Introduction
Osteoarthritis (OA) is a degenerative and
progressive disease that affects mainly joint cartilage and
subchondral bone (1). Conventional
drug therapy for OA includes non-opioid analgesics such as
paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs),
topical and opioid analgesics, and intra-articular steroid
injections (2,3). These treatments may be ineffective in
certain patients and NSAIDS frequently have serious adverse
effects, including gastrointestinal complications (4,5). Thus,
drugs with good efficacy and low toxicity are required for the
treatment of OA.
Calcium (Ca) salts have anti-inflammatory activities
(6,7), and various Ca salts have demonstrated
preventive or therapeutic effects on osteoporosis (8,9). Polycan
is purified β-glucan from Aureobasidium pullulans SM-2001,
and is comprised mostly of β-1,3/1,6-glucan (10). It has been found that Polycan has
anti-osteoporotic effects by inhibiting bone loss and accelerating
bone formation (11,12), as well as promoting fracture healing
(13) with anti-inflammatory effects
(14,15). In addition, the individual effects of
Polycan and a Ca salt in a rat model of OA have been evaluated and
revealed beneficial effects following anterior cruciate ligament
transection and partial medial meniscectomy, respectively (16,17).
In a previous study, it was found that a mixture of
Polycan and Ca lactate-gluconate in a 1:9 weight ratio, designated
Polycalcium, had favorable and synergistic effects on osteoporotic
rats compared with two other Polycan/Ca lactate-gluconate mixtures
(5:95 and 1:99) (18). In the
present study, the beneficial and synergistic effects of
Polycalcium on a rat model of OA were compared with the effects of
Polycan or Ca lactate-gluconate alone.
Materials and methods
Animals
A total of 80 Sprague-Dawley specific pathogen-free
male rats (6 weeks old upon receipt; Japan SLC, Inc., Hamamatsu,
Japan) were used following acclimatization for 8 days. Animals were
allocated five per polycarbonate cage in a temperature (20–25ºC)
and humidity (30–35%) controlled room. The light:dark cycle was 12
h:12 h, and commercial feed (Samyang Foods Co., Ltd, Wonju, Korea)
and water were supplied ad libitum. The total of the 80 rats
were randomly assigned to each of the 8 groups. The study was
approved by the Ethics Committee of Daegu Haany University
(Gyeongsan, Korea).
Preparation and drug
administration
Polycan and Ca lactate-gluconate were supplied by
Glucan Corporation (Busan, Korea) as brown and white powders,
respectively. Diclofenac sodium was purchased from Wako (Osaka,
Japan). All test materials were stored in a refrigerator to protect
them from light and moisture. Three different doses (50, 100 and
200 mg/kg) of Polycalcium (containing Polycan and calcium
lactate-gluconate in a 1:9 weight ratio) were dissolved in
distilled water and administered orally once per day for 28 days at
5 ml/kg from 1 week after OA. In the sham and OA control groups,
only 5 ml/kg distilled water was administered orally from 1 week
after the OA-induction surgery. As a reference control, 2 mg/kg
diclofenac sodium (dissolved in saline at a volume of 1 ml/kg) was
administered subcutaneously once per day for 28 days from 1 week
after OA.
Grouping
A total of 80 rats were divided randomly into eight
groups as follows: Sham control, OA control, diclofenac-treated,
polycan-treated, Ca-LG and polycalcium-treated (with 50, 100 and
200 mg/kg dose) groups.
Induction of OA
Rats were anesthetized with 25 mg/kg Zoletile
intraperitoneally (Zoletile 50; Virbac, Carros, France). The
surgical procedure was performed as follows. The OA treatment group
underwent open surgery in which anterior cruciate ligament
transection and partial medial meniscectomy were conducted via an
incision on the medial aspect of the joint capsule of the left
knee, anterior to the medial collateral ligament. Following
surgery, the incision was closed in two layers. The joint capsule
was sutured independently from peripheral tissues using dissolvable
5-0 Vicryl sutures (Ethicon, Inc., Somerville, NJ, USA), and the
skin was closed with interrupted silk sutures. This surgery induced
OA pathogenesis in the operated knees. The second group of rats
underwent a sham surgery in which a similar incision in the joint
capsule was made, but anterior cruciate ligament transection and
partial medial meniscectomy were not performed.
Changes in body weight
Body weights were measured weekly from the start of
treatment until sacrifice using an automatic electronic balance
(Precisa Instruments, Dietikon, Switzerland). In addition, body
weight gains for 12 weeks after test material treatment were
calculated.
Knee thickness measurement
The thickness of the OA-operated left hind knee was
measured using an electronic digital caliper (Mitutoyo Corporation,
Kawasaki, Japan) and recorded weekly after treatment with test
materials. In addition, the knee thickness was measured following
joint capsule exposure upon sacrifice to reduce differences from
surrounding tissues using the same methods.
Measurement of the maximum extensor
angle
OA-operated knees were dissected from the
coxofemoral region to the ankle region, leaving the articular
capsule intact. Following dissection, the maximum extension angle
of each knee was measured as described previously (19), with 0° corresponding to the maximum
possible extension. To minimize bias, all surgeries and
measurements of the extension level were performed by the same
veterinarian.
Measurement of cartilage
glycosaminoglycan (GAG) content
The quantities of cartilage GAGs, namely heparin
sulfate, chondroitin sulfate and hyaluronic acid, were measured as
described previously (20) using an
ultraviolet spectrophotometer (Model 22: Angstrom Advanced Inc.,
Braintree, MA, USA) at absorbances of 478, 480 and 650 nm,
respectively.
Histopathology
The knee joints were sampled with joint capsule
preservation and fixed in 10% neutral-buffered formalin. After 5
days of fixation, the knee joints were decalcified using a
decalcifying solution (24.4% formic acid and 0.5 N sodium
hydroxide) for 5 days, with exchange of the mixed decalcifying
solution once per day over the 5 days. Next, median joints were
longitudinally trimmed and embedded in paraffin, sectioned (3–4 µm)
and stained with Safranin O for cartilage visualization as
described previously (21–23). Histological profiles of the knee
joints were evaluated using Mankin scoring (24,25), and
the thickness of articular cartilage was compared with that of the
intact control.
5-Bromo-2’-deoxyuridine (BrdU) uptake
measurement
To assess the effects of Polycalcium on the
proliferation of cells, proliferating cells were labeled by means
of an intraperitoneal injection of BrdU (Sigma, St. Louis, MO,
USA). One hour prior to test material treatment (on day 25 of
treatment), rats were administered intraperitoneal injections of
BrdU at 50 mg/kg dissolved in saline, and the animals were
sacrificed 72 h later, as described previously (26). BrdU uptake was detected with an
anti-BrdU antibody as described by Moore et al (27). Fixed tissues were prepared, embedded
in paraffin, and sectioned as described above. Tissues were
de-paraffinized through a series of washes with xylene and graded
alcohols. Following epitope retrieval by pretreatment with trypsin
(Sigma) and 2 N HCl, as described previously, sections were
immunostained (28,29) using primary BrdU antiserum (anti-BrdU
monoclonal antibody; VP-B209; Vector Laboratories, Inc.,
Burlingame, CA, USA), Vectastain Universal Elite ABC kit (PK-6200;
Vector Laboratories, Inc.) and DAB Peroxidase Substrate kit
(SK-4100; Vector Laboratories, Inc.).
