despite the mice undergoing training
a few days prior to the experiment (27). In addition, the images used for
strain analysis must be of high resolution, with clear
visualization of the endocardium and epicardium borders. Therefore,
it is necessary that the mice be immobile, which would not have
been feasible if the mice had remained conscious. Secondly, Doppler
parameters were not measured due to the high HR, as Doppler
waveforms merge together when there is a reduction in the diastolic
period (28,29). Thirdly, bradycardic mice with a low
HR (<500 bpm) and HF in the severe TAB group were excluded from
the study. Therefore, the least healthy mice with HF were omitted
from the study and the effects of anesthesia on HF mice may have
been underestimated.
In conclusion, the present study conducted a novel
investigation into the effects of anesthesia on conventional
echocardiography and STE parameters in healthy mice and
pathological models. The pathological model is of particular
clinical relevance, as anesthesia may severely reduce cardiac
contraction in patients with HF. In addition, the present study
examined the effects of anesthesia in a mouse model of pressure
overload/aortic stenosis and assessed whether an anesthetic was
able to limit the effectiveness of echocardiography in the
evaluation of cardiac function (30). Under LA conditions, relatively
precise conventional and STE measurements may be obtained without a
significant reduction in cardiac function. This approach offers a
potentially valuable method for accurately measuring conventional
and STE parameters with good reproducibility. Thus, immobility and
sedation may widen the applicability of conventional
echocardiography and STE in healthy and diseased mice.
Acknowledgements
The current study was supported by grants from the
Hubei Science & Technology Pillar Program (no. 2012DCA12007)
and the National Natural Science Foundation of China (no.
81300141).
References
|
1
|
Yu L, Ruifrok WP, Meissner M, et al:
Genetic and pharmacological inhibition of galectin-3 prevents
cardiac remodeling by interfering with myocardial fibrogenesis.
Circ Heart Fail. 6:107–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lairez O, Calise D, Bianchi P, et al:
Genetic deletion of MAO-A promotes serotonin-dependent ventricular
hypertrophy by pressure overload. J Mol Cell Cardiol. 46:587–595.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asai K, Yang GP, Geng YJ, et al:
Beta-adrenergic receptor blockade arrests myocyte damage and
preserves cardiac function in the transgenic G(salpha) mouse. J
Clin Invest. 104:551–558. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guellich A, Gao S, Hong C, et al: Effects
of cardiac overexpression of type 6 adenylyl cyclase affects on the
response to chronic pressure overload. Am J Physiol Heart Circ
Physiol. 299:H707–H712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stanton T and Marwick TH: Assessment of
subendocardial structure and function. JACC Cardiovasc Imaging.
3:867–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cottrell C and Kirkpatrick JN:
Echocardiographic strain imaging and its use in the clinical
setting. Expert Rev Cardiovasc Ther. 8:93–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abduch MC, Alencar AM, Mathias W Jr and
Vieira ML: Cardiac mechanics evaluated by speckle tracking
echocardiography. Arq Bras Cardiol. 4:403–412. 2014.
|
|
8
|
Kurt M, Tanboga IH and Aksakal E:
Two-dimensional strain imaging: Basic principles and technical
consideration. Eurasian J Med. 46:126–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roth DM, Swaney JS, Dalton ND, Gilpin EA
and Ross J Jr.: Impact of anesthesia on cardiac function during
echocardiography in mice. Am J Physiol Heart Circ Physiol.
282:H2134–H2140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Collins KA, Korcarz CE and Lang RM: Use of
echocardiography for the phenotypic assessment of genetically
altered mice. Physiol Genomics. 13:227–239. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lairez O, Lonjaret L, Ruiz S, et al:
Anesthetic regimen for cardiac function evaluation by
echocardiography in mice: comparison between ketamine, etomidate
and isoflurane versus conscious state. Lab Anim. 47:284–290. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szczesny G, Veihelmann A, Massberg S,
Nolte D and Messmer K: Long-term anaesthesia using inhalatory
isoflurane in different strains of mice-the haemodynamic effects.