BrdU immunoreactive cell counts were determined as
follows: Among 100 chondrocytes, cells accounting for >10% of
BrdU immunoreactivity were detected in the inner articular membrane
and surface articular cartilage of the femur and tibia using an
automated digital image analyzer (DMI-300; DMI, Daegu, Korea). The
histopathologist was blinded to group distribution during this
analysis.
Statistical analyses
Multiple comparison tests for different dose groups
were conducted. Variance homogeneity was examined using the Levene
test. If the Levene test showed no significant deviations from
variance homogeneity, the data were analyzed using one-way analysis
of variance followed by least-significant differences (LSD)
multi-comparison tests to determine which pairs of group
comparisons were significantly different. When significant
deviations from variance homogeneity were observed based on the
Levene test, a non-parametric comparison test, the Kruskal-Wallis H
test, was conducted. When a significant difference was observed in
the Kruskal-Wallis H test, the Mann-Whitney U test was conducted to
identify specific pairs of group comparisons that differed
significantly. Statistical analyses were conducted using SPSS for
Windows (Release 14K, SPSS Inc., Chicago, IL, USA). In addition,
the percentage changes compared with the OA control were calculated
to increase the understanding of the efficacy of test materials,
and the percentage changes between sham and OA control were
calculated to determine the induction status of OA.
Results
Changes in body weight
No significant changes in body weight were detected
in test material-treated groups compared with the OA controls (data
not shown).
Changes in knee thickness prior to
articular capsule exposure
Significant (P<0.01) increases in OA-operated
knee thickness were detected in the OA controls compared with the
sham controls from the initial treatment day until day 28. However,
the OA-induced increases in induced knee thickness were
significantly (P<0.01 or P<0.05) attenuated from 7 days after
treatment with test materials, including diclofenac sodium. The
Polycalcium (50 mg/kg)-treated group exhibited an inhibitory effect
of the OA-induced increase in knee thickness similar to that in the
Ca lactate-gluconate-treated group. The least significant reversal
in knee thickness increase was observed in the Polycan-treated
group (Fig. 1).
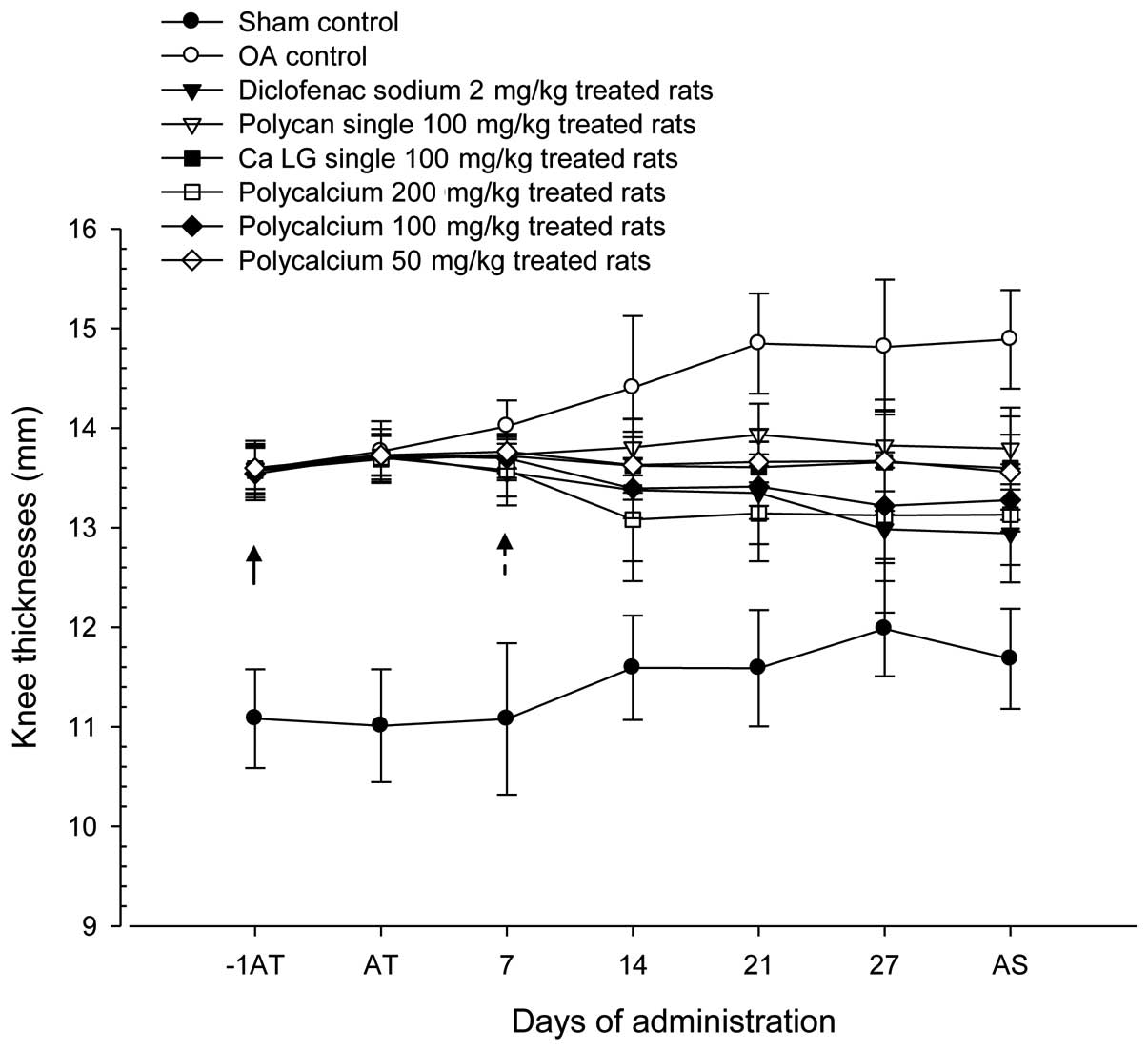 | Figure 1.Changes in OA-induced knee thickness
during 28 days of continuous oral treatment with test materials in
a rat model of OA. Note that significant (P<0.01) increases in
OA-operated knee thickness were detected in the OA control from 1
day prior to test material treatment as compared with the sham
control (arrow). However, these increases in OA-induced knee
thickness were significantly (P<0.01) attenuated in polycan (#;
P<0.05 on day 14), PC 100 mg/kg, 200 mg/kg, diclofenac-treated
groups and were significantly (P<0.05) attenuated in PC 50
mg/kg, Ca LG 100 mg/kg treated groups from 7 days after the start
of treatment as compared with the OA control (dot arrow). Values
are expressed as the mean ± SD of ten rats. OA, osteoarthritis; Ca,
calcium; LG, lactate-gluconate; −1AT; 1 day before the start of
test material treatment; AT, start of test material treatment; AS,
at sacrifice. All animals were fasted overnight prior to OA
induction, the start of test material treatment and sacrifice.
P<0.05, diclofenac sodium vs. sham control by least-significant
differences (LSD) test. P<0.05, Ca LG vs. sham control by LSD
test. P<0.05, polycalcium 50 mg/kg vs. sham control by LSD
test. |
The knee thickness of the OA control was increased
by 27.45% compared with that of the sham control. Reductions in
knee thickness of 13.08, 7.36, 8.69, 11.82, 10.83 and 8.95% were
observed following treatment with diclofenac sodium, Polycan alone,
Ca lactate-gluconate alone and Polycalcium (50, 100 and 200 mg/kg),
respectively, compared with the knee thickness in the OA control
group.
Changes in knee thickness following
capsule exposure
The thickness of the OA-operated knees following
joint capsule exposure was significantly (P<0.01) increased in
all OA-induced groups compared with the sham control. However, the
OA-induced increases in knee thickness following joint capsule
exposure were significantly (P<0.01 or P<0.05) reversed in
all test material-treated groups. The Polycalcium (50
mg/kg)-treated group exhibited a reversal effect on the increases
in knee thickness similar to those of Polycan alone and Ca
lactate-gluconate alone. Joint capsule exposure to Polycan alone
exhibited the least significant reversal of the increase in knee
thickness (Table I).
 | Table I.Knee thickness after joint capsule
exposure and maximum extensor angles detected at sacrifice after 28
days of continuous oral treatment with test materials in OA
rats. |
Table I.
Knee thickness after joint capsule
exposure and maximum extensor angles detected at sacrifice after 28
days of continuous oral treatment with test materials in OA
rats.
| Groups | Knee thickness
(mm) | Maximum extensor
angle (°) |
|---|
| Controls |
|
|
| Sham | 7.85±0.29 | 29.80±2.66 |
| OA |
9.26±0.37a |
57.60±4.25a |
| Diclofenac |
8.83±0.42a, b |
45.90±6.64a, b |
| Polycan alone |
8.94±0.25a,c |
49.40±4.03a, b |
| Ca LG alone |
8.85±0.32a, b |
47.70±3.06a, b |
| Polycalcium |
|
|
|
| 200
mg/kg |
8.67±0.36a, b |
38.30±5.29a, b |
| 100
mg/kg |
8.73±0.34a, b |
40.30±4.47a, b |
| 50
mg/kg |
8.85±0.28a, b |
46.30±6.46a, b |
Knee thickness following joint capsule exposure in
the OA control was increased by 17.96% compared with that in the
sham control. Reductions of 4.69, 3.42, 4.43, 6.40, 5.77 and 4.45%
were observed following treatment with diclofenac sodium, Polycan
alone, Ca lactate-gluconate alone and Polycalcium (50, 100 and 200
mg/kg), respectively, compared with the knee thickness in the OA
control group.
Changes in knee maximum extension
angles
The maximum extensor angle of the knee was
significantly (P<0.01) increased in the OA control as compared
with that in the sham control. However, this angle were
significantly (P<0.01) decreased in all test material-treated
groups as compared with that in the OA control. The Polycalcium (50
mg/kg)-treated group exhibited an inhibitory effect on the maximum
extensor angle increases similar to that in the group treated with
Ca lactate-gluconate alone. Polycan alone resulted in the least
significant reduction in the extensor angle (Table I).
The maximum extensor angle of the knee in the OA
group was increased by 93.29% compared with that in the sham
control. Reductions of 20.31, 14.14, 17.19, 33.51, 30.03 and 19.62%
were observed following treatment with diclofenac sodium, Polycan
alone, Ca lactate-gluconate alone, and Polycalcium (50, 100 and 200
mg/kg), respectively, compared with the maximum extensor angle in
the OA control group.
Changes in cartilage GAG contents
The levels of GAGs in the knee cartilage were
significantly (P<0.01) decreased in the OA control as compared
with those in the sham control. However, cartilage GAG contents
were significantly (P<0.01 or P<0.05) higher in all test
material-treated groups compared with those in the OA control. The
Polycalcium (50 mg/kg)-treated group exhibited an attenuation
effect on the reductions in cartilage GAG content similar to that
in the group treated with Ca lactate-gluconate alone. Polycan alone
resulted in the least significant increase in GAG content (Table II).
 | Table II.Knee cartilage GAG contents at
sacrifice after 28 days of continuous oral treatment with test
materials in OA rats. |
Table II.
Knee cartilage GAG contents at
sacrifice after 28 days of continuous oral treatment with test
materials in OA rats.
|
| Cartilage GAGs
contents (mg/g defatted tissues) |
|---|
|
|
|
|---|
| Groups | Chondroitin
sulfate | Heparan
sulfate | Hyaluronic
acid |
|---|
| Controls |
|
|
|
|
Sham | 77.28±10.67 | 224.59±32.68 | 2.35±0.41 |
| OA |
45.77±10.47a |
133.12±26.26a |
1.36±0.33e |
| Diclofenac |
57.19±9.04a, d |
164.56±20.38a, c |
1.69±0.20e, h |
| Polycan alone |
56.42±10.17a, d |
163.60±14.81a, c |
1.64±0.17e, h |
| Ca LG alone |
58.96±14.92a, d |
173.35±15.26a, c |
1.78±0.28e, h |
| Polycalcium |
|
|
|
| 200
mg/kg |
66.08±12.93b, c |
195.51±22.36a, c |
1.97±0.23f, g |
| 100
mg/kg |
63.12±12.34a, c |
183.05±17.69a, c |
1.89±0.16e, g |
| 50
mg/kg |
58.23±7.67a, d |
174.16±19.89a, c |
1.78±0.20e, g |
The cartilage chondroitin sulfate content of the
knee in the OA control was decreased by 40.77% compared with that
in the sham control. Increases of 24.94, 23.28, 28.81, 44.37, 37.91
and 27.22% relative to the content in the OA control were observed
following treatment with diclofenac sodium, Polycan alone, Ca
lactate-gluconate alone and Polycalcium (50, 100 and 200 mg/kg),
respectively.
The knee cartilage content of heparan sulfate in the
OA control was decreased by 40.72% compared with that in the sham
control. However, increases of 23.61, 22.89, 30.22, 46.86, 37.50
and 30.83% relative to those in the OA control were observed
following treatment with diclofenac sodium, Polycan alone, Ca
lactate-gluconate alone, and Polycalcium (50, 100 and 200 mg/kg),
respectively.
The knee cartilage content of hyaluronic acid in the
OA control was increased by 41.94% compared with that in the sham
control. Increases of 23.80, 20.43, 30.18, 44.54, 38.17 and 30.47%
relative to those in the OA control, were observed following
treatment with diclofenac sodium, Polycan alone, Ca
lactate-gluconate alone and Polycalcium (50, 100 and 200 mg/kg),
respectively.
Changes in the Mankin score
Various degrees of articular cartilage surface
damage, hypocellularity, clones and Safranin O staining intensity
were detected in the OA-induced groups; the total Mankin scores for
the tibia and femur of the OA control were significantly
(P<0.01) increased compared with those of the sham control.
However, the individual scores varied in all tested groups, and the
total Mankin scores in both the tibia and femur of all test
material-treated groups were significantly (P<0.01) decreased
compared with those in the OA control. The Polycalcium (50
mg/kg)-treated group demonstrated a reversal of cartilage damage
based on the Mankin score similar to that in the group treated with
Ca lactate-gluconate alone. Polycan alone resulted in the least
significant decrease in Mankin score (Tables III and IV; Fig.
2).