Lab Anim. 38:64–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuda Y, Ohsaka K, Yamamoto H, et al:
Comparison of newly developed inhalation anesthesia system and
intraperitoneal anesthesia on the hemodynamic state in mice. Biol
Pharm Bull. 30:1716–1720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weytjens C, DHooge J, Droogmans S, et al:
Influence of heart rate reduction on Doppler myocardial imaging
parameters in a small animal model. Ultrasound Med Biol. 35:30–35.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka N, Dalton N, Mao L, et al:
Transthoracic echocardiography in models of cardiac disease in the
mouse. Circulation. 94:1109–1117. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gentry-Smetana S, Redford D, Moore D and
Larson DF: Direct effects of volatile anesthetics on cardiac
function. Perfusion. 23:43–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takamura T, Dohi K, Onishi K, et al: Left
ventricular contraction-relaxation coupling in normal,
hypertrophic, and failing myocardium quantified by speckle-tracking
global strain and strain rate imaging. J Am Soc Echocardiogr.
23:747–754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riha H, Papoušek F, Neckář J, Pirk J and
Ošt'ádal B: Effects of isoflurane concentration on basic
echocardiographic parameters of the left ventricle in rats. Physiol
Res. 61:419–423. 2012.PubMed/NCBI
|
|
19
|
Weidemann F, Jamal F, Kowalski M, et al:
Can strain rate and strain quantify changes in regional systolic
function during dobutamine infusion, B-blockade, and atrial pacing
- implications for quantitative stress echocardiography. J Am Soc
Echocardiogr. 15:416–424. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morris JJ III, Pellom GL, Murphy CE,
Salter DR, Goldstein JP and Wechsler AS: Quantification of the
contractile response to injury: assessment of the work-length
relationship in the intact heart. Circulation. 76:717–727. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weidemann F, Jamal F, Sutherland GR, et
al: Myocardial function defined by strain rate and strain during
alterations in inotropic states and heart rate. Am J Physiol Heart
Circ Physiol. 283:H792–H799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davies LA, Gibson CN, Boyett MR, Hopkins
PM and Harrison SM: Effects of isoflurane, sevoflurane, and
halothane on myofilament Ca2+ sensitivity and sarcoplasmic
reticulum Ca2+ release in rat ventricular myocytes. Anesthesiology.
93:1034–1044. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davies LA, Hamilton DL, Hopkins PM, Boyett
MR and Harrison SM: Concentration-dependent inotropic effects of
halothane, isoflurane and sevoflurane on rat ventricular myocytes.
Br J Anaesth. 82:723–730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki R, Matsumoto H, Teshima T and
Koyama H: Influence of heart rate on myocardial function using
two-dimensional speckle-tracking echocardiography in healthy dogs.
J Vet Cardiol. 15:139–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woodworth RS: Maximal contraction,
“staircase” contraction, refractory period, and compensatory pause
of the heart. Am J Physiol. 8:213–249. 1902.
|
|
26
|
Katz AM: Ernest Henry Starling, his
predecessors, and the “Law of the Heart”. Circulation.
106:2986–2992. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uechi M, Asai K, Osaka M, et al: Depressed
heart rate variability and arterial baroreflex in conscious
transgenic mice with overexpression of cardiac Gsalpha. Circ Res.
82:416–423. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schaefer A, Meyer GP, Brand B,
Hilfiker-Kleiner D, Drexler H and Klein G: Effects of anesthesia on
diastolic function in mice assessed by echocardiography.
Echocardiography. 22:665–670. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rottman JN, Ni G, Khoo M, et al: Temporal
changes in ventricular function assessed echocardiographically in
conscious and anesthetized mice. J Am Soc Echocardiogr.
16:1150–1157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weiss RM, Ohashi M, Miller JD, Young SG
and Heistad DD: Calcific aortic valve stenosis in old
hypercholesterolemic mice. Circulation. 114:2065–2069. 2006.
View Article : Google Scholar : PubMed/NCBI
|