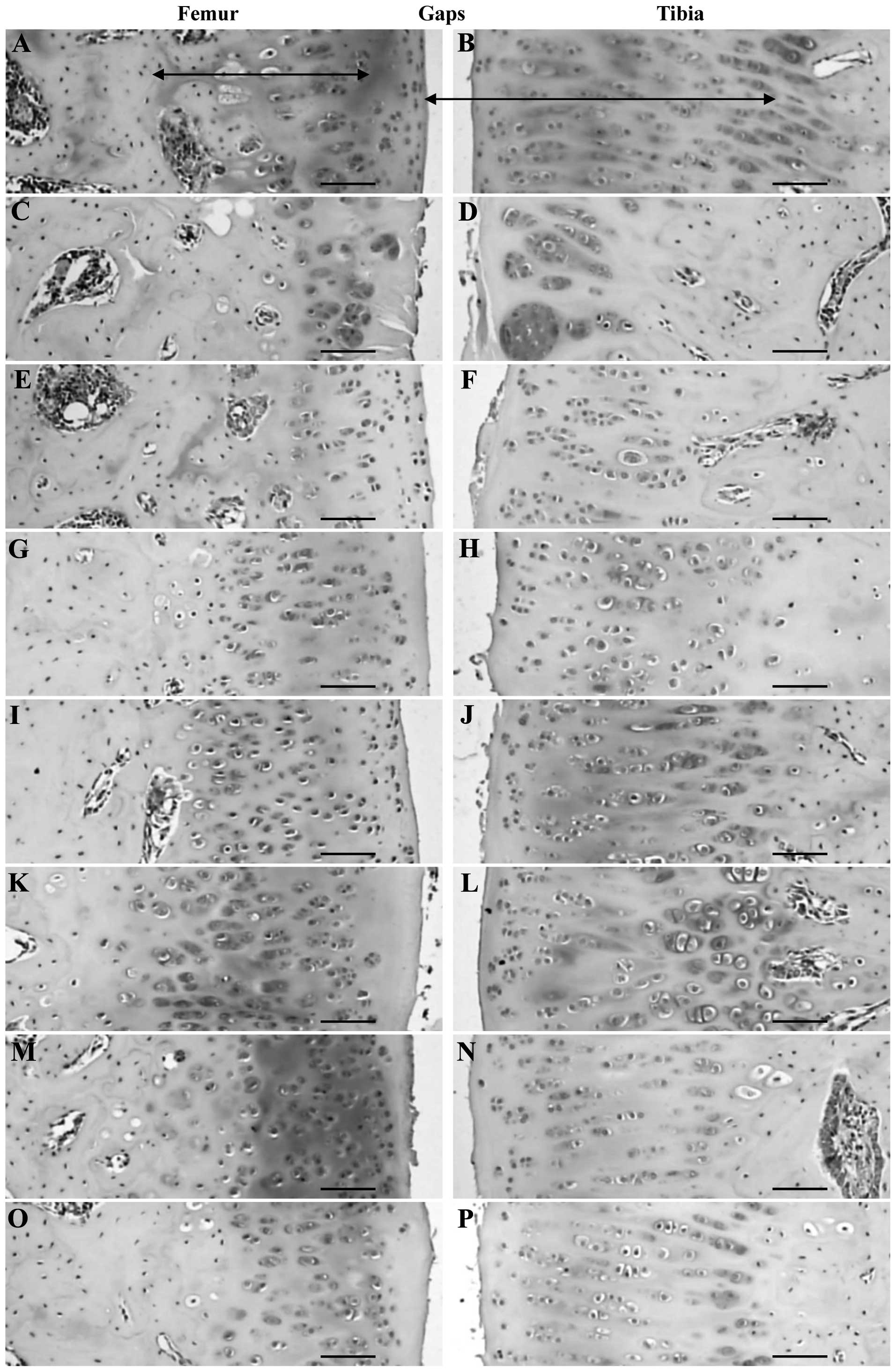 | Figure 2.Histopathological observations in the
articular surface cartilage of the femur and tibia of the (A and B)
sham control, (C and D) OA control, (E and F) diclofenac sodium, (G
and H) Polycan alone, (I and J) Ca LG alone and polycalcium at (K
and L) 200, (M and N) 100 and (O and P) 50 mg/kg groups. Note that
the articular surface of the femur was markedly damaged (based on
the Mankin scoring system, which showed abnormal changes on the
surface, hypocellularity, multiple clones and reductions in
Safranin O staining intensity) in the OA control with reductions in
cartilage thickness compared with the sham control. However, this
femur damage was markedly inhibited by all test materials. The
polycalcium (50 mg/kg)-treated group showed a similar inhibition of
articular histopathological damage as the Ca LG (100 mg/kg)-treated
group. Polycan alone showed the least favorable effects on
articular histopathological damage. OA, osteoarthritis; Ca,
calcium; LG, lactate-gluconate. Arrows indicate the thickness of
the articular cartilages. All Safranin O stained. Scale bars=160
µm. |
 | Table III.Mankin scores detected in the femur
at sacrifice after 28 days of continuous oral treatment with test
materials in OA rats. |
Table III.
Mankin scores detected in the femur
at sacrifice after 28 days of continuous oral treatment with test
materials in OA rats.
| Groups | Surface |
Hypocellularity | Clones | Safranin O | Totalsa |
|---|
| Controls |
|
Sham | 0.20±0.42 | 0.20±0.42 | 0.00±0.00 | 0.40±0.52 | 0.80±0.79 |
| OA |
2.60±0.52b |
2.10±0.88e |
2.40±0.70e |
2.60±0.52e |
9.70±1.42e |
| Diclofenac |
1.60±0.52b, d |
1.50±0.53e |
1.10±0.74e, g |
1.40±0.70e, g |
5.60±1.84e, g |
| Polycan single |
1.40±0.52b, d |
1.60±0.70e |
1.80±0.79e |
1.50±0.53e, g |
6.30±1.64e, g |
| Ca LG single |
1.30±0.82b, d |
1.40±0.97e |
1.20±0.63e, g |
1.40±0.52e, g |
5.30±2.31e, g |
| Polycalcium |
|
|
|
|
|
|
| 200
mg/kg |
0.90±0.88c, d |
0.70±0.67g |
0.80±0.79f, g |
0.30±0.48g |
2.70±2.50g |
| 100
mg/kg |
1.00±0.82b, d |
0.80±0.42f, g |
0.80±0.79e, g |
0.90±0.32g |
3.50±1.78e, g |
| 50
mg/kg |
1.50±0.71b, d |
0.80±0.63g |
1.40±0.52e, g |
1.30±0.48e, g |
5.00±1.56e, g |
 | Table IV.Mankin scores detected in tibia at
sacrifice after 28 days continuous oral treatment of test materials
in OA rats. |
Table IV.
Mankin scores detected in tibia at
sacrifice after 28 days continuous oral treatment of test materials
in OA rats.
| Groups | Surface |
Hypocellularity | Clones | Safranin O | Totalsa |
|---|
| Controls |
|
|
|
|
|
|
Sham | 0.20±0.42 | 0.30±0.67 | 0.10±0.32 | 0.10±0.32 | 0.70±1.06 |
| OA |
2.50±0.71b |
2.40±0.70b |
2.30±0.48e |
2.40±0.70e |
9.60±2.12e |
| Diclofenac |
1.90±0.88b |
1.30±0.67b, d |
0.90±0.57e, g |
1.50±0.85e, h |
5.60±2.55e, g |
| Polycan single |
1.20±0.63b, d |
1.20±0.92c, d |
1.50±0.71e, h |
1.60±0.70e, h |
5.50±2.55e, g |
| Ca LG single |
1.20±1.03b, d |
1.10±0.88c, d |
0.90±0.88f, g |
1.40±1.07e, h |
4.60±3.53e, g |
| Polycalcium |
| 200
mg/kg |
0.60±0.70d |
0.40±0.70d |
0.20±0.42g |
0.70±0.48f, g |
1.90±1.79g |
| 100
mg/kg |
0.80±0.79d |
0.90±0.88d |
0.40±0.52g |
1.00±0.94f, g |
3.10±2.81f, g |
| 50
mg/kg |
1.50±0.97b, d |
0.90±1.10d |
1.20±0.92e, g |
1.10±0.74e, g |
4.70±3.20e, g |
The total Mankin scores of the articular cartilage
of the femur in the OA control group were increased by 1,112.50%
compared with those in the sham control group. Reductions of 42.27,
35.05, 45.36, 72.16, 63.92 and 48.45% were observed after treatment
with diclofenac sodium, Polycan alone, Ca lactate-gluconate alone
and Polycalcium (50, 100 and 200 mg/kg), respectively, compared
with the total Mankin scores of the OA control group.
The total Mankin scores of induced tibia articular
cartilages in the OA control were increased by 1,271.43% compared
with those in the sham control. However, reductions of 41.67,
42.71, 52.08, 80.21, 67.71 and 51.04% were observed following
treatment with diclofenac sodium, Polycan alone, Ca
lactate-gluconate alone and Polycalcium (200, 100 and 50 mg/kg),
respectively, compared with the total Mankin scores in the OA
control group.
Changes in articular cartilage
thickness
Significant (P<0.01) reductions in articular
cartilage thickness were detected in the OA control compared with
those in the sham control in both the tibia and femur. However,
these reductions in cartilage thickness in the tibia and femur were
significantly inhibited by all test materials. The Polycalcium (50
mg/kg)-treated group demonstrated inhibitory effects on the
reductions in articular cartilage thickness that were similar to
those observed in the Ca lactate-gluconate group. Polycan alone
exhibited the least significant increases in articular cartilage
thickness (Table V, Fig. 2).
 | Table V.Histomorphometrical scores detected
at sacrifice after 28 days continuous oral treatment of test
materials in OA rats. |
Table V.
Histomorphometrical scores detected
at sacrifice after 28 days continuous oral treatment of test
materials in OA rats.
|
| Thickness of
articular cartilage (µm) |
|---|
|
|
|
|---|
| Groups | Femur | Tibia |
|---|
| Controls |
|
|
|
Sham | 703.29±96.20 | 962.58±119.30 |
| OA |
259.34±54.14a |
380.38±120.17a |
| Diclofenac |
336.46±45.30a |
485.19±70.07a, d |
| Polycan alone |
426.19±87.28a, c |
595.24±96.73a, c |
| Ca LG alone |
505.66±133.03a, c |
684.20±149.07a, c |
| Polycalcium |
|
|
| 200
mg/kg |
620.82±112.59b, c |
844.02±130.13b, c |
| 100
mg/kg |
588.41±79.31a, c |
758.48±105.01a, c |
| 50
mg/kg |
508.78±62.95a, c |
686.56±124.70a, c |
In the OA control group, the articular cartilage
thickness of the femur was decreased by 63.13% compared with that
in the sham control group. Increases of 29.74, 64.34, 94.98,
139.39, 126.89 and 96.18% were observed following treatment with
diclofenac sodium, Polycan alone, Ca lactate-gluconate alone and
Polycalcium (50, 100 and 200 mg/kg), respectively, compared with
the articular cartilage thickness in the OA control group.
The articular cartilage thickness of the tibia in
the OA control group was found to be decreased by 60.48% compared
with that in the sham control group. However, increases in
articular cartilage thickness of 27.55, 56.48, 79.87, 121.89, 99.40
and 80.49% were observed following treatment with diclofenac
sodium, Polycan alone, Ca lactate-gluconate alone and Polycalcium
(50, 100 and 200 mg/kg), respectively, compared with the articular
cartilage thickness in the OA control.
Changes in BrdU uptake
Significant (P<0.01) reductions in the numbers of
BrdU-immunoreactive cells were detected in the inner articular
membrane and the articular cartilage of the tibia and femur of the
OA control group compared with the those in sham control group.
However, these reductions in BrdU-immunoreactive cell numbers were
significantly (P<0.01) inhibited by treatment with test
materials, with the exception of diclofenac sodium; the BrdU
immunoreactivities detected in the inner articular membrane, femur
and tibia of the diclofenac sodium-treated group were similar to
those observed in the OA control. The Polycalcium (50
mg/kg)-treated group exhibited a reversal of the reduction in BrdU
immunoreactivity similar to that in the group treated with Ca
lactate-gluconate alone. Polycan alone resulted in the least
significant reversal in the reduction in BrdU immunoreactivity
(Table VI, Fig 3).
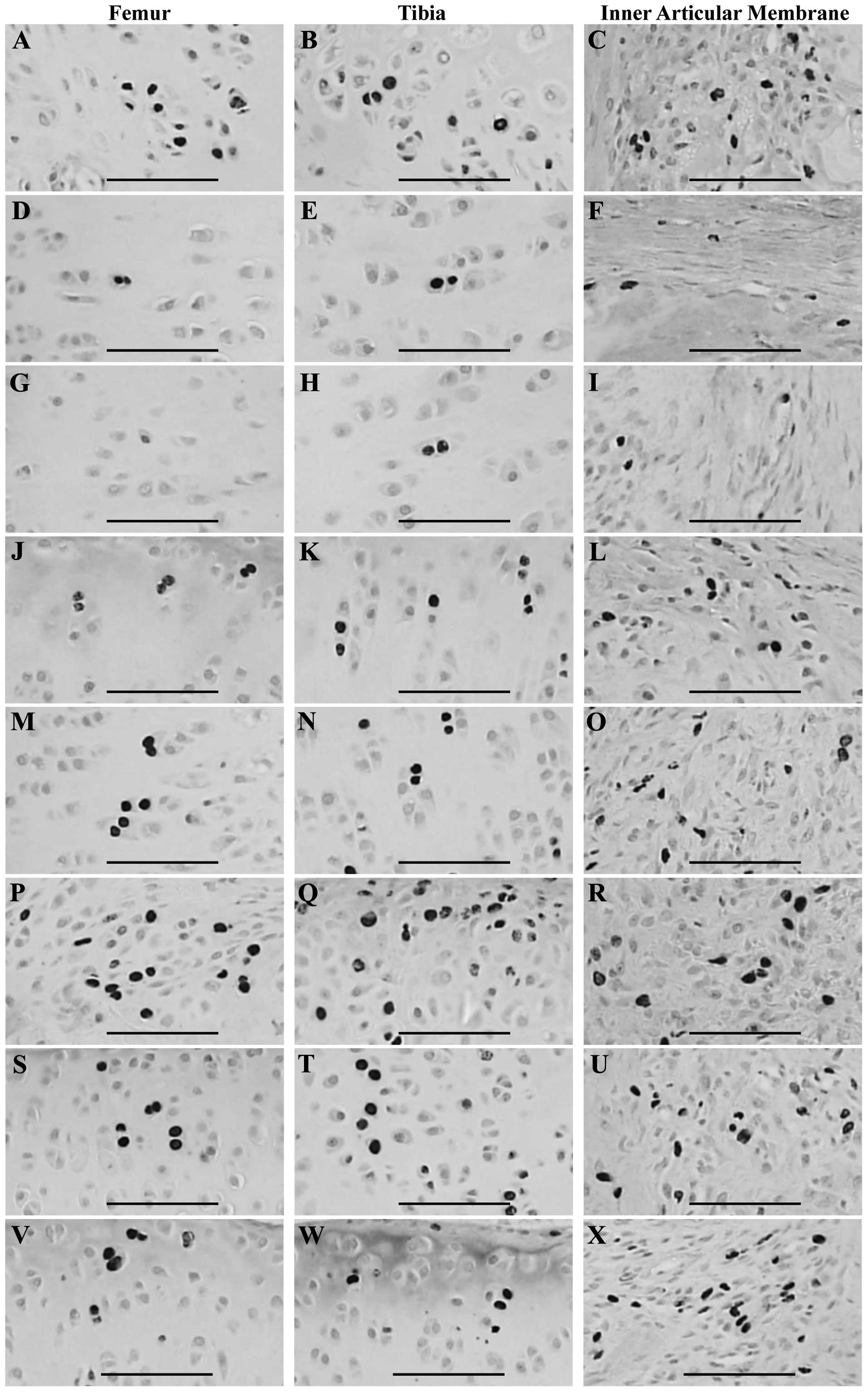 | Figure 3.BrdU-immunoreactive cells detected in
the articular surface cartilage of the femur and tibia and the
inner articular membranes in the (A–C) sham control, (D–F) OA
control, (G–I) diclofenac sodium, (J–L), Polycan alone, (M–O) Ca LG
alone and polycalcium at (P–R) 200, (S–U) 100 and (V–X) 50 mg/kg
groups. Note that similar numbers of BrdU-immunoreactive cells were
detected in the articular cartilage of the femur and tibia and
inner articular membrane of the OA control compared with the sham
control. However, these reductions in BrdU-immunoreactive cells
were significantly (P<0.01) inhibited by all test materials,
with the exception of diclofenac sodium, in which the BrdU
immunoreactivities detected in the inner articular membrane and
both femur and tibia were similar to those in the OA control. The
polycalcium (50 mg/kg)-treated group showed a similar inhibitory
effect on the reductions in BrdU immunoreactivity to those in the
group treated with Ca LG alone (100 mg/kg). Polycan alone showed
the least significant reversal of the reduction in BrdU
immunoreactivity. OA, osteoarthritis; Ca, calcium; LG,
lactate-gluconate. All processed by avidin biotin peroxidase
complex (ABC) methods. Scale bars=160 µm. |
 | Table VI.BrdU-immunoreactive cell numbers
detected at sacrifice after 28 days of continuous oral treatment of
test materials in OA rats. |
Table VI.
BrdU-immunoreactive cell numbers
detected at sacrifice after 28 days of continuous oral treatment of
test materials in OA rats.
|
| Number of
BrdU-immunoreactive cells (%) |
|---|
|
|
|
|---|
| Groups | Femur | Tibia | Inner articular
membrane |
|---|
| Controls |
|
|
|
|
Sham | 18.70±2.71 | 23.60±4.17 | 55.30±13.74 |
| OA |
3.80±3.01a |
5.20±2.44c |
14.30±5.08c |
| Diclofenac |
3.90±2.96a |
5.30±2.31c |
14.40±6.00c |
| Polycan single |
7.20±2.49a, b |
8.80±1.32c, e |
28.00±4.29c, e |
| Ca LG single |
9.50±1.58a, b |
11.00±1.63c, e |
31.70±7.60c, e |
| Polycalcium |
|
|
|
| 200
mg/kg |
17.80±2.82b |
26.70±5.74e |
51.70±9.59e |
| 100
mg/kg |
13.70±3.27a, b |
23.20±3.88e |
44.39±9.39d, e |
| 50
mg/kg |
10.80±2.10a, b |
12.90±3.67c, e |
32.90±5.38c, e |
The numbers of BrdU-immunoreactive cells in the
articular cartilage of the femur in the OA control were decreased
by 79.68% compared with those in the sham control. Increases of
2.63, 89.47, 150.00, 368.42, 260.53 and 184.21% were observed
following treatment with diclofenac sodium, Polycan alone, Ca
lactate-gluconate alone or Polycalcium (50, 100 and 200 mg/kg),
respectively, compared with the BrdU-immunoreactive cell numbers in
the OA control.
The BrdU-immunoreactive cell numbers in the
articular cartilage of the tibia of the OA control were decreased
by 77.97% compared with those in the sham control. Increases in
BrdU-immunoreactive cell numbers of 0.70, 95.80, 121.68, 261.54,
209.79 and 130.07% were observed following treatment with
diclofenac sodium, Polycan alone, Ca lactate-gluconate alone and
Polycalcium (50, 100 and 200 mg/kg), respectively, compared with
those in the OA control.
The BrdU-immunoreactive cell numbers in the inner
articular membranes of the OA control were decreased by 74.14%
compared with those in the sham control. Increases of 1.92, 69.23,
111.54, 413.46, 346.15 and 148.08% were observed following
treatment with diclofenac sodium, Polycan alone, Ca
lactate-gluconate alone and Polycalcium (50, 100 and 200 mg/kg),
respectively, compared with the BrdU-immunoreactive cell numbers in
the OA control.
Discussion
Osteoarthritis (OA) is a progressive rheumatic
disease in which the articular cartilage degenerates. It is the
most common rheumatic disorder and is likely to become one of the
most prevalent and costly diseases (30). Paracetamol may be used as a drug
therapy for OA, alone or in combination with codeine. Topical and
oral NSAIDs, such as diclofenac and ibuprofen, are also used to
mitigate pain and improve function in patients with OA (2,3).
However, the dose and duration of treatment with oral NSAIDs should
be minimized to reduce the risk of associated morbidities, such as
cardiovascular, gastrointestinal, liver or renal complications
(31). Therefore, there is a
requirement for drugs with good efficacy and low toxicity to treat
OA.
In a previous study, Polycalcium, which comprises a
mixture of Polycan and Ca lactate-gluconate in a 1:9 ratio by
weight, showed the most favorable and synergistic effects on
osteoporotic rats among three Polycan and Ca lactate-gluconate
mixtures (1:99, 5:95 and 10:90 by weight) (18). In the present study, the beneficial
effects of Polycalcium on OA were confirmed in comparison with
Polycan and Ca lactate-gluconate alone.
In the present study, no significant changes in body
weight were detected in the test material-treated groups compared
with the OA control.
OA is a degenerative joint disease and a chronic
inflammatory condition. Cartilage damage in OA can lead to
edematous changes in the surrounding tissues and increases in the
thickness of affected joints (32).
The thickness of the operated knees increased significantly in the
present study. However, the surgery-induced changes in knee
thickness were reversed by the test materials. The
Polycalcium-treated group showed more favorable inhibitory effects
on the increases in knee thickness, regardless of capsule exposure,
compared with those in the groups treated with Polycan or Ca
lactate-gluconate alone. The Polycalcium (50 mg/kg)-treated group
demonstrated a similar reversal of the increase in knee thickness
to that observed in the group treated with Ca lactate-gluconate
alone. Polycan alone showed the least significant reduction in knee
thickness. These favorable effects on knee thickness may be due to
the anti-inflammatory properties of Polycan (14,15)
and/or Ca salts (6,7), as described previously. These results
demonstrate that appropriate mixtures of Polycan and Ca
lactate-gluconate are able to induce favorable synergistic
anti-inflammatory effects in OA rats.
Anterior cruciate ligament transection and partial
medial meniscectomy increases the maximum extension angles (limited
extension values), causes edematous changes in the knees and
capsule thickness, and decreases chondrocyte proliferation
(detected by BrdU uptake) and the levels of cartilage GAGs
(chondroitin sulfate, heparan sulfate and hyaluronic acid). It also
causes marked degenerative changes in the cartilage, affect the
Mankin score and reduce articular cartilage, which are classic
symptoms of osteoarthritis.
However, these OA-associated changes were inhibited
after 28 days of continuous oral treatment with three doses of
Polycalcium compared with the OA control. Favorable anti-OA effects
were detected in Polycalcium-treated rats compared with those in
rats treated with Polycan and Ca lactate-gluconate alone.
Additionally, Polycalcium (50 mg/kg) showed favorable effects
similar to those of Ca lactate-gluconate (100 mg/kg) alone.
Fibrosis in OA is the result of chronic inflammatory
processes that limit joint motion; joint stiffness is a major
symptom of OA. Joint stiffness has been evaluated using the maximum
extension angle of the joint, considering 0° as the maximum
extension, with lower values representing better knee function
(19). Increases in maximum
extension angles in the knees were inhibited by all test materials,
suggesting that they are able to ameliorate OA.
Although diclofenac sodium did not affect BrdU
uptake, the number of BrdU-immunoreactive cells increased following
treatment with Polycalcium, suggesting that Polycalcium induces the
proliferation of chondrocytes in the inner articular membranes and
surface articular cartilage of the tibia and femur. In addition,
higher BrdU-immunoreactivities were detected in Polycalcium-treated
rats than in rats treated with Polycan and Ca lactate-gluconate;
Polycalcium (50 mg/kg) showed BrdU-immunoreactivities similar to
those of Ca lactate-gluconate alone in rats in the present
study.
As OA progresses, the loss of various components of
the extracellular cartilage matrix, particularly GAGs, has been
reported (33). Extracellular
cartilage matrix contains sulfide proteoglycans such as chondroitin
sulfate, heparin sulfate, keratin sulfate and dermatan sulfate
(34), and changes in sulfide
proteoglycans in cartilage or blood have been used as markers of OA
progression because they are released from damaged cartilage to the
blood during OA (35). In addition,
hyaluronic acid is a relatively large polysaccharide secreted by
type B synoviocytes in the joints. Therefore, a reduction in the
hyaluronic acid content of cartilage is a useful marker of OA
(36). In the present study, the
cartilage GAG levels of the operated knees decreased significantly.
However, these changes in cartilage GAGs were reduced by treatment
with the test materials. The Polycalcium-treated group showed
inhibitory effects on the reductions in cartilage GAG content that
were more favorable than those in the groups treated with Polycan
or Ca lactate-gluconate alone, and the Polycalcium (50
mg/kg)-treated group exhibited inhibitory effects on the reductions
in cartilage GAG content similar to those in the group treated with
Ca lactate-gluconate alone. Polycan alone showed the least
significant beneficial effects on cartilage GAG content.
The Mankin scoring system is a common
histopathological evaluation method used to detect articular
cartilage injuries. In this system, the higher the score, the
higher the level of OA (19,24). Favorable reductions in the Mankin
score were observed following treatment with the test materials in
the present study; thus, the test materials ameliorated OA. Marked
reductions in articular cartilage thickness in OA have been
reported (27). In the present
study, all test materials effectively inhibited reductions in
articular cartilage thickness. The Polycalcium-treated group showed
more favorable inhibition effects on the increases in Mankin scores
and cartilage loss compared with those in the groups treated with
Polycan or Ca lactate-gluconate alone, and the Polycalcium (50
mg/kg)-treated group exhibited similar inhibition effects on the
increases in Mankin score and cartilage loss to those observed in
the group treated with Ca lactate-gluconate alone. Polycan alone
showed the least significant reductions in Mankin score and
cartilage preservation.
Among the various methods of detecting cell
proliferation in histological sections, immunohistochemistry for
BrdU is preferable (27,37). BrdU staining is easier to interpret
and reflects the cell proliferation more specifically than other
staining (26). In addition, BrdU
uptake can be used to detect chondrocyte proliferation in
OA-affected cartilage (27). Cells
containing BrdU represent proliferated or proliferating cells. In
the present study, the number of BrdU-immunoreactive cells
decreased significantly in the inner articular membrane and surface
articular cartilage of the femur and tibia in the OA control,
suggesting that the proliferation of chondrocytes was inhibited
significantly. Although diclofenac sodium did not affect BrdU
uptake, BrdU-immunoreactive cells increased following treatment
with all three doses of Polycalcium, suggesting that Polycalcium
induces the proliferation of chondrocytes in the inner articular
membranes and surface articular cartilage of the tibia and femur.
In addition, higher BrdU-immunoreactivity was detected in
Polycalcium-treated rats than was detected in rats treated with
Polycan and Ca lactate-gluconate alone; Polycalcium (50 mg/kg)
showed BrdU-immunoreactivities comparable with those of Ca
lactate-gluconate alone (100 mg/kg) in the present study. The
cartilage proliferative effects of Polycan (16) and Ca salts (17) have been reported previously.
The results obtained in this study suggest that
28-day continuous oral treatment with Polycalcium reduces articular
stiffness and histological cartilage damage compared with that in
OA controls, and may induce chondrocyte proliferation based on BrdU
uptake. More favorable anti-OA effects based on chondrocyte
proliferation were detected in Polycalcium-treated rats than were
detected in rats treated with Polycan or Ca lactate-gluconate
alone; Polycalcium (50 mg/kg) showed favorable effects on OA
similar to those of Ca lactate-gluconate alone (100 mg/kg).
Therefore, a mixture of Polycan and Ca lactate-gluconate exhibited
favorable synergistic anti-OA effects.
References
|
1
|
De Silva V, El-Metwally A, Ernst E, et al:
Arthritis Research UK Working Group on Complementary and
Alternative Medicines: Evidence for the efficacy of complementary
and alternative medicines in the management of osteoarthritis: a
systematic review. Rheumatology (Oxford). 50:911–920. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Long L, Soeken K and Ernst E: Herbal
medicines for the treatment of osteoarthritis: a systematic review.
Rheumatology (Oxford). 40:779–793. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walker J: Management of osteoarthritis.
Nurs Older People. 23:14–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buchanan WW: Implications of NSAID therapy
in elderly patients. J Rheumatol Suppl. 20:29–32. 1990.PubMed/NCBI
|
|
5
|
Tramèr MR, Moore RA, Reynolds DJ and
McQuay HJ: Quantitative estimation of rare adverse events which
follow a biological progression: a new model applied to chronic
NSAID use. Pain. 85:169–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piller NB: Assessment of anti-inflammatory
activity of calcium dobesilate. Effect on macrophages attaching to
subcutaneously implanted cover slips in guinea pigs.
Arznemittelforchung. 40:698–700. 1990.
|
|
7
|
Smith MM, Ghosh P, Numata Y and Bansal MK:
The effects of orally administered calcium pentosan polysulfate on
inflammation and cartilage degradation produced in rabbit joints by
intraarticular injection of a hyaluronate-polylysine complex.
Arthritis Rheum. 37:125–136. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sosa M and Bregni C: Metabolism of the
calcium and bioavailability of the salts of most frequent use. Boll
Chim Farm. 142:28–33. 2003.PubMed/NCBI
|
|
9
|
Heaney RP, Recker RR, Watson P and Lappe
JM: Phosphate and carbonate salts of calcium support robust bone
building in osteoporosis. Am J Clin Nutr. 92:101–105. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo HP, Kim JM, Shin HD, Kim TK, Chang HJ,
Park BR and Lee JW: Production of β-1,3/1,6-glucan by Aureobasidium
pullulans SM-2001. Korean Journal of Biotechnology and
Bioengineering. 17:376–380. 2002.(In Korean).
|
|
11
|
Song HB, Park DC, Do GM, Hwang SL, Lee WK,
Kang HS, Park BR, Jang HJ, Son CW, Park EK, Kim SY and Huh TL:
Effect of exopolymers of Aureobasidium pullulans on improving
osteoporosis induced in ovariectomized mice. J Microbiol
Biotechnol. 16:37–45. 2006.
|
|
12
|
Shin HD, Yang KJ, Park BR, Son CW, Jang HJ
and Ku SK: Antiosteoporotic effect of Polycan, beta-glucan from
Aureobasidium, in ovariectomized osteoporotic mice. Nutrition.
23:853–860. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HS, Cho HR, Moon SB, Shin HD, Yang KJ,
Park BR, Jang HJ, Kim LS and Ku SK: Effect of β-glucan from
Aureobasidium pullulans on rat rib fracture healing. Lab Anim Res.
24:39–44. 2008.
|
|
14
|
Kim HD, Cho HR, Moon SB, Shin HD, Yang KJ,
Park BR, Jang HJ, Kim LS, Lee HS and Ku SK: Effect of exopolymers
from Aureobasidum pullulans on formalin-induced chronic paw
inflammation in mice. J Microbiol Biotechnol. 16:1954–1960.
2006.
|
|
15
|
Kim HD, Cho HR, Moon SB, Shin HD, Yang KJ,
Park BR, Jang HJ, Kim LS, Lee HS and Ku SK: Effects of β-glucan
from Aureobasidum pullulans on acute inflammation in mice. Arch
Pharm Res. 30:323–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JW, Cho HR and Ku SK: Efficacy test of
Polycan, a geta-glucan originated from Aureobasidium pullulans
SM-2001, on anterior cruciate ligament transection and partial
medial meniscectomy-induced-osteoarthritis Rats. J Microbiol
Biotechnol. 22:274–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang SJ, Kim JW, Kim KY, Ku SK and Lee YJ:
Protective effects of calcium gluconate on osteoarthritis induced
by anterior cruciate ligament transection and partial medial
meniscectomy in Sprague-Dawley rats. J Orthop Surg Res. 9:142014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi JS, Kim JW, Kim KY, Cho HR, Choi IS
and Ku SK: Anti-osteoporotic effects of Polycan in combination with
calcium lactate-gluconate in ovariectomized rats. Exp Ther Med.
8:957–967. 2014.PubMed/NCBI
|
|
19
|
Rezende MU, Gurgel HM, Vilaça Junior PR,
et al: Diacerhein versus glucosamine in a rat model of
osteoarthritis. Clinics (Sao Paulo). 61:461–466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Homer KA, Denbow L and Beighton D:
Spectrophotometric method for the assay of glycosaminoglycans and
glycosaminoglycan-depolymerizing enzymes. Anal Biochem.
214:435–441. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Camplejohn KL and Allard SA: Limitations
of safranin ‘O’ staining in proteoglycan-depleted cartilage
demonstrated with monoclonal antibodies. Histochemistry.
89:185–188. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kahveci Z, Minbay FZ and Cavusoglu L:
Safranin O staining using a microwave oven. Biotech Histochem.
75:264–268. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tran D, Golick M, Rabinovitz H, Rivlin D,
Elgart G and Nordlow B: Hematoxylin and safranin O staining of
frozen sections. Dermatol Surg. 26:197–199. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Armstrong S, Read R and Ghosh P: The
effects of intraarticular hyaluronan on cartilage and subchondral
bone changes in an ovine model of early osteoarthritis. J
Rheumatol. 21:680–688. 1994.PubMed/NCBI
|
|
25
|
Lovász G, Park SH, Ebramzadeh E, Benya PD,
et al: Characteristics of degeneration in an unstable knee with a
coronal surface step-off. J Bone Joint Surg Br. 83:428–436. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang YI, Yoo YB and Baik SH: Comparative
study of rat thyroid regeneration using PCNA and BrdU
immunohistochemistry. Korean J Anat. 33:247–254. 2000.
|
|
27
|
Moore EE, Bendele AM, Thompson DL, Littau
A, Waggie KS, Reardon B and Ellsworth JL: Fibroblast growth
factor-18 stimulates chondrogenesis and cartilage repair in a rat
model of injury-induced osteoarthritis. Osteoarthritis Cartilage.
13:623–631. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ito T, Mitui H, Udaka N, Hayashi H,
Okudela K, Kanisawa M and Kitamura H: Ki-67 (MIB 5) immunostaining
of mouse lung tumors induced by 4-nitroquinoline 1-oxide. Histochem
Cell Biol. 110:589–593. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang X, Falls DL, Li X, Lane T and Luskin
MB: Antigen-retrieval procedure for bromodeoxyuridine
immunolabeling with concurrent labeling of nuclear DNA and antigens
damaged by HCl pretreatment. J Neurosci. 27:5837–5844. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brooks PM and March LA: New insights into
osteoarthritis. Med J Aust. 163:367–369. 1995.PubMed/NCBI
|
|
31
|
National Institute for Health and Clinical
Excellence: COX II Inhibitors for the Treatment of Osteoarthritis
and Rheumatoid Arthritis. NICE Technology Appraisal TA27. 2001.
|
|
32
|
Guo JS, Ou L, Zhou J, Wang XJ and Guo X:
Impact on the model of rat osteoarthritis of jingu tablet. Zhongguo
Zhong Yao Za Zhi. 31:232–235. 2006.(In Chinese). PubMed/NCBI
|
|
33
|
Reddy GK and Dhar SC: Metabolism of
glycosaminoglycans in tissues of adjuvant arthritic rat. Mol Cell
Biochem. 106:117–124. 1991.PubMed/NCBI
|
|
34
|
Haraoui B, Thonar EJ, Martel-Pelletier J,
Goulet JR, Raynauld JP, Ouellet M and Pelletier JP: Serum keratan
sulfate levels in rheumatoid arthritis: inverse correlation with
radiographic staging. J Rheumatol. 21:813–817. 1994.PubMed/NCBI
|
|
35
|
Thonar EJ, Lenz ME, Klintworth GK,
Caterson B, Pachman LM, Glickman P, Katz R, Huff J and Kuettner KE:
Quantification of keratan sulfate in blood as a marker of cartilage
catabolism. Arthritis Rheum. 28:1367–1376. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Emlen W, Niebur J, Flanders G and Rutledge
J: Measurement of serum hyaluronic acid in patients with rheumatoid
arthritis: correlation with disease activity. J Rheumatol.
23:974–978. 1996.PubMed/NCBI
|
|
37
|
Ganey T, Libera J, Moos V, Alasevic O,
Fritsch KG, Meisel HJ and Hutton WC: Disc chondrocyte
transplantation in a canine model: a treatment for degenerated or
damaged intervertebral disc. Spine (Phila Pa 1976). 28:2609–2620.
2003. View Article : Google Scholar : PubMed/NCBI
|

